International bi-monthly journal of cell signaling, tissue protection, and translational research.
Extracellular matrix peptides as theranostic mediators of ischemic stroke neuroprotection and repair: lessons from studies in remote ischemic preconditioning and exercise
Kathleen E. Salmeron1,2, Katherine Poinsatte4, Tony J. Parker5,6, Ann M. Stowe4, Gregory J Bix1,2,3
Author Affiliations


- 1Sanders-Brown Center on Aging, University of Kentucky, Lexington, Kentucky, USA
- 2Department of Neuroscience, University of Kentucky, Lexington, Kentucky, USA
- 3Department of Neurology, University of Kentucky, Lexington, Kentucky, USA
- 4Department of Neurology & Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, Texas, USA
- 5Tissue Repair and Translational Physiology Program, Institute of Health and Biomedical Innovation
- 6School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, Queensland, Australia
Abstract
Ischemic stroke remains one of the leading causes of long-term disability and death worldwide, despite being a preventable neurological injury. Efforts in prevention have included urging patients toward a healthier lifestyle, including a healthy diet and regular exercise. Experimental and clinical evidence supporting these healthy-living recommendations found that systemic interventions like exercise impart protection for both rodents and humans. Despite these data, patients who are at the highest risk for stroke are unlikely to make these long-term lifestyle changes. Herein lies a challenge for clinicians and researchers alike to identify pharmaceutical or physical substitutions for exercise (e.g. limb remote ischemic conditioning (RIC)) that can be administered upon identification of a stroke, or a high risk of stroke, in the clinic. One impact of exercise is to promote vascular remodeling in the skeletal muscle. A chief component of this remodeling is proteolysis of the vascular extracellular matrix. Likewise, similar remodeling of the extracellular matrix occurs in the brain following focal ischemia. Among the most heavily proteolyzed matrix components is perlecan, a heparan sulfate proteoglycan. Importantly, we found that this proteolysis generates neuroprotective fragments, including the LG3 peptide, following experimental stroke. Exercise also increases LG3 peptide levels in urine and serum, making this not only a mediator of exercise-mediated neuroprotection, but also a potential theranostic biomarker for an efficacious delivery of systemic interventions like exercise. In this review, we discuss the potential benefits and mechanisms of exercise and RIC, and propose that the LG3 peptide could be a theranostic for stroke recovery.
Abstract
Ischemic stroke remains one of the leading causes of long-term disability and death worldwide, despite being a preventable neurological injury. Efforts in prevention have included urging patients toward a healthier lifestyle, including a healthy diet and regular exercise. Experimental and clinical evidence supporting these healthy-living recommendations found that systemic interventions like exercise impart protection for both rodents and humans. Despite these data, patients who are at the highest risk for stroke are unlikely to make these long-term lifestyle changes. Herein lies a challenge for clinicians and researchers alike to identify pharmaceutical or physical substitutions for exercise (e.g. limb remote ischemic conditioning (RIC)) that can be administered upon identification of a stroke, or a high risk of stroke, in the clinic. One impact of exercise is to promote vascular remodeling in the skeletal muscle. A chief component of this remodeling is proteolysis of the vascular extracellular matrix. Likewise, similar remodeling of the extracellular matrix occurs in the brain following focal ischemia. Among the most heavily proteolyzed matrix components is perlecan, a heparan sulfate proteoglycan. Importantly, we found that this proteolysis generates neuroprotective fragments, including the LG3 peptide, following experimental stroke. Exercise also increases LG3 peptide levels in urine and serum, making this not only a mediator of exercise-mediated neuroprotection, but also a potential theranostic biomarker for an efficacious delivery of systemic interventions like exercise. In this review, we discuss the potential benefits and mechanisms of exercise and RIC, and propose that the LG3 peptide could be a theranostic for stroke recovery.
Introduction
Ischemic stroke remains one of the leading causes of long-term disability and death worldwide, despite being a preventable neurological injury. Very few interventions can successfully protect against ischemic injury, but to date, exercise and a healthy diet are among the best modifiable interventions that a person can do to reduce the risk of stroke and other cardiovascular diseases (Thompson et al., 2003). While researchers and clinicians alike agree that prophylactics like exercise are a powerful way to prevent ischemic injury, convincing patients to change their lifestyle presents a formidable challenge. Because of this obstacle, researchers continue to attempt to find new, more immediate interventions to protect at-risk populations. One promising intervention is remote ischemic conditioning (RIC). During RIC, patients undergo, for example, triplicate cycles of 5-min inflation of a blood pressure cuff placed around the upper arm (~200 mmHg) and 5 min of deflation (Loukogeorgakis et al., 2005). This intervention is non-invasive, requires minimal effort from individuals, and can be safely used in at-risk populations. In this review, we propose that exercise and RIC share both humoral and neuronal mechanisms, including remodeling of the extracellular matrix (ECM), that promote neuroprotection against injury.
During ischemic injury, the ECM in the brain is heavily and rapidly processed by proteases. The breakdown of the ECM contributes to the breakdown of the blood-brain barrier (BBB), ultimately resulting in the development of edema and contributing to neuroinflammation in the brain (Salmeron et al., 2017). Given its contribution to stroke pathology, ECM degradation has been almost exclusively viewed in a negative light. However, brain ECM proteolysis may also lead to the release of components of the BBB that contribute to repair and recovery (Roberts et al., 2012). Perlecan, a major heparan sulfate proteoglycan (HSPG) ECM component of the BBB, is rapidly proteolyzed after stroke (Fukuda et al., 2004; Bix, 2013). We previously found that a bioactive 85-kDa protein fragment of perlecan called domain V (DV), as well as a smaller (25-kDa) portion of DV known as LG3, are immediately and persistently upregulated after stroke and exert a neuroprotective effect on the brain through mechanisms shared between exercise and RIC (Saini and Bix, 2012; Moon et al., 2016). This, combined with our earlier evidence indicating that LG3 is elevated in the urine of physically active individuals (Parker et al., 2012), suggests an attractive, exercise-associated (and potentially RIC-associated) “theranostic” for more effective “prescription” of rehabilitation regimens like exercise, both prior to and following stroke.
The benefits of systemic interventions in post-stroke outcomes
Studying exercise as prophylaxis for acute incidents began in the context of ischemia in the heart, specifically protection from myocardial infarction (MI) (Murray et al., 1986). Multitudes of clinical studies focused on outcomes in physically active individuals compared to their sedentary peers following MI, and consistently demonstrated that the more “physically fit” the patient is, the better they will fare during recovery (Warburton et al., 2006). Soon after this discovery, similar phenomena were observed in the brain, specifically in acute neurological events such as TBI (Taylor et al., 2015) and stroke (Meng et al., 2012; Meng et al., 2015; Shamsaei et al., 2017). Regular exercise reduces the risk of stroke, decreases stroke severity, and improves functional outcome (Lee et al., 2003; Alevizos et al., 2005; Diep et al., 2010). Based on these observations in the clinic, basic and translational studies began using animal models to study how pre-stroke exercise training induced neuroprotection against stroke (see reviews (Zhang et al., 2011; Wang et al., 2014). Two major forms of exercise training are used in rodent stroke models – forced and voluntary exercise -- each with distinct differences in the resulting neuroprotection. While forced exercise (short, intense bursts of exercise) leads to greater reductions in infarct volumes (Egan et al., 2014), it is stressful for the animals, as shown by increased corticosterone levels in the serum (Ke et al., 2011). Conversely, rats that underwent voluntary exercise (unrestricted access to running wheels) had higher hippocampal brain-derived neurotrophic factor (BDNF, a factor that supports neuronal growth and survival) levels and improved motor recovery (Ke et al., 2011).
At the same time as research began to delve into mechanisms behind exercise-induced neuroprotection, a new intervention, RIC, arrived on the scene. During RIC, an individual (or animal) undergoes brief repetitive periods of ischemia at a remote site before a longer, pathological ischemic injury in a target organ. Typically, in the clinic, a patient will undergo a 5-minute inflation (200 mmHg) of a blood pressure cuff on the upper arm or leg 3 times, separated by 5 minutes of deflation (Loukogeorgakis et al., 2005). Three major kinds of RIC have been developed to induce neuroprotection – remote ischemic preconditioning (RIPC), perconditioning (RIPerC), and postconditioning (RIPostC) – and these are used before, during, and after stroke, respectively. Numerous animal studies show reductions in infarct volumes, as well as improvements in functional recovery, following these interventions (Pan et al., 2016). Recent clinical trials have supported the benefits of pre- and per-conditioning.
Briefly, as outlined in Table 1, RIPC has shown benefit for patients at high risk of having recurrent stroke (those who have symptomatic internal carotid artery stenosis (IAS) caused by atherosclerosis) (Meng et al., 2012) or for patients who are over 80 years of age with no history of stroke (Meng et al., 2015). In the context of symptomatic IAS, patients who received RIPC experienced reduced recurrent stroke rates, reduced time to recovery, and improved cerebral perfusion upon administration of intravenous (IV) tissue plasminogen activator (tPA), still the only approved post-stroke pharmacological intervention in the United States. Furthermore, patients over 80 with no history of stroke experienced fewer strokes and transient ischemic attacks (TIAs) and had overall reduced cerebral inflammation.
RIPerC, delivered at or near the time of stroke onset, also demonstrates benefit for those patients diagnosed with (Blauenfeldt et al., 2017), or suspected of having (Hougaard et al., 2014), an acute ischemic stroke. Patients with a confirmed ischemic stroke, and who had reported having exercised the week prior to stroke, received added benefit from RIPerC. They experienced decreased 24-hour infarct development and smaller infarcts one month following stroke (Blauenfeldt et al., 2017). Patients with a suspected ischemic stroke receiving RIPerC as an adjunct to IV tPA also presented with more TIAs (and thus not acute ischemic or hemorrhagic infarcts), lower functional deficit scores, and increased tissue survival after one month following stroke (Hougaard et al., 2014). These results suggest the RIC induces neuroprotection even when delivered at the time of stroke onset.
Table 1
|
Arial |
Patients | Stimuli | Results | Reference |
|
Preconditioning |
Patients with symptomatic atherosclerotic IAS who had previously had an IS or TIA |
5 cycles of bilateral upper limb ischemia (2000 mm Hg) for 5 minutes followed by reperfusion for another 5 minutes, 2x a day for 300 consecutive days |
Reduced recurrent stroke rate at 90 and 300 days; Reduced time to recovery; Improved cerebral perfusion status |
|
|
Patients with IAS, >80 years of age, no previous stroke |
5 cycles of bilateral arm ischemia followed by reperfusion for 5 minutes, 2x a day for 180 consecutive days |
Reduced strokes and TIAs; Reduced inflammation |
||
|
Perconditioning |
Acute IS patients |
Protocol not described |
Physical activity the week before the stroke correlated with decreased infarct development at 24 hours and smaller infarct size at 1 month post-stroke |
|
|
Patients with suspected acute stroke |
5 min inflations of upper limb blood cuff to either 200 or 25 mm Hg above patient’s systolic blood pressure, with 5 min deflations in between delivered in ambulance |
Increased TIAs and lower NIHSS scores; Increased tissue survival at 1 month; No change in final infarct size or infarct growth |
||
|
IAS - intracranial arterial stenosis; IS - ischemic stroke; TIA - transient ischemic attack; |
||||
Shared mechanisms of RIC and exercise-induced neuroprotection
Despite the dramatic differences in stimuli, both exercise and RIC result in systemic, global changes throughout the body, and thus may share several common mechanisms. Both interventions induce changes to remote organs, in particular the muscle, leading to the production of protective signals that are transmitted to the brain predominantly through two pathways: humoral and neuronal (Pan et al., 2016).
Humoral mechanisms of protection
Both RIC and exercise modulate blood flow throughout the body. During RIC, brief periods of reperfusion follow short ischemic events in remote muscle. These alterations in blood flow could allow for blood to “wash out” protective signals from the muscle and transport them to a distal organ, such as the brain, where they can induce protection against injury (Pan et al., 2016). During exercise, increased metabolic demands induce vasodilation, promoting the movement of blood from the active muscle to remote sites throughout the body, also potentially allowing for the transport of humoral factors to the brain (Thomas et al., 2004). Exercise remotely alters blood flow, including increased cerebral blood flow (CBF) up to ~60% of maximal oxygen uptake (Ogoh and Ainslie, 2009).
Many studies examining humoral mechanisms of protection have investigated RIC in the context of myocardial infarcts. One seminal study found that blood taken from a subject (human or rabbit) who had previously undergone RIC (via repeated inflation of a blood pressure cuff (human) or tourniquet (rabbit)), and then subsequently injected into a naïve subject (rabbit), conveyed significant protection after cardiac ischemic reperfusion injury, hinting at the presence of protective factors within the blood (Shimizu et al., 2009). Further studies of the heart found that occluding the femoral vein, and thus blocking the wash-out of cardioprotective signals from the ischemic limb, ablated RIC-induced protection against myocardial infarct (Lim et al., 2010). Studies of RIC in stroke models confirmed several neuroprotective humoral factors including erythropoietin (EPO), adenosine, heme oxygenase-1, and nitric oxide (NO) (Hu et al., 2012; Peng et al., 2012; Hess et al., 2013; Pignataro et al., 2013; Pan et al., 2016). Unfortunately, fewer studies have investigated which humoral factors contribute to exercise-induced neuroprotection. However, similar to RIC, one study found that endothelial nitric oxide synthase (eNOS) may be an important mediator for ischemic tolerance (Endres et al., 2003). In wild-type mice, exercise prior to stroke increased eNOS expression and augmented nitric oxide-dependent vasodilation, enhancing regional cerebral blood flow compared to sedentary mice and decreasing infarct volumes (Endres et al., 2003). This protection was contingent on eNOS, as eNOS knockout mice were not protected by physical activity prior to stroke (Endres et al., 2003).
Neuronal mechanisms of protection elucidated an important role for the neuronal pathway in protection against stroke. Denervation of the afferent pathway with pretreatment of capsaicin blocks RIC-induced protection (Ren et al., 2009). Furthermore, two studies demonstrated that ganglionic blockers (hexamethonium) ablated protection when RIC was given either before or after stroke (Ren et al., 2009; Malhotra et al., 2011). It is less clear how neural mechanisms could contribute to exercise-induced neuroprotection, though there is likely a strong connection between neural and humoral mechanisms of protection after exercise training. Neuronal systems control blood flow to skeletal muscle during exercise. In fact, increased somatomotor nerve activity is associated with inducing skeletal muscle contraction during exercise, while the release of norepinephrine (NE) by the sympathetic nerves causes vasoconstriction (McCorry, 2007). During exercise, these two neuronal control systems could work together to control the flow of neuroprotective humoral factors from working have Despite the strong evidence for a humoral mechanism of neuroprotection following systemic interventions, it is unlikely that humoral factors are the sole contributors to protection following either exercise or RIC. Instead, studies muscle to the brain. Further underscoring the relationship between the humoral and neural systems, NO modulates NE release in skeletal muscle, with decreased NE release after intense ischemic exercise (Costa et al., 2001).
The role of perlecan in post-exercise musculovascular ECM remodeling
Exercise as an activity is predicated on concerted and controlled muscle contractions. While it is obvious that muscle tissue is required for contractions to occur during exercise, skeletal muscle fiber contractions cannot occur without innervation and associated nervous stimulation. Consequently, stimulation of muscle fiber contraction by motor neurons is also tightly controlled and regulated, in part, via the involvement of the ECM within the neuromuscular junction (NMJ). For a long time, the ECM was regarded as merely a cellular scaffold and served a primarily structural function (Farach-Carson and Carson, 2007). We now know that the ECM is a tightly regulated signaling environment which is critical for the maintenance of proper tissue function beyond mere structural support. In muscle tissue generally, elements of the ECM, such as dystroglycan, collagen, and HSPGs such as perlecan, are critical for the maintenance of not only the vascular basement membrane, but also the NMJ (Smirnov et al., 2005). Perlecan, in conjunction with dystroglycan in particular, plays a key role in anchoring acetylcholinesterases to the neuromuscular basal lamina which degrade the neurotransmitter acetylcholine in the NMJ, leading to the cessation of muscle contraction (Peng et al., 1999; Jacobson et al., 2001; Smirnov et al., 2005). The loss or mutation of perlecan’s structure and/or function results in a range of negative outcomes ranging from musculoskeletal abnormalities to lethality (Olsen, 1999; Stum et al., 2006).
Similarly, these specialized ECM components exist within the vascular basement membrane and are critical for the maintenance of muscle health through guidance and support of vascular remodeling in muscle. For instance, during exercise, muscles undergo periods of ischemia and reperfusion which drives structural changes in the muscle vasculature. Indeed, it is clear that regular exercise training results in increased blood flow through the vasculature of actively involved muscles. This response is due in part to a slight increase in the diameter of major conduit arteries due to hyperemia, and therefore increased shear stress, but also as a result of vascular adaptation of associated capillary beds (Brown and Hudlicka, 2003). The latter occurs through sprouting and intussusception angiogenesis, which are thought to be induced as a result of muscle tissue and associated endothelial cell hypoxia (Prior et al., 2004). Vascular basement membrane remodeling is necessary for these angiogenic processes to occur and is in turn mediated by several classes of enzymes, including various matrix metalloproteinases (MMPs), the urokinase and tissue plasminogen activators (uPA and tPA), cathepsins, and bone morphogenetic protein-1/tolloid-like proteinases (BMP1/TLP) (Prior et al., 2004; Vadon-Le Goff et al., 2015; Fonovic and Turk, 2014a; Fonovic and Turk, 2014b). The ECM of the vascular basement membrane consists largely of collagen type IV, laminins, nidogen, and perlecan (Glentis et al., 2014). Interestingly, we previously found that the c-terminal protein fragment of DV portion of perlecan, the so-called LG3 peptide, was elevated in the urine of a cohort of physically active mining workers (largely maintenance staff) compared to their more sedentary colleagues (plant operators) (Parker et al., 2012). In addition, our recent unpublished observations suggest that LG3 is also elevated in serum following exercise.
Perlecan consists of 5 unique domains (I-V), and while all except Domain I have well-established proteolysis sites, perlecan’s C terminal DV is the most proteolytically labile, as are DV’s three laminin-type globular domains (LG1, 2, and 3). More recent evidence suggests that many other enzymes play a part in perlecan processing, including the cysteine proteases cathepsin B and L as well as BMP-1/tolloid-like proteinases which have been strongly implicated in the proteolysis of perlecan DV to release perlecan’s smallest bioactive fragment, the LG3 peptide (Figure 1) (Gonzalez et al., 2005; Farach-Carson and Carson, 2007; Roberts et al., 2012, Saini and Bix, 2012). Thus, taken together, the findings suggest that it is possible that the LG3 peptide is proteolytically released during neuromuscular activity and/or during muscle tissue hypoxia induced by physical activity and exercise, or in the latter case RIC.
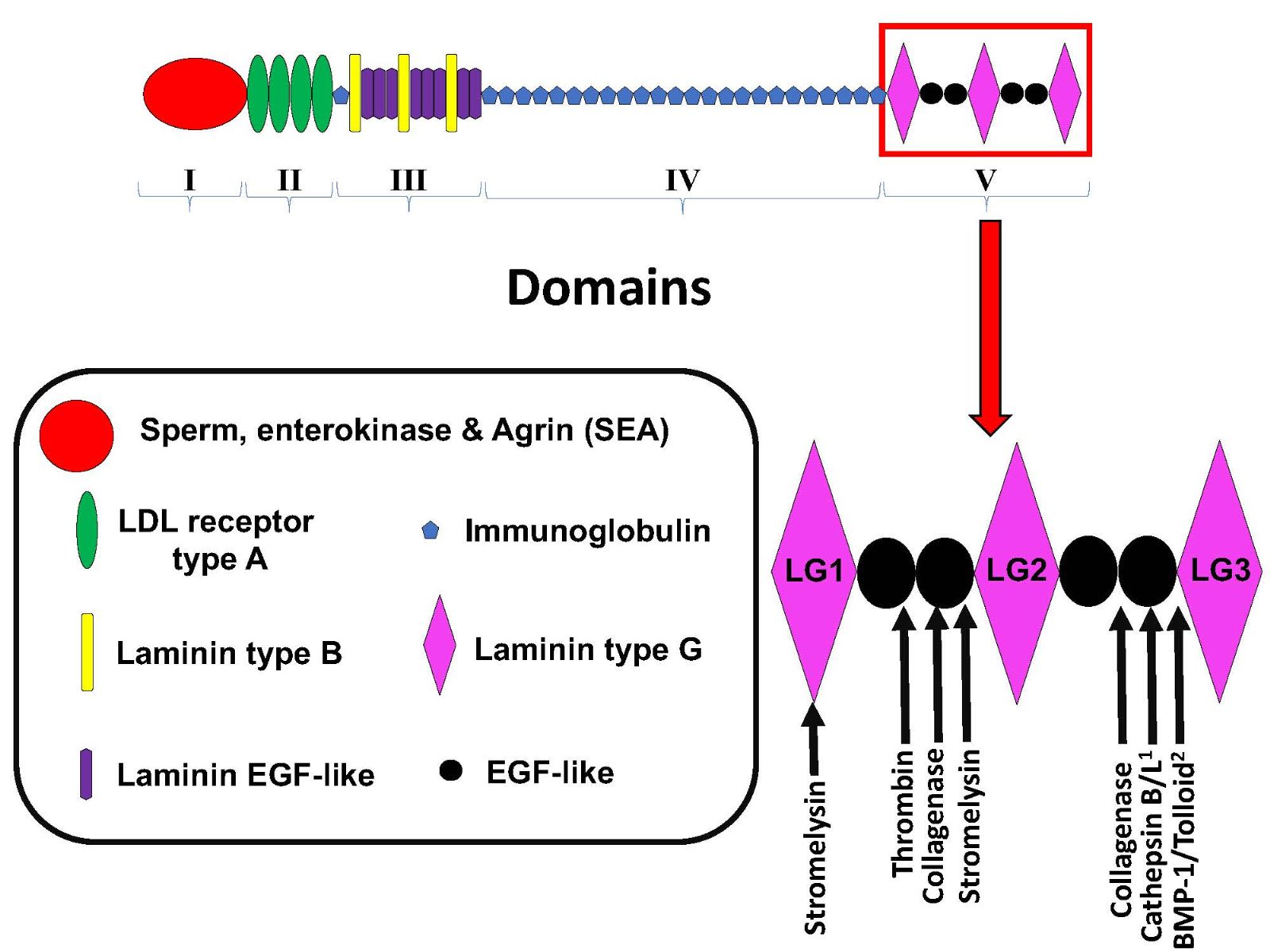
In a new window | Download PPT
Figure 1: Schematic of perlecan’s domain structure and sites of Domain V proteolysis. Adapted from Roberts et al, 2012 1) Saini and Bix, 2012 2) Gonzalez et al, 2005.
Perlecan in stroke
As alluded to above, the ECM in the brain is rapidly and heavily processed following ischemic injury, just as in the muscle after exercise. Interestingly, post-stroke processing of brain ECM is thought to be primarily driven by plasmin, thrombin, MMP2 and MMP9, and cathepsins (Gasche et al., 1999; Fukuda et al., 2004; Roberts et al., 2012, Heo et al., 1999). Consequently, the ECM components most affected by the upregulation of the enzymes listed above are various forms of collagen (primarily type IV but also type XVIII), laminin, and perlecan (Roberts et al., 2012). Perlecan, a major component of the BBB, is not only the most rapidly proteolyzed of these ECM components after experimental stroke (in non-human primates), but it is also the most rapidly upregulated following proteolysis, indicating its likely critical role in response to acute hypoxic injury (Fukuda et al., 2004). Importantly, recent research has shown that the major peptide fragments of perlecan, namely DV and LG3, are both protective and reparative following stroke (Lee et al., 2011; Clarke et al., 2012; Saini and Bix, 2012). Additional evidence suggests that exogenous, intravenously-administered DV, and to a lesser extent LG3, can traffic to the site of ischemic injury in experimental models of stroke in rodents (Lee et al., 2011; Clarke et al., 2012). These data show that both peptides survive further significant proteolysis while in the circulation. This, combined with our earlier evidence indicating that LG3 can be detected in the urine (and serum) of active individuals, suggests that it could be an attractive exercise-associated “theranostic” for more effective “prescription” of exercise-based rehabilitation regimens following stroke (Figure 2) (Sampson et al., 2014).

In a new window | Download PPT
Figure 2: Schematic summarizing the overall hypothesis of this review.
Clinical trials for RIC and exercise in stroke-related neuroprotection
As mentioned above, both RIC and exercise are clinically relevant interventions that can reduce the risk of stroke (Lee et al., 2003; Meng et al., 2012). Individuals that engage in high levels of physical activity prior to stroke have milder strokes and better functional recovery (Diep et al., 2010). In cases of a suspected stroke, RIC administered in the ambulance did not reduce infarct volume, but this treatment increased survival in tissues at high risk for death (Hougaard et al., 2014). Interestingly, in patients treated with RIC in conjunction with intravenous thrombolysis, physical activity in the week prior to the stroke was associated with decreased infarct development and a smaller infarct size at 1 month post-stroke, hinting that there could be an interaction between pre-stroke exercise and the neuroprotective effects of remote ischemia (Blauenfeldt et al., 2017). Despite these mixed results, there are several ongoing clinical trials examining RIPC, RIPerC, and RIPostC. Some studies are investigating whether RIPC protects against stroke in at-risk individuals (NCT: NCT03004820; NCT02971462; NCT02971462), while others are examining whether treatment with remote ischemia coupled with endovascular treatment could improve post-stroke outcome (NCT: NCT03045055; NCT03210051). While many of these clinical trials are focusing on exercise and RIC as separate interventions, the beneficial effects of exercise and RIC, and the mechanisms that confer these benefits, do not have to be studied in isolation. Blood flow restriction exercise (BFRE) may be able to bridge the gap between these two interventions (Sprick and Rickards, 2017). During BFRE, individuals complete a bout of exercise while blood flow is restricted to the exercising muscle, thus combining a brief period of ischemia with exercise (Slysz et al., 2016). In healthy individuals, BFRE increases muscle strength and size, as well as altering the cardiovascular response, including increased sympathetic activity and decreasing blood pressure (Park et al., 2015; Slysz et al., 2016). The effect of BFRE on the brain is not as well-characterized, but one study found that BFRE increases corticomotor excitability (Brandner et al., 2015). One of the advantages of BFRE over conventional resistance exercise is that low-intensity exercise (<25% maximal capacity) is sufficient to induce these changes (Slysz et al., 2016). Given that high-intensity training is difficult in elderly individuals, particularly those who have experienced a severe stroke, maximizing the benefits of exercise at a low intensity could increase the number of patients who can participate in post-stroke exercise rehabilitation and improve compliance to exercise regimens (Sprick and Rickards, 2017). BFRE may be a useful way of examining the synergistic effects of exercise and RIC on stroke patients, or those at a high-risk of stroke, but significantly more research is necessary to establish whether this type of training would exert a neuroprotective effect.
Biomarkers to identify protective systemic interventions
RIC to induce beneficial adaptive epigenetics has long been acknowledged as a potential therapeutic tool in the clinic. Recent discoveries on the molecular basis of the observed benefit of RIC have led to models of forced muscular damage and repair using RIC. RIC has clear clinical translatability and can be implemented with tools readily available in virtually every clinic around the world. It is even more relevant as it can be implemented at various times before, during, and even following stroke for potentially significant benefit (Zhao, 2009; Hougaard et al., 2014; Blauenfeldt et al., 2017). The idea of using urine or even serum biomarkers as prognostic indicators of patient outcome is also not new (Kouriefs et al., 2009). However, there have not been any easily accessible biomarker options for determination of stroke severity or progression, or of efficacious rehabilitation paradigms. In this review, we outlined the process of discovering only one potential protein biomarker for exercise and hypothesized as to this protein’s implications as a theranostic biomarker for stroke with the confidence that more such discoveries will follow.
Given the evidence that RIC and exercise, as well as the combination of the two in BFRE, affect muscular remodeling, we propose here that factors such as the LG3 peptide and potentially other ECM-derived molecules delivered from the site of remote muscular ischemia to the brain via the circulation could be used as blood or urinary biomarkers for more accurate prescription of exercise programs for stroke patients. Furthermore, we propose the idea that those biomarkers could then be supplemented as needed to impart pharmacological protection in the presence or absence of exercise.
References
Kathleen E. Salmeron
1Sanders-Brown Center on Aging; 2Department of Neuroscience; University of Kentucky, Lexington, Kentucky, USA.
Katherine Poinsatte
4Department of Neurology & Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Tony J. Parker
5Tissue Repair and Translational Physiology Program, Institute of Health and Biomedical Innovation. 6School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, Queensland, Australia.
Ann M. Stowe
4Department of Neurology & Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Gregory J. Bix
1Sanders-Brown Center on Aging; 2Department of Neuroscience; 3Department of Neurology, University of Kentucky, Lexington, Kentucky, USA.
Kathleen E. Salmeron and Katherine Poinsatte contributed equally to this work.
Corresponding author:
Gregory J. Bix
Email: gregorybix@uky.edu

In a new window | Download PPT
Figure 1: Schematic of perlecan’s domain structure and sites of Domain V proteolysis. Adapted from Roberts et al, 2012 1) Saini and Bix, 2012 2) Gonzalez et al, 2005.

In a new window | Download PPT
Figure 2: Schematic summarizing the overall hypothesis of this review.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 12091 | 26 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA