Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Sex and Gender Differences in Cardiovascular Diseases
Time:2021-01-17
Number:9240
Pasquale Pagliaro1,2, Saveria Femminò2, Cecilia Thairi1, Stefano Comità1, Giuseppe Alloatti3, Claudia Penna1
Author Affiliations


- 1Department of Clinical and Biological Sciences, University of Turin. Regione Gonzole 10, 10043, Orbassano (TO), Italy.
- 2Department of Medical Sciences, University of Turin. Corso Dogliotti 14, 10126, Turin, Italy.
- 3Uni-Astiss, Polo Universitario Rita Levi Montalcini. Piazzale Fabrizio de Andrè, 14100 Asti, Italy.
Conditioning Medicine 2020. 3(6): 274-284.
Abstract
Cardiovascular diseases have such a devastating and increasing impact on the entire world population. In the last decade, sex differences have been found in the epidemiology, pathophysiology, treatment, and resolution of these pathologies. Therefore, research is focused on improving the knowledge of the mechanisms underlying these discrepancies. In this minireview we summarize some of the main processes that are involved in the development of cardiovascular diseases, pointing out the major differences between male and female. We discuss disparities in the exposure to risk factors, in the electrophysiology of the heart, and in the maintenance of contractile functions by Ca(2+) regulation. In addition, due to the importance of sexual hormones in the regulation of metabolism and maintenance of cardiac function, the role of estrogen on different parameters has been analyzed. We focus on endothelial factors and NOD-, LRR, and pyrin domain- containing 3 protein (NLRP3) modulation by estrogen. In conclusion, many molecular factors and cellular mechanisms that result in cardiovascular disease are different in men and women. Therefore, further research is necessary to enhance the understanding of all the sex related differences.
Keywords: cardiovascular, mechanisms, sex, gender
Abstract
Cardiovascular diseases have such a devastating and increasing impact on the entire world population. In the last decade, sex differences have been found in the epidemiology, pathophysiology, treatment, and resolution of these pathologies. Therefore, research is focused on improving the knowledge of the mechanisms underlying these discrepancies. In this minireview we summarize some of the main processes that are involved in the development of cardiovascular diseases, pointing out the major differences between male and female. We discuss disparities in the exposure to risk factors, in the electrophysiology of the heart, and in the maintenance of contractile functions by Ca(2+) regulation. In addition, due to the importance of sexual hormones in the regulation of metabolism and maintenance of cardiac function, the role of estrogen on different parameters has been analyzed. We focus on endothelial factors and NOD-, LRR, and pyrin domain- containing 3 protein (NLRP3) modulation by estrogen. In conclusion, many molecular factors and cellular mechanisms that result in cardiovascular disease are different in men and women. Therefore, further research is necessary to enhance the understanding of all the sex related differences.
Keywords: cardiovascular, mechanisms, sex, gender
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in Western countries with a relevant impact on public health (Virani et al., 2020). A series of factors play a role in the development of CVDs: lifestyle, environment, and different biological aspects such as sex, epigenetic factors, sex-specific gene expression, age, and hormonal status in pre- and post- menopausal women. There are a lot of differences between men and women both in the susceptibility to these factors and in the pathophysiological mechanisms of CVDs. The role of sex in different pathological manifestations is not always clear and the underlying cause of these differences between males and females remains still unknown. Moreover, the prevalence and the mortality of CVDs appear different between men and women, thus stressing the necessity for gender and sex-specific intervention to address the gap in outcomes.
For instance, a PubMed search for “cardiovascular diseases and sex” results in 7,394 review articles. If we search "cardiovascular diseases and sex" and "physiological mechanisms" the results are just 16 review articles (https:// pubmed.ncbi.nlm.nih.gov/?term=%22cardiovascular+disea ses+and+sex%22+and+%22physiological+mechanisms%2 2&filter=pubt.review&sort=date; accessed on October 27th 2020). Therefore, it appears that there is a high interest in sex differences in cardiovascular disease, but relatively few studies considered the physiology and pathophysiology at the basis of these differences. The main purpose of this short article is to examine sex differences in cardiovascular patho-physiology, report and briefly discuss representative aspects of sexual dimorphism and sex-related mechanisms. Then we examine risk factors that may facilitate sex- and gender-specific quality care in CVDs. Finally, sex-related differences in the main CVDs are briefly considered.
Sexual dimorphism in cardiovascular physiology
Cardiovascular system anatomy
Focusing on the anatomy of the cardiovascular system, males and females share the same forming components, whose anatomy is not significantly different between the two sexes. In fact, the morphology and anatomical location of the heart, lungs, and major blood vessels are comparable in men and women (except for slight variations among individuals). However, a considerable difference between males and females exists in the size, and in particular, men have bigger hearts and vessels. The anatomical discrepancy in the heart size between males and females is not relevant, but it has important effects on how they regulate cardiovascular function and homeostasis. It has been demonstrated that stroke volume (SV) is smaller in females, but this does not translate to significant differences in cardiac index (cardiac output (CO) per unit of body surface area) between the two sexes (Huxley, 2007). The reason is that women compensate for their smaller SV with a higher heart rate. This is a clear example of how men and women maintain homeostasis in different ways.
Autonomic nervous system
The relationship between the autonomic nervous system and the cardiovascular system is another way in which sexual dimorphism becomes evident. It has been demonstrated that females maintain a lower sympathetic tone on the heart at rest, with the parasympathetic system prevailing sharply. However, post-menopausal women show a relationship between markers of whole body sympathetic activity and vascular resistance, and sympathetic traffic rises progressively in both sexes with aging (Joyner et al. 2015). Yet, young men show a higher degree of sympathetic stimulation at rest both on the heart and on the vasculature, as demonstrated by the higher levels of circulating norepinephrine. The higher degree of parasympathetic stimulation at rest in females is believed to play a role in their increased susceptibility to orthostatic hypotension: when changing position from clinostatic to orthostatic, blood is pooled in the veins (especially those of the lower limbs) due to their capability to dilate, accommodating changes in volume with only minor changes in pressure. It causes a decrease in venous return to the heart, which in turn determines a transient decrease in CO. As a result, the baroreceptors are less stimulated and they withdraw the baroreflex, inducing a sympathetic discharge, which results in enhanced venous return to the heart and increased heart rate and contractility. This mechanism is usually swift, and these adjustments do not give any symptoms. However, females are more likely to experience the symptoms of orthostatic hypotension (such as dizziness, blurry vision, and even fainting), and this is believed to be a result of their reduced sympathetic tone at rest (among other causes, such as a lower muscle mass to help maintain a consistent venous return to the heart). Even under conditions of stress, such as when exercising, the cardiovascular systems of men and women respond differently. Several studies have shown that men respond to stress by increasing total peripheral resistance, whereas women respond by increasing the heart rate. In case of prolonged stress, men are subject to vessel remodeling and hypertension, whereas in women the extra workload is placed almost entirely on the heart.
Cardiac electrophysiology
One fascinating point about sexual dimorphism in humans concerns the electrophysiology of the heart. It has been observed that the QT interval of post-pubertal females is, on average, longer than that of males (Salama and Bett, 2014) and interestingly, this gap was not observed at birth nor in pre-pubertal individuals, suggesting the involvement of sex hormones. In fact, the QTc (i.e. the QT corrected for the heart rate) of males was found to shorten during puberty and then progressively lengthen with aging, resulting in males having the same QTc of females both during the pre-pubertal and senile ages. The duration of the QT interval is strictly dependent upon the duration of the action potential, and more specifically upon the duration of phases 2 and 3. Phase 2 is mostly regulated by the inward Ca(2+) current generated by L-type Ca(2+) channels, whereas phase 3 is regulated by the outward potassium currents generated by several K+ channels (rapid delayed rectifier K+ current [IKr], slow delayed rectifier K+ current [IKs], inward rectifier K+ current [Ik1]). Since testosterone and progesterone act on these channels in a nongenomic way, several studies have been conducted on the relationship between sexual hormones and QTc length (Sedlak et al., 2012). In particular, testosterone is known to shorten the action potential, by lowering the Ca(2+) current and increasing the potassium one; this results in an overall shortening of the QT interval. Similarly, interesting results have been reached analyzing the relationship between female sex hormones (estrogen and progesterone) and QTc. Estrogen has been found to have no impact on the length of QTc in humans, but progesterone seems to shorten the QT interval in females. It is demonstrated that women have cyclic variations in the QT interval corresponding to different phases of the menstrual cycle (Sedlak et al., 2012): in the luteal phase, in which there is a burst of progesterone, repolarizing K+ currents increases and the QT interval shortens. Despite the similar effects of testosterone and progesterone, post-pubertal males show a shortened QT in comparison to post-pubertal female; concordant to this, female hearts have shown a lower density of repolarizing K+ currents, i.e. a lower repolarization reserve, and more variance in the L-type Ca(2+) channels (Gaborit et al., 2010). This difference in the patterns of cardiac repolarization could explain why females have a longer action potential, resulting in a longer QT interval. The consequences for women are a sex-specific higher prevalence in cardiac arrhythmias such as the Torsade des pointes, a high-risk cardiac arrhythmia associated with long QT interval, and possible differences in responses to drugs used in the treatment of CVDs. Further study of the mechanisms that lead to QT length variation between the two sexes can be helpful in clarifying pathological implications.
In summary, it seems evident that sexual dimorphism in humans is much more complex than it appears on the bare anatomical level. Males and females show different ways of regulating several pathophysiological processes, including neural control and electrophysiology features (Figure 1).
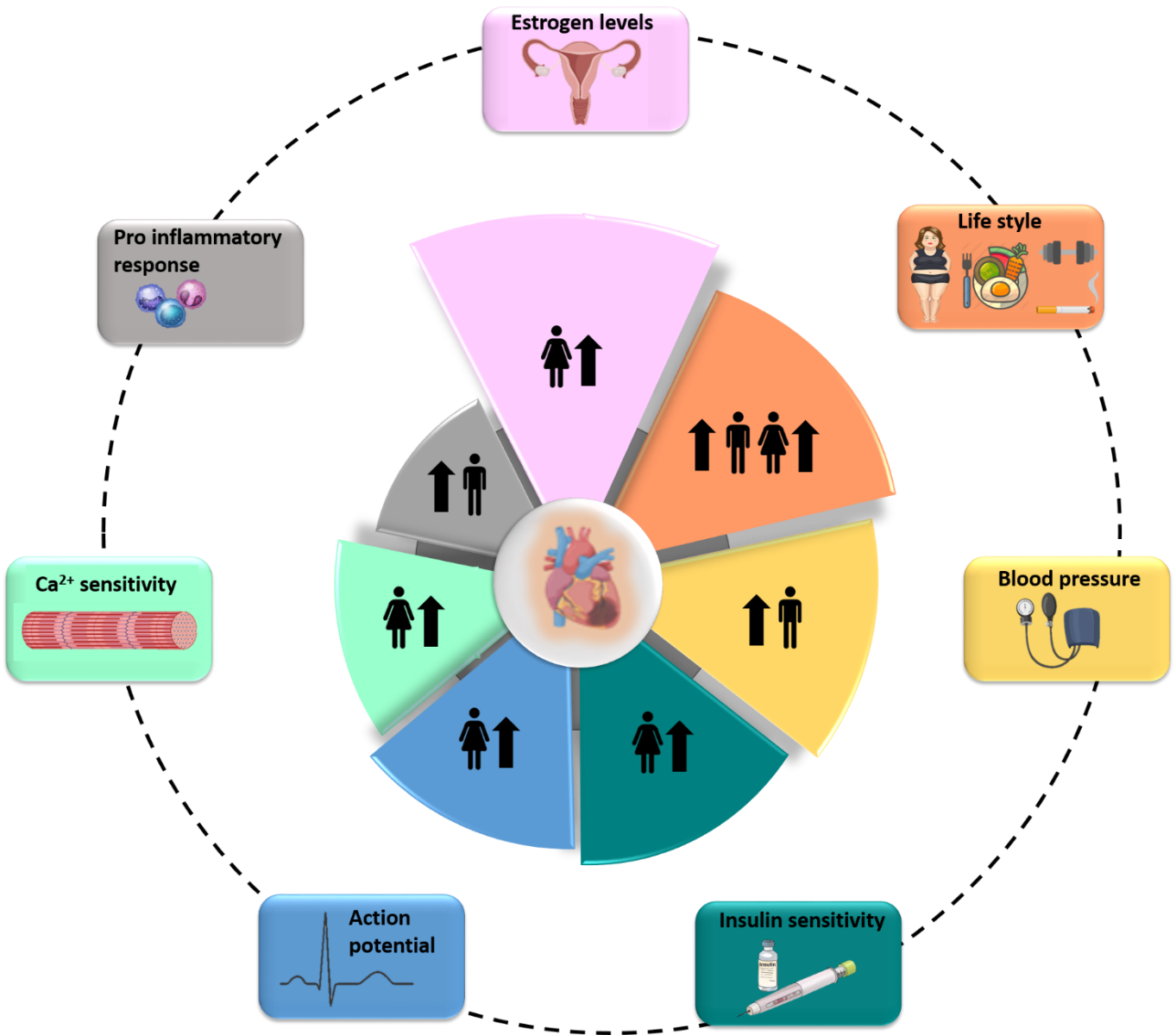
In a new window | Download PPT
Figure 1. Main Factors Determining Sex-Related Differences in Cardiovascular Diseases.
Sex-differences in pathophysiological mechanisms
Calcium regulation
One of the fundamental mediators for heart function is Ca(2+) which is crucial for starting the contractile function and regulating the intensity of contractility at each beat. Ca(2+) is involved in the regulation of contractility, excitability, conductivity, and rhythmicity of the heart. Ca(2+) is also the leading factor in the regulation of metabolism, hypertrophic cell growth, and apoptosis, acting as a second messenger in signal transduction pathways (del Monte and Hajjar, 2003). Calcium handling alterations can lead to cell dysfunction and death and, consequently, to cardiac dysfunction and CVDs such as arrhythmias and heart failure. In the literature, several authors have addressed the difference in cell Ca(2+) management between men/males and women/females. Schwertz et al. (2004) examined these differences with different techniques using male and female Sprague-Dawley rats, and provided a specific picture of sex differences for the Ca(2+) sensitivity of contractile proteins and myofibrillar Ca(2+) transport in the myocardial ventricle. In particular, they have shown that there are sex divergences in the contractile ventricular response to extracellular Ca(2+) and they rely on the activity of all the Ca(2+) modulating proteins (channels, pumps, and transporters) that contribute in varying degrees to the availability of Ca(2+) for contractile proteins. Their work showed significant differences in Ca(2+)-stimulated Mg(2+)-dependent adenosine triphosphatase (ATPase) activity in isolated myofibrils from male and female rat heart ventricles. Specifically, they demonstrated that myofibrillar ATPase activity was higher in females than males and the Ca(2+) EC50 for myofibrillar ATPase was significantly lower in female ventricular homogenate with respect to male's; therefore, Ca(2+) sensitivity of contractile proteins from female hearts is greater than contractile proteins from male hearts. Besides, at high concentrations of Ca(2+) the rate of shortening and strengthening in isolated myocytes of female rats reaches a plateau while it continues to rise in male myocytes. In addition, they showed a greater influx of Ca(2+) current in female myocytes heart cells compared to male cells. These results provide scientific evidence for sex differences in Ca(2+) responsiveness and lead to a better understanding of whether these alterations may be at the basis of mechanisms harmful to the contractile properties of myocardial cells.
In recent years, research on sex-dependent differences on the susceptibility to CVDs has intensified, efforts analyzing aspects that have not yet been investigated. Fels and Manfredi (2019) analyzed sex differences in ischemia and reperfusion injury and the role of mitochondrial permeability transition pores (mPTPs), framing the study as a possible correlation between intracellular and intra mitochondrial Ca(2+) levels in response to ischemic and reperfusion injury and the protection provided by estrogen in preventing the opening of mPTPs. Indeed, the loss of Ca(2+) homeostasis represents a key point in the fate of cells and tissues that undergo ischemic stress, and the opening of mPTPs marks the border between life and death of cells. In summary, they showed that there is a different response in men and women to ischemic injury, and these differences lie in the modulatory effects of estrogen receptors. The sexual differences found in this study model highlighted the modulatory/protective effect of estrogen in the manipulation of Ca(2+) and in preventing the opening of mPTPs in ischemia and reperfusion.
Estrogen effects in cardiovascular pathophysiology
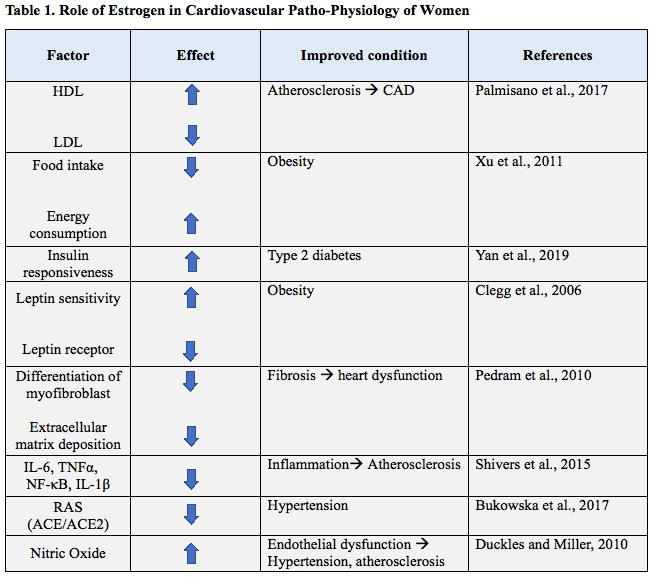
Menopause and sex hormone levels are thought to play a crucial role in the onset of CVDs; this assumption is based on the fact that the risk of many CVDs, such as atherosclerosis and hypertension, is significantly different in premenopausal and postmenopausal women (Table 1). In particular, a postmenopausal loss of cardioprotection is observed, suggesting a central role in sex hormones and, in particular, estrogen, on the cardiovascular system (Dosi et al., 2014). Estrogen is a steroid molecule that reaches quite high levels in females of reproductive age, but it is nevertheless present in all individuals at different concentration. Estrogen’s role in reproductive health is well known while its effect on other systems has been only investigated recently. Estrogen receptors ER-α and ER-β are present in many tissues and when they are activated, they dimerize and bind DNA response elements, which code for different proteins and macromolecules that can induce distinct cellular processes. Estrogen response is not always mediated by genetic expression, but rapid, non-nuclear signaling is also employed. For example, it has been shown that estrogen is able to bind to an orphan G-protein coupled receptor, GPR30, leading to rapid activation of kinase signaling pathways such as phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) (Simoncini et al., 2000). In Table 1, we summarize some of the protective effects of estrogen on the main factors that mediate cardiovascular pathophysiology.
Endothelial function
The maintenance of endothelial function plays an important role in the homeostasis of the cardiovascular system and endothelial dysfunction is a major cardiovascular risk factor associated with the majority of CVDs. In particular, endothelial dysfunction promotes the development of vascular diseases such as atherosclerosis, that is strongly associated with aging, hypertension, and hyperlipidemia (Davignon and Ganz 2004). The main alteration characterizing endothelial dysfunction is an imbalance in the activity of endothelial nitric oxide synthase (eNOS), which instead of leading to release of nitric oxide (NO) may release reactive oxygen species (ROS) (i.e. uncoupled NOS) (Lam et al., 2006; Morales et al., 2015). Indeed, estrogen therapy may restore vascular tetrahydrobiopterin levels and reduce oxidative stress in ovariectomized rats (Lam et al., 2006). Besides that, other mechanisms are involved in the impairment of endothelial function, and differences between the two sexes may be relevant.
Endothelial cells extracted from men and women have shown different transcriptional profiles in normal conditions. In particular, female endothelial cells have higher expression of immune-related genes, including genes regulating the major histocompatibility complex (MHC), the complement system, and chemokine and cytokine release (Lorenz et al., 2015). Endothelial dysfunction and related pathologies are directly correlated to inflammation of the endothelium (Galle et al., 2003); therefore, the understanding of sex differences in the immune gene profile may be pivotal in the discovery of sex differences in CVDs. Sex-specific expression patterns of endothelial cells were also found in response to shear stress, the force applied on the endothelial surface resulting from the friction of the blood on the vessel wall. In particular, a stronger flow-dependent vasodilation is characteristic of female endothelial cells, that are therefore more protected against endothelial dysfunction induced by oscillatory shear stress (Tremblay et al., 2019). Additional information regarding sex differences in endothelial function may be found in the recent review article by Stanhewicz et al. (2018). Overall, sex differences play important roles in the control of endothelial function and specific recommendations for the treatment or prevention of endothelial dysfunction and associated CVDs are needed for the mitigation of morbidity and mortality in men and women.
Nitric Oxide signaling
It is well known that the vasodilator NO has protective effects on cardiovascular tissue, but it is still undetermined whether sexual differences are present in eNOS activity and NO function in endothelial cells. Cattaneo et al. (2017) studied the expression of eNOS in human male and female endothelial cells, and they found that female endothelial cells had higher eNOS mRNA and protein levels. NO signaling is enhanced by estrogen by different mechanisms, both by stimulating the expression of eNOS by increasing its activity via phosphorylation and by limiting eNOS uncoupling (Lam et al., 2006; Morales et al., 2015). Estrogen also upregulates eNOS through the classical genomic action of ER-α (Stanhewicz et al., 2018). Besides these mechanisms, estrogen infusion may induce a rapid vasodilation (in a few minutes) attributable to a concomitant increase in vascular cyclic adenosine monophosphate (cAMP) content (Barton, 2016). This suggested that estrogen could regulate vascular tone via a couple of non-genomic actions, including through the phosphorylation and activation of eNOS as a consequence of activation of PI3K signaling (Duckles and Miller, 2010). In addition, the downstream signaling of G-protein-coupled estrogen receptor (GPER), previously termed GPR30, may be another mechanism promoting eNOS activity in a non-genomic way. Indeed, G1, a selective GPER ligand, exerts cardiovascular effects through activation of the PI3K-Akt pathway and Notch signaling. It was recently reported that G1 may induce cardioprotection via Notch1 signaling and is able to reduce ischemia/reperfusion (I/R) injury in female hypertensive rats (Rocca et al., 2018). Finally, nitrergic nerves are of particular importance in the relaxation of corpus cavernosum and NO is known as the key factor involved in initiating and maintaining an erection (Förstermann & Sessa 2012). NO is involved in a plethora of pathophysiological mechanisms influenced by hormonal regulation and deserves to be further studied in relation to sex differences (Morales et al., 2015; Andreadou et al., 2020).
Endothelial dependent hyperpolarization
Other than the NO/cyclic guanosine monophosphate (cGMP) pathway, the regulation of the vascular tone is mediated also by another event, called endothelium-dependent hyperpolarization (EDH). EDH refers to the relaxation of vascular smooth muscle cells that occurs as a consequence of a hyperpolarizing stimulus, that in turn is promoted by the opening of the intermediate conductance calcium-activated potassium (IKCa) and small conductance calcium-activated potassium (SKCa) channels (Kong et al., 2015). These potassium channels open upon the elevation of intracellular Ca(2+) in endothelial cells, therefore EDH is stimulated by all ionotropic stimuli, such as acetylcholine. EDH represents a vasodilation mechanism that occurs independently of the production of endothelium- derived NO. Physiologically the increased ability of a blood vessel to activate EDH occurs mostly when the NO signaling pathway is inhibited, i.e. in cases of endothelial dysfunction. It is hypothesized that EDH may intervene in endothelial dysfunction to partially compensate for the loss of NO vasodilator effect (Ozkor et al., 2011). Despite this, during pathologic conditions such as aging and hypertension the ability to respond to endothelial dysfunction is reduced, and that is correlated with the fact that a selective reduction of EDH- mediated relaxation has been observed (Ozkor et al., 2011). Again, estrogen may have a determining role in the modulation of EDH, as suggested by some evidence summarized in the review of Leung and Vanhoutte (2017). For example, in female rats 44–45 weeks old, as well as ovariectomized rats, mesenteric arteries showed a reduced EDH response and impaired relaxation (Liu et al., 2001). Instead, young females appear to have a stronger EDH response in parallel to an improved IKCa channel activity (Wong et al., 2014). The administration of 17β-estradiol to female rats subjected to ovariectomy is protective against vascular diseases, since it has been shown to improve EDH-type relaxations, other than NO vasodilation signaling (Chan et al., 2012).
Renin angiotensin system
Angiotensin-converting enzyme (ACE), the main enzyme involved in the renin angiotensin system (RAS), is able to convert angiotensin I to angiotensin II, which not only can induce systemic vasoconstriction and sodium retention, but also has a crucial role on a local level in tissues expressing the angiotensin II type 1 (AT1) receptor, including the heart and vessels (Lavoie and Sigmund, 2003). Angiotensin II induces processes such as fibrosis, hypertrophy, vasoconstriction, and increased ROS production. All these factors are involved in the mechanisms underlying coronary pathologies (which include systemic issues like hypertension and more tissue-specific ones such as fibrosis and inflammation).
Women are protected from excessive activation of RAS, as demonstrated by the fact that they show lower ACE activity and expression, and lower AT1 receptor expression levels in comparison with men. That seems to be a consequence of the estrogen modulation of RAS. Indeed, estrogen is able to downregulate ACE and upregulate ACE2 expression, probably by targeting the promoter of these genes (Bukowska et al., 2017). ACE2 can convert angiotensin II to angiotensin-(1-7), which upon the binding to Mas receptors, can in turn phosphorylate eNOS and Akt, thus increasing vasodilation and inhibiting apoptosis. Therefore, estrogen is able to drive the shift of RAS towards the formation of angiotensin (1-7), which, in contrast with angiotensin II, mediates protection against pathological cardiovascular conditions.
To sum-up two counter-regulatory axes that form the RAS: the ACE/Angiotensin II/AT1 receptor axis the and ACE2/ angiotensin-(1-7)/Mas receptor axis. The binding of angiotensin II to the AT1 receptor mediates vasoconstriction, inflammation, fibrosis, and tissue injury. The binding of angiotensin-(1-7) to the Mas receptor limits proliferation, inflammation, and fibrogenic effects and, thus, promotes organ protection. It seems that females have the RAS balance towards the ACE2/ angiotensin-(1-7)/Mas receptor axis.
Metabolism
Estrogens are able to modulate the energy balance through different mechanisms, acting on multiple tissues from the central nervous system to metabolism-regulating organs such as the adipose tissue, the muscle, the liver, and the pancreas. Regarding the central nervous system, the hypothalamus is the main site in which metabolic homeostasis is regulated. In particular, estrogen suppresses food intake by activation of ERα in the neurons of the arcuate nucleus and it promotes physical activity and controls body fat distribution though the stimulation of neurons in the ventromedial nucleus of hypothalamus (Xu et al., 2011). In these areas of the hypothalamus, ERα is co-
localized with the receptor for leptin, LEP-R (Diano et al., 1998). Leptin is a hormone produced by adipose tissues with the aim to send catabolic signals to the brain, inhibiting food intake and limiting fat storage. It has been reported that estrogen, through a direct interaction on the LEP-R gene, down- regulates the expression of the leptin receptor in the arcuate nucleus of the hypothalamus (Bennett et al., 1999). Therefore, estrogen is associated with increased leptin sensitivity, as demonstrated by the fact that ovariectomy reduces sensitivity to central leptin, that can be reversed by exogenous estrogen treatment. Exogenous estrogen administration increases leptin sensitivity in male rats (Clegg et al., 2006). According to these results, estrogen and leptin have a synergic action promoting energy expenditure and fighting fat accumulation, which in turn protect against metabolic alterations and obesity, relevant risk factors for CVDs. Another important effect on metabolism that could protect against CVDs, is the interaction of estrogen and insulin. Premenopausal women show increased insulin sensitivity compared with age-matched men, reflecting also the reduced incidence of type 2 diabetes mellitus (T2DM) in women (Park et al., 2003). In postmenopausal women or in ovariectomized patients, it is possible to observe a rapid decay in insulin sensitivity, along with fat mass elevation and increases in low-density lipoprotein (LDL), triglycerides, and fatty acids. These findings suggest that estrogen positively affects insulin action and protects against the development of T2DM in women. Estrogen effect on the lipid profile is also well known. It upregulates high-density lipoprotein (HDL) and downregulates LDL, shifting the lipid metabolism to an anti- atherosclerotic profile, thus delaying eventual plaque formation in the coronary arteries (Palmisano et al., 2017). Lower LDL levels are achieved by increasing its clearance from plasma and upregulating LDL receptor on the surface of cells, while high HDL levels are a consequence of the decreased hepatic lipase activity and the increased synthesis of apolipoprotein, the major protein component of HDL particles in plasma (Guetta and Cannon, 1996).
Inflammation
Inflammation has a role in many pathological conditions that can contribute to the development of CVD, for example the inflammatory component of the atherosclerotic plaque is fundamental in the progression and final outcome of atherosclerosis. In particular, vascular inflammation plays a central role in initiating and perpetuating atherosclerotic and ischemic diseases, both in men and women (Shabbir et al., 2020). Estrogen is able to modulate inflammation through some mechanisms that occur upon activation of ERα, such as the inhibition of adhesion molecules such as E-selectin, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), by decreasing the synthesis of pro-inflammatory molecules, and through activation of the degradation of damaged proteins through the proteasome (Villa et al., 2015) . For example, Ghisletti et al. (2005) investigated how estrogen was able to prevent the nuclear translocation of nuclear factor-kappa B (NF-κB), after lipopolysaccharide stimulation of mouse monocytes. They found that the blockage of this inflammatory mediator was exerted by a direct interaction of estrogen receptor with PI3K. Instead estrogen, through the transcriptional modulation of ERα, is able to downregulate the expression of some pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor alpha (TNFα), and IL-1β (Shivers et al., 2015). Sex differences have been described in preclinical and experimental medicine studies in the inflammatory response and several recent studies underlined a clear sex difference in the resolution of inflammation, offering pharmacological opportunities for sex- related therapies in coronary artery disease (CAD) (Reviewed in Shabbir et al., 2020).
Inflammasome
NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) the predominant inflammasome, is a high molecular weight complex present in the cytosol of variously stimulated cells. The assembled NLRP3 platform determines the activation of inflammatory caspases, in particular caspase-1, leading to the production of pro-inflammatory cytokines IL-1β and IL-18 (Schroder and Tschopp, 2010). These cytokines may induce pro-hypertensive effects in endothelial cells and vascular smooth muscle cells, such as ROS production, endothelial dysfunction, and vasoconstriction (Krishnan et al., 2014). The NLRP3 inflammasome seems to play a pivotal role in the progression of many CVDs, specifically in the pathologies that are more affected by chronic inflammation and vascular damage such as hypertension, metabolic syndrome, and atherosclerosis (Mastrocola et al., 2018; Pasqua et al., 2018). For example, in rodent atherosclerosis models, the deletion of NLRP3 or other players of the inflammasome such as caspase-1, led to a significant reduction in atherosclerotic plaques (Duewell et al., 2010).
In the literature, evidence as to whether the NLRP3 inflammasome is regulated differently in men and women is still scarce and should be integrated, but some insights are gathered below. It has been demonstrated that estrogen is able to drive the M2 phenotype polarization of macrophages (Keselman et al., 2017). The M1 phenotype is predominant in males, along with greater expression of NLRP3 and IL-1β. Cowie et al. (2019) demonstrated that after a surgical incision the production of NLRP3 mRNA was similarly increased in both males and females; despite this, following the deletion of NLRP3, the level of IL-1β was decreased only in males, suggesting that females have a NLRP3-independent mechanism of IL-1β production (Cowie et al., 2019).
NLRP3 has been found to be regulated by estrogen during allergic airway inflammation, against which estrogen seems to have a protective effect (Matsubara et al., 2008). In the work of Cheng et al. (2019), ovariectomized mice sensitized with ovalbumin to establish airway inflammation, showed higher expression of the NLRP3 inflammasome and downstream effectors caspase-1 and IL-1β. The long term administration of 17β-estradiol on the same mice markedly reduced ovalbumin- induced airway inflammation and led to a suppression of both the mRNA expression and protein expression of NLRP3, caspase-1, and IL-1β (Cheng et al., 2019). Further studies are needed to elucidate the role of estrogen and to find the molecular pathways involved in the regulation of NLRP3 that are able to reduce inflammasome formation and inflammatory pathologies, including CVDs.
Fibrosis
Cardiac fibrosis refers to a pathological remodeling of the extracellular matrix, as a consequence of aging, injury, or diseases. The fibrotic scars in the heart alter cardiac function, by thickening of the myocardial matrix or by impairing electric conductance, resulting in reduced ejection fraction (Hinderer and Schenke-Layland, 2019). Cardiac fibrosis is induced in fibroblasts by angiotensin II and endothelin-1, which stimulate transforming growth factor beta 1 (TGFβ1) synthesis, thereby activating c-Jun N-terminal kinase followed by Sma- and Mad- related protein 3 phosphorylation, which in turn translocate to the nucleus and mediate the transition of the cardiac fibroblast to a myofibroblast phenotype, synthesizing procollagens I and III (Ruiz-Ortega et al., 2007). Estrogen seems to exert an anti-fibrotic action in cardiac fibrosis; in particular, it has been demonstrated that through cAMP and protein kinase A, estrogen is able to inhibit c-Jun N-terminal kinase and prevent myofibroblast formation. Estrogen administration in ovariectomized female mice, was able to prevent angiotensin II-induced cardiac fibrosis; this rescue was not observed in ERβ knockout rodents (Pedram et al., 2010).
Aging
Aging promotes some changes in the heart and blood vessels that predispose individuals to an increased risk of developing CVDs. Left ventricle (LV) chamber morphology, higher vascular stiffness, cardiomyocyte loss, and myocellular hypertrophy are age and sex-specific factors that can promote CVD onset, particularly in postmenopausal women (Oneglia et al., 2020). The role of aging and sex as modulators of I/R injury development, cardioprotection signaling, and efficacy of the therapeutic interventions has been reviewed elegantly by Ruiz- Meana et al. (2019a). In this review intracellular and functional changes involved in I/R susceptibility and cardioprotection, as well as variations in inflammatory cells, and in extracellular matrix during aging, have been analyzed. Of note, aging induces a certain mismatch in the cell energy supply and demand, which may be secondary to deficient sarcoplasmic reticulum- mitochondria Ca(2+) exchange and defective supramolecular assembly of the respiratory chain supercomplexes. Whether this Ca(2+) handling is different between sexes is unclear. Also inadequate transfer of intracellular energy and progressive metabolic remodeling (i.e., partial replacement of OXPHOS by glycolysis) have been proposed as a central mechanism of aging (Fernandez-Sanz et al., 2014; Ramirez-Camacho et al., 2019; Ruiz-Meana et al., 2019a, 2019b). Whether these mechanisms influence the outcome of CAD in aging patients of both sexes remains to be ascertained. Nevertheless, cardiomyocyte death rates during aging increases to a greater extent in men compared to women, and this is probably due to the fact that women have a greater pool of cardiac stem cells, as well as a lower myocyte turnover in comparison to men. On the other hand, women during aging show increased vascular stiffening, an event that is dependent on a series of factors including endothelial dysfunction, elastin-collagen content, inflammation, and neurohormonal signaling (Oneglia et al., 2020).
Risk factors, gender, and sex
Cardiac risk factor management may be a smart strategy to decrease overall the rates of CVDs in both men and women. Other than the conventional risk factors such as hypertension, diabetes, smoking, and dyslipidemia, it is important to evaluate the risk related to healthcare, and social and cultural behaviors. The sex-specific susceptibility to these factors is not easy to determine, but it is fundamental to improving the prevention, diagnosis, treatment, and prognosis of CVD.
Men and women may be exposed to different cardiac risk factors. For instance, men are more susceptible than women to unfavorable risk factors including smoking, low-fiber diet, and low vitamin C levels, while women are more prone to diabetes mellitus, hypertension, hypercholesterolemia, and obesity (Manfrini et al., 2020). CVD is estimated to be responsible for approximately 65% of deaths in diabetic patients, and the relative mortality risk from CVDs is higher in women with diabetes than in men (Harreiter et al., 2020). Besides, diabetic women show a 3- to 7-fold increased CVD onset risk compared with a 2- to 3 fold elevated risk in men; despite this, evidence in the literature are controversial, as pointed out in the systematic review by Chase-Vilchez et al. (2020), in which they show that diabetes is not associated with a higher risk in women for peripheral arterial disease compared to men (Chase-Vilchez et al., 2020).
Social and psychological aspects can have a relevant impact on the determination of sex/gender differences in CVDs. First of all, women are less aware that heart disease is a leading cause of death for them and they are also less informed about the typical clinical manifestation of CVDs. The recognition of symptoms of myocardial infarction (MI) for women can be more challenging, because often they do not experience the typical chest pain, instead MI in women is characterized by non-specific symptoms such as abdominal pain, dyspnea, nausea, back and neck pain, and palpitations (Stehli et al., 2020). That could be the reason why women experience longer time from the MI onset to hospital admission. The time between symptoms presentation and treatment affects patient outcome in cases of MI. Besides women, compared to men, are less prone to undergo invasive diagnostic testing or percutaneous coronary intervention (Manfrini et al., 2020). All of these factors can contribute to the poorer cardiovascular outcomes that often affect women more than men. Sex and gender specific prevention and diagnosis could be the first steps in order to partially fill this gap.
Sex-related differences in the main CVDs
Atherosclerosis and CAD
Atherosclerosis is a condition characterized by narrowed and hardened arteries as a consequence of the formation of lipid plaques. At a younger age, men are more susceptible to develop atherosclerosis than women, but it has been observed that in the elderly the prevalence for women increases, in particular in the presence of autoimmune diseases (Fairweather, 2014). The explanation is that sex hormones, inflammation, lipid profile, shear stress, and all other risk factors common in CVDs such as obesity, smoke, and life style, can expose both sexes to atherosclerosis risk in different ways (Shabbir et al., 2020). Men are affected more by occlusive CAD, while women suffer more from the nonobstructive form. In men, CAD is mainly due to atherosclerosis of coronary epicardial arteries, while in women CAD is a consequence of pathological vasoreactivity, such as spasm and endothelial dysfunction (Regitz-Zagrosek and Kararigas, 2017).
Hypertension
Globally, it was estimated that 31.1% of the world’s population is affected by hypertension (Mills et al., 2020). Hypertension represents the primary reversible risk factor in the development of CVD. It has been observed that blood pressure in men is higher than women, resulting in a higher prevalence of hypertension in young men (Ramirez and Sullivan, 2018). Despite this, in older individuals the percentage of hypertensive women reaches that of men. This may be a consequence of the change in the hormonal status of pre- and post-menopausal women (Nwankwo et al., 2013). Both in women and in men, the development of hypertension is often correlated with diabetes and metabolic syndromes (Henry et al., 2012). Clearly, the metabolic syndrome accelerates arterial aging and amplifies hypertension-related cardiac and renal alterations. Studies from all over the world support this association between sex, age, hypertension and the related complications (e.g. Campbell et al., 2020).
Myocardial infarction
It has been observed that myocardial infarction has a higher impact on mortality in women than age-matched men (Johansson et al., 2017). Also experimental studies have shown a substantial difference in myocardial I/R injury susceptibility between the two sexes (Penna et al., 2009). MI in women is often a consequence of plaque erosion compared with the plaque rupture that occurs most of the time in men and older women (Stehli et al., 2020). Another cause of MI typically detected in women rather than men is the spontaneous coronary artery dissection, that determines an infarction event in up to 25% of women younger than 60 years of age. Besides, the coronary arteries of women are more susceptible to microvascular dysfunction and impaired coronary flow reserve compared to vessels in men (Taqueti et al., 2018). Women mortality post MI is more frequently associated with cardiac rupture, while arrhythmia is the main complication leading to death in men (Mirić et al., 2008). These observations on mortality do not depend on the type of treatment used. Moreover, heart failure (HF) following MI is more frequent in women than in men.
Heart failure
Regarding heart failure, which is often a complication of MI, some observations indicate the presence of peculiar differences between women and men. In particular, women are mainly affected by HF with preserved ejection fraction (HFpEF). On the contrary, HF with reduced ejection fraction is typically found in men (Regitz-Zagrosek et al., 2007). According to many authors, the analysis of a series of physiological and mechanical properties of the heart, such as ventricular diastolic distensibility, vascular stiffness, ventricular/vascular coupling, and skeletal muscle adaptation to HF, is useful to describe the main sex differences in HF. Campbell et al. (2019) focused on the threshold of body mass index (BMI) and sex-specific waist circumference on the increased risk of HFpEF. They proposed different caist circumference and BMI thresholds for the increased incidence of HFpEF that may be useful for prevention strategies. The waist circumference thresholds of 100 cm or less in men and 90cm or less in women and a BMI threshold of 27.5kg/m2 or less were demonstrated from this study as a cutoff point. The BMI and waist circumference below these thresholds may reduce the incidence rate of HFpEF by about 50%. Sex differences in HF have been recently reviewed by Lam et al. (2019).
COVID-19 and Sex
The virus responsible for coronavirus disease 2019 (COVID-19) binds to ACE2 and the pathophysiology of COVID-19 infection might rely on a large extent on imbalance of the RAS. As many CVDs display an unbalance of RAS, COVID-19 may be considered a pneumonia highly associated with CVDs or a systemic disease (Pagliaro and Penna, 2020; Penna et al., 2020). It has been shown that COVID-19 has the worst outcomes when present in individuals with pre-existing cardiovascular pathologies, in particular the most frequent comorbidities are hypertension (15.6%), diabetes (7.7%), and CVDs (4.7%) (Lumpuy-Castillo et al., 2020; Moccia et al., 2020). The severity and lethality of COVID-19, as measured by admission to intensive care units and the death prevalence, have been reported to be about 2 times greater for men than for women worldwide (Penna et al., 2020; Richardson et al., 2020). These data suggest that sex plays a role in COVID-19 severity and that women of all ages are protected against COVID-19. In particular, among COVID-19 affected patients, fewer women than men presented with cardiac damage, but those women with cardiac damage had higher levels of systemic inflammatory markers, suggesting a male inflammatory phenotype (Shi et al., 2020). We also believe that COVID-19 sex differences should be taken into account because they could reveal specific therapeutic approaches, useful to improve outcomes for both sexes.
Conclusions
In conclusion, males and females exhibit some differences in the cardiovascular anatomic structures, electrophysiology, autonomic nervous system, metabolism, inflammation, and in some molecular mechanisms such as Ca(2+) homeostasis, NO signaling, NLRP3, and RAS. It is well established that sex hormones, in particular estrogen, play a crucial role in regulating all these processes. Estrogen decline after menopause results in changes that predispose post-menopause women to CVD, such as reduced insulin and leptin responsiveness, altered lipid profile, and increased inflammatory state, which in turn increase the risk for obesity and diabetes, higher propensity to fibrosis, enhanced blood pressure and others. Therefore, along with all the traditional risk factors for CVD, menopause, especially the early onset form, represents a sex-specific risk factor. In recent years, many researchers investigated the possibility to reverse the negative effect of menopause with hormone replacement therapy. Although this treatment has been effective, it has not demonstrated a clear protective effect on the development of CVD. This suggests that research should be focused on new mechanisms that result in CVD differences in men and women. The knowledge of sex differences may be useful for both women and men, and efforts are also needed to bring sex and gender to the attention of modern medical research, clinical practice, and biomedical education. This research, with an accurate re-evaluation of diagnostic procedures, could lead to a more efficient management of all patients. In conclusion, physicians should be aware of these issues and educate medical students and patients for a future greater awareness of sex and gender differences in CVDs.
Declaration of Conflicting Interests
Authors have no relationships/conditions/circumstances that present a potential conflict of interest.
Acknowledgement
P.P. is a Members of COST Action EU-CARDIOPROTECTION CA16225. C.P. and P.P. report grants from the University of Torino, Ricerca Locale Ex-60% (Grants: PAGP_RILO; PENC_ RILO) and from MIUR (PAGP_FFABR_17_01 and by PENC_ FFABR_17_01). CP reports “Fondi di beneficenza Intesa San Paolo No. 375-2019 (PENC_RIC_COMP_20_01)”.
References
Pasquale Pagliaro1,2
1Department of Clinical and Biological Sciences, University of Turin. Regione Gonzole 10, 10043, Orbassano (TO), Italy. 2Department of Medical Sciences, University of Turin. Corso Dogliotti 14, 10126, Turin, Italy.
Saveria Femminò2
2Department of Medical Sciences, University of Turin. Corso Dogliotti 14, 10126, Turin, Italy.
Cecilia Thairi1
1Department of Clinical and Biological Sciences, University of Turin. Regione Gonzole 10, 10043, Orbassano (TO), Italy.
Stefano Comità1
1Department of Clinical and Biological Sciences, University of Turin. Regione Gonzole 10, 10043, Orbassano (TO), Italy.
Giuseppe Alloatti3
3Uni-Astiss, Polo Universitario Rita Levi Montalcini. Piazzale Fabrizio de Andrè, 14100 Asti, Italy.
Claudia Penna1
1Department of Clinical and Biological Sciences, University of Turin. Regione Gonzole 10, 10043, Orbassano (TO), Italy.
Corresponding author:
Pasquale Pagliaro
Email: pasquale.pagliaro@unito.it

In a new window | Download PPT
Figure 1. Main Factors Determining Sex-Related Differences in Cardiovascular Diseases.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9240 | 19 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA