International bi-monthly journal of cell signaling, tissue protection, and translational research.
Pulmonary hypertension associated with left side heart disease
Przemysław Leszek1, Marcin Kurzyna2, Michał Mączewski3
Author Affiliations


- 1Heart Failure and Transplantology Department, The Cardinal Stefan Wyszyński National Institute of Cardiology, Warsaw, Poland.
- 2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education in Warsaw, European Health Center Otwock, Poland.
- 3Department of Clinical Physiology, Centre of Postgraduate Medical Education, Warsaw, Poland.
Abstract
Pulmonary hypertension (PH) secondary to left-sided heart disease (PH-LHD) is a heterogeneous phenotypic disorder. The development of PH and right ventricular (RV) dysfunction, independent of left ventricular dysfunction, have an important impact on disease progression, morbidity, and mortality. PH-LHD represents the most common form of PH, accounting for 65–80% of cases. Despite recent advances in the pathophysiological understanding and treatment of PH, there are currently no evidence-based recommendations for the management of PH-LHD. In this review, we highlight the prevalence and significance of PH and RV dysfunction, as well as insights into the complex pathophysiology of cardiopulmonary interaction in LHD. We also provide information for therapeutic options in PH-LHD and the necessity for future developments.
Keywords: pulmonary hypertension, left side heart disease
Abstract
Pulmonary hypertension (PH) secondary to left-sided heart disease (PH-LHD) is a heterogeneous phenotypic disorder. The development of PH and right ventricular (RV) dysfunction, independent of left ventricular dysfunction, have an important impact on disease progression, morbidity, and mortality. PH-LHD represents the most common form of PH, accounting for 65–80% of cases. Despite recent advances in the pathophysiological understanding and treatment of PH, there are currently no evidence-based recommendations for the management of PH-LHD. In this review, we highlight the prevalence and significance of PH and RV dysfunction, as well as insights into the complex pathophysiology of cardiopulmonary interaction in LHD. We also provide information for therapeutic options in PH-LHD and the necessity for future developments.
Keywords: pulmonary hypertension, left side heart disease
Introduction
Pulmonary hypertension (PH) is a common condition, being a consequence of pulmonary microvascular disease, chronic left heart failure (HF), lung disease, pulmonary embolism, and additional disorders. The clinical classification of PH categorizes multiple clinical conditions into five main groups related to their pathological findings, hemodynamic parameters, and possible treatment strategy. Among the various PH groups, PH related to left heart disease (PH-LHD), designated as Group 2 by the European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines (Galiè et al., 2016), represents by far the most common form of PH (Al-Omary et al., 2020). It accounts for 65–80% of all PH cases. PH occurs in response to an increase in left-sided filling pressures. It is quite common and a life-threatening complication that remains underestimated (Miller et al., 2013; Vachiéry et al., 2013). However, still little is known about the physiopathology and mechanisms underlying PH-LHD. Moreover, LHD is often associated not only with PH, but also right ventricle (RV) dysfunction, which has an important impact on disease progression, morbidity, and mortality. Furthermore, while there are approved therapies that exhibit at least some efficacy in the treatment of other forms of PH, there is no specific treatment for PH-LHD apart from optimization of therapy for the LHD itself. Last but not least, the pathophysiology of PH-LHD is still not completely understood. These considerations emphasize the need for further studies that would provide rationale for targeting PH as a potential additional treatment option in left sided HF treatment (Rosenkranz et al., 2016).
Pulmonary hypertension in left heart disease – definitions and measurements
The pulmonary circulation is a low-pressure and high- capacity system, and it can handle large increases in blood flow, as seen in healthy individuals during exercise with little or even no increase in pressures. The normal resting mean pulmonary artery pressure (mPAP) is 14.0 ± 3.3 mmHg and is largely independent of age, ethnicity, or posture (Kovacs et al., 2009). This is not the case with PAP during exercise that rises significantly with age (19.4 ± 4.8 mmHg in subjects aged <50 yrs compared with 29.4 ± 8.4 mmHg in subjects ≥50 yrs) (Kovacs et al., 2009). Recently, the 6th World Symposium on Pulmonary Hypertension has recommended that PH be redefined as mPAP >20 mmHg (two standard deviations above the mean) on resting right heart catheterization (RHC) and adding the criterion of pulmonary vascular resistance (PVR) with the threshold at 3 Wood Units (WU) (Simonneau et al., 2019).
Within PH patients, according to the classification provided by ESC/ERS Guidelines, Group 2 includes PH associated with left heart disease (PH-LHD) (Table 1). PH-LHD results from the passive backward transmission of elevated left-sided filling pressures - determined either as LV end-diastolic pressure (LVEDP), left atrial pressure (LAP), or pulmonary artery wedge pressure (PAWP), into the pulmonary circulation. Initially, elevated left sided filling pressure causes a proportionate increase of the mPAP - maintaining a normal transpulmonary pressure gradient (TPG) defined as mPAP minus PAWP < 12 mmHg and usually normal pulmonary PVR < 3 WU. PVR is mPAP – PAWP divided by cardiac output. However mPAP is influenced by PAWP at any level of stroke volume. In contrast, diastolic PAP (dPAP) is less dependent, so therefore the diastolic transpulomonary pressure gradient (DPG) defined as the difference between dPAP and the mean PAWP appears to be the best approach to determine PH.
Table 1: Classification of Pulmonary Hypertension (PH) associated with left heart disease from Galiè N. et al.(Galiè et al., 2016)
Heart failure with reduced left ventricle ejection fraction (ejection fraction ≤ 50%; systolic dysfunction)
– Ischemic cardiomyopathy
– Dilated cardiomyopathy
Heart failure with preserved left ventricle ejection fraction (ejection fraction > 50%; diastolic dysfunction)
– Hypertensive heart disease
– Coronary heart disease
– Diabetic cardiomyopathy
– Hypertrophic cardiomyopathy
– Restrictive cardiomyopathy
– Constrictive pericarditis
Valvular diseases
– Aortic valve stenosis
– Aortic valve regurgitation
– Mitral valve stenosis
– Mitral valve regurgitation
– Persistent/residual PH after effective valvular defect correction
Other causes
– Cor triatriatum
– Cor Myxoma or left atrial thrombus
PH-LHD can be classified into two main types: isolated postcapillary PH (IpcPH) and combined post- and precapillary PH(CpcPH).LowDPG(<7mmHg)andTPG(≤12mm Hg) or PVR ≤ 3 WU defines IpcPH, while elevation of these parameters suggests underlying pulmonary vascular disease (Vachiéry et al., 2013; Simonneau et al., 2019). Recently DPG > 7 mmHg has been reported to be associated with a worse prognosis in a subgroup of patients with TPG > 12 mmHg, and also correlates with pulmonary vascular remodeling (Vachiéry et al., 2013; Simonneau et al., 2019). The correct assessment of PH plays a very important role in classifying PH to the proper group, and also has direct implications on treatment possibilities. Proper PAWP measurements have direct implications on PH values. PAWP measurements are prone to significant errors and uncertainties that include: the lack of proper standardizations of calibration (zero level), the method of proper PAWP reading in relation to the respiratory cycle, and several others (Table 2) (Rosenkranz et al., 2016).
Table 2: Potential pitfalls in interpretation of pulmonary artery wedge pressure (PAWP) tracings
Modified, based on Rosenkranz S. et al. (Rosenkranz et al., 2016)
|
Factors related to the patient and his clinical condition |
|
|
Volume status |
Volume overload – artificially increase PAWP |
|
Rhythm disturbances |
Atrial fibrillation/frequent ventricular/supraventricular arrhythmia cause important beta to beat variation |
|
Tricuspid/mitral valve regurgitation |
Right/left atrial pressure respectively are increased due to regurgitation reflected as v wave |
|
COPD/dyspnea |
Caused prominent respiratory swings |
|
Thorax deformation |
Technical problems during measurements and zero point assessment |
|
Obesity |
Increase in intra-abdominal and intra-thoracic pressure |
|
Pitfalls in measurement and reading of pressure tracings |
|
|
Zero point |
Should be mid-thoracic |
|
Zero point – too low |
Values of central vein pressure artificially high |
|
Zero point – too high |
Values of central vein pressure artificially low |
|
Partial balloon occlusion (Swan-Ganz catheter) |
Values of PAWP artificially high |
|
Respiratory variation |
PAWP swinging curve - unreliable values |
Pathophysiology of pulmonary hypertension associated with left side heart disease
PH in LHD results from the passive backward transmission of elevated left-sided filling pressures resulting from all three principle causes of PH-LHD (Table 1): HF with reduced LV ejection fraction (HFrEF), HF with preserved LV ejection fraction (HFpEF), and left heart valvular disease. Initially, mPAP is normal at rest, and increases only during exercise (exercise PH), then later the PH elevation persists also at rest. In addition, in LHD, perturbations in left ventricular function have important effects on the geometry and structural properties of the left atrium (LA). Both systolic and diastolic HF results in elevation of left atrial pressures, leading to atrial hypertrophy and dilation. Furthermore the alteration of LA structure - left atrial dilation, increased left atrial mass, loss of myofibrillar atrial cardiomyocytes, and atrial fibrosis will cause reduced compliance and increased stiffness, and lead to contractility impairment, which in turn will contribute to LA remodeling and dysfunction. This also contributes to the passive backward transmission of elevated pressures into the pulmonary circulatory system (Rossi et al., 2014; Melenovsky et al., 2015). Additionally, the loss of LA hemodynamic function due to atrial fibrillation contributes to pathogenic alteration in PH and subsequently RV failure (Gorter et al., 2018). Furthermore if functional mitral regurgitation (MR) appeared, this would result in further PH increase affecting diastolic and systolic LA and LV properties (Tigges et al., 2018). Further events are understood less clearly. Passive backward transmission of the sustained elevated left – sided filling pressure cause increases in pulmonary venous pressure. Such increase in pulmonary pressures may be accompanied by an ‘alveolarcapillary stress failure,’ a barotrauma altering the endothelial barrier, causing leakage of proteins, red cells, and fluid into the alveolar lumen with interstitial, alveolar edema, as well as local inflammation. The alveolar edema may also induce matrix proteoglycan degradation (activation of metalloproteinases) and alter the composition of the endothelial membrane. Pulmonary vascular endothelial dysfunction is another pathology potentially contributing to increased PVR and CpcPH. Both physical factors (reduced velocity of blood flow) and chemical agents (oxygen free radicals and mediators of inflammation) are able to induce endothelial dysfunction in pulmonary vessels, reducing production of vasodilatory and anti-inflammatory nitric oxide, as well as decreasing its half life and stimulating its conversion to a highly toxic peroxynitrite, and promoting release of endothelin that favors vasoconstriction and inflammation. Moreover, abnormalities of natriuretic peptide receptors related to endothelial dysfunction have been implicated in the development of pulmonary vascular pathology (Figure 1) (Vachiéry et al., 2013; Melenovsky et al., 2015; Rosenkranz et al., 2016; Egom et al., 2017a; Egom et al., 2017b).
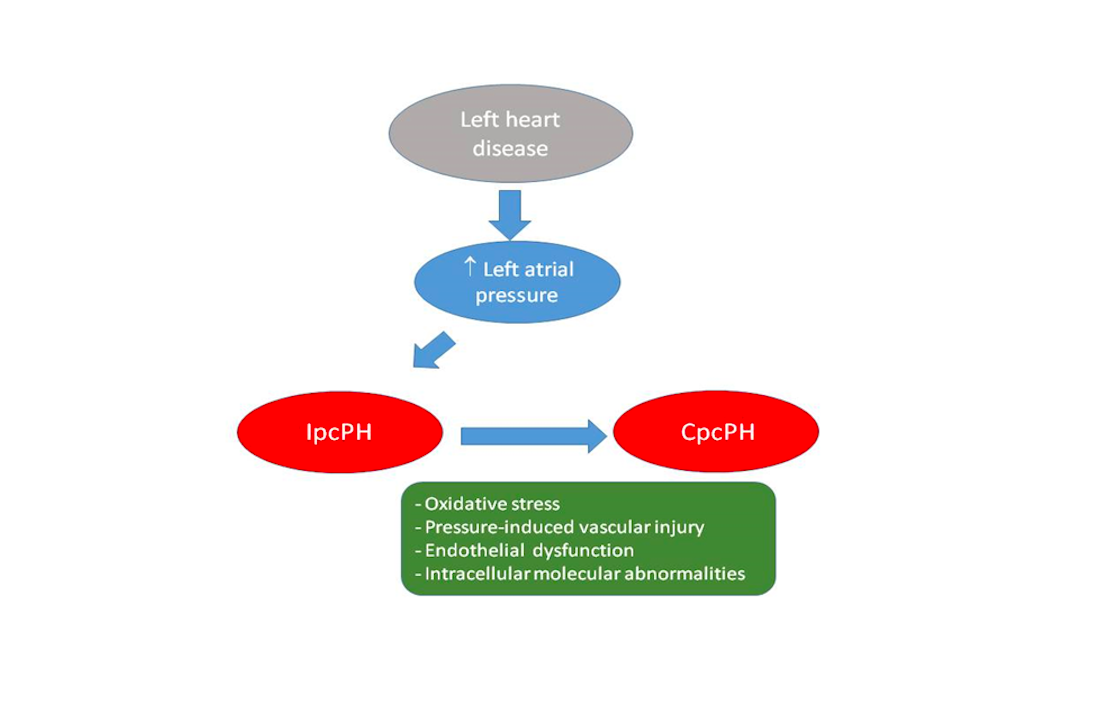
In a new window | Download PPT
Figure 1: Postulated pathophysiology of pulmonary hypertension related to left heart disease (PH-LHD)
Backward transmission of elevated left atrial pressure results in isolated postcapillary PH (IpcPH). Time and additional factors, such as oxidative stress, pressure induced pulmonary vascular injury, endothelial dysfunction, and intracellular molecular abnormalities, such as reduced activation of PTEN (phosphatase-and-tensin homolog on chromosome 10) result in pulmonary vasculopathy, increased pulmonary vascular resistance (PVR), and eventually combined post- and precapillary PH (CpcPH).
Recently several preclinical animal studies provided evidence that oxidative stress, hypoxia, and peroxynitrate may be involved in the downregulation of phosphatase-and- tensin homolog on chromosome 10 (PTEN), a crucial regulator of cell proliferation/apoptosis through multiple intracellular pathways, in pulmonary vascular smooth muscle cells (Ravi et al., 2013b), resulting in adverse remodeling of pulmonary vascular walls, intimal fibrosis, and medial hypertrophy. This may be a hallmark of all types of PH and hence therapies aimed at prevention of oxidative stress or activation of PTEN (e.g. curcumin, the principal component of the popular Indian spice turmeric (the rhizome of Curcuma Longa)) offer a promise as a targeted therapy, especially for CpcPH (Ravi et al., 2013a; Ravi et al., 2013b; Egom et al., 2017a; Egom et al., 2017b).
The alterations in pulmonary circulation are partly reversible at least in some patients, especially in HFrEF patients, by implanting left ventricular assist device (LVAD) (Tsukashita et al., 2015). However, the pulmonary arterial remodeling finally contributes to the increase in PVR, RV pressure overload, and deterioration of RV function, often accompanied by tricuspid valve insufficiency (Gerges et al., 2015; Tsukashita et al., 2015).
Irrespective of the cause of the left ventricle dysfunction, the presence of PH, especially in the presence of compromised RV function is significantly associated with disease progression, decreased exercise tolerance and quality of life, as well as a poor outcome. Moreover it should also be mentioned that in HFrEF, severe PH is the important contraindication for heart transplantation whereas RV failure is a contraindicator for LVAD implantation (Copeland et al., 2004; Cook et al., 2015). As PH and RV function assessment brings important prognostic impact in patients with LHD, they are both targeted as a potential treatment options in left sided HF (Figure 2). However, in comparison to the recent advances in pulmonary artery hypertension (Group 1 pulmonary arterial hypertension (PAH)) no significant progress has been made for PH-LHD.
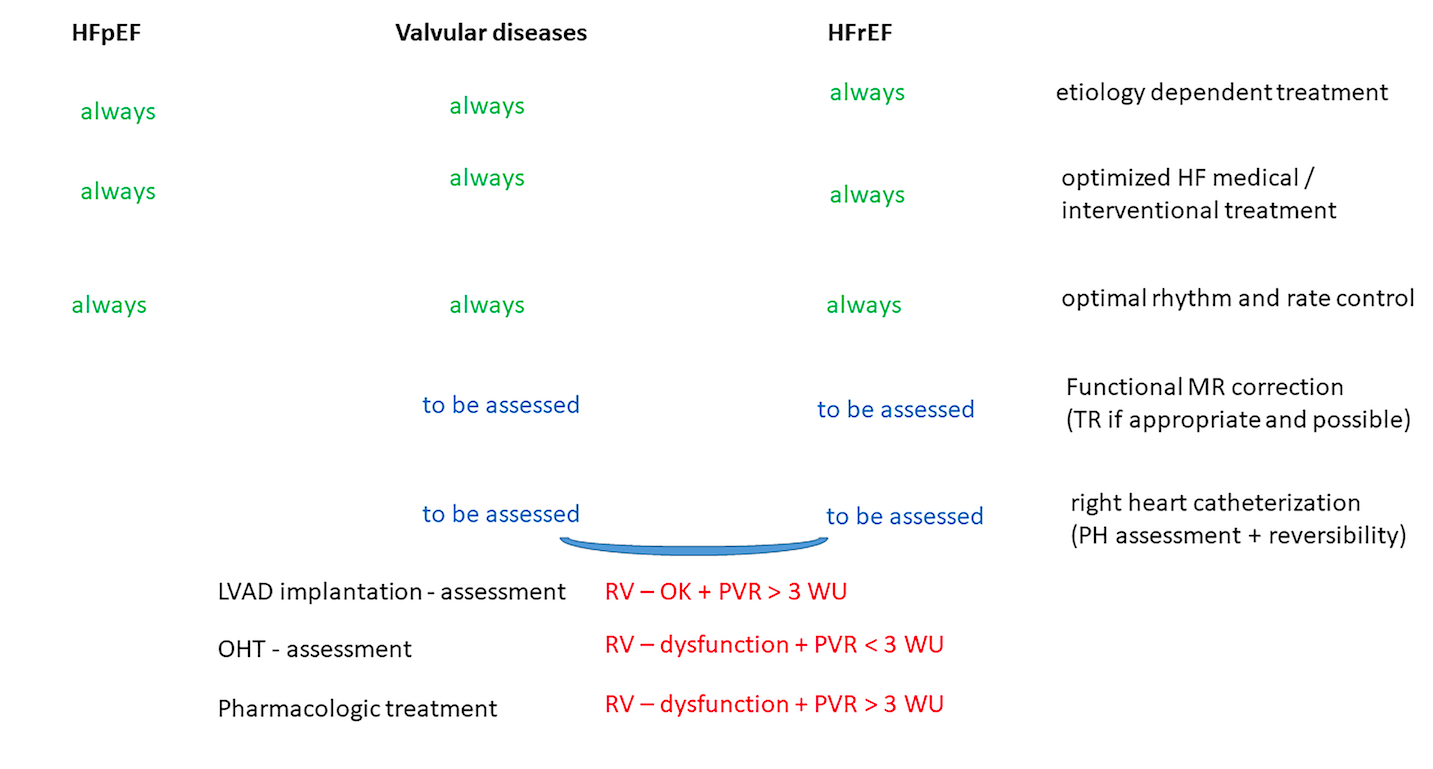
In a new window | Download PPT
Figure 2: Treatment possibilities in pulmonary hypertension related to left heart disease. In pulmonary hypertension due to left heart disease (PH-LHD) the treatment possibilities are related to the cause of PH-LHD. Etiology dependent correction, heart failure treatment optimization and appropriate rhythm control are independent from the initial cause of PH-LHD. However further steps as mitral / tricuspid regurgitation correction (if appropriate and possible) and referral for left ventricle assist device (LVAD) implantation or orthotropic heart transplantation (OHT) should be individualized but are also related to the initial cause of PH-LH.
Pulmonary hypertension in left heart disease - epidemiology
PH is a common complication of any left heart disorder, such as HF, valvular heart diseases, or congenital defects. The prevalence of PH in LHD increases with the progression of HF or degree of valve impairment. In epidemiological studies the definition of PH has been based on RHC or echocardiography, with a variety of cut-off so the true prevalence of PH-LHD in left HF remains unknown. Epidemiological data regarding PH in HFrEF are limited mostly to populations with advanced HF, so the data does not reflect the entire HF population. Most available data are based on RHC. The prevalence of PH in HFrEF ranges between 33% and 68%. While Gerges et al. (2015) in a study from a large cohort of 664 HFrEF patients, referred for RHC, reported a prevalence of PH of 68%, in another study Miller et al. (2013) found PH prevalence in ambulatory HFrEF group to be 33%.
The etiology of HFrEF can be different, but the most common ones are ischemic or non-ischemic dysfunction. The prognosis in the HFrEF population depends on the cause of the LV damage. However, regardless of the HFrEF etiology, the appearance of PH significantly worsens the prognosis. Most epidemiological studies consistently indicate an inverse correlation between PH and survival in HFrEF. Moreover, a combination of PH together with reduced RV function worsen the prognosis and is particularly associated with an unfavorable outcome in HFrEF patients (Guazzi and Naeije, 2017). Of note in the HFrEF group, PH and RV dysfunction not only worsens the prognosis for survival, but also reduces the treatment possibilities. Whereas heart transplantation is an accepted treatment for end-stage HFrEF, severe PH is an absolute contraindication to heart transplantation. In such situations, for patients with even ‘irreversible’ PH, the use of mechanical circulatory support, particularly LVAD should be considered with subsequent re-evaluation to establish candidacy. The evaluation of RV function is crucial since postoperative RV failure greatly increases perioperative mortality and reduces survival (Copeland et al., 2004; Cook et al., 2015; Tsukashita et al., 2015).
Unlike HFrEF, most data on the prevalence of PH in HFpEF are based on non-invasive Doppler assessment. The prevalence of PH in HFpEF ranges between 54% and 83%. While Gerges et al. (2015) in a study including 399 HFpEF patients, found a prevalence of PH in HFpEF of 54%. In another study, Lam et al. (2009) reported data from a prospective study of randomly recruited 1413 adults and found the prevalence to be 83%. However, we can only estimate the prevalence as the definition of PH has been based on echocardiography, with large variety of cut-offs used. In HFpEF patients, PH is relatively more frequent in the group of elderly patients with hypertension, obesity, diabetes, atrial fibrillation, and chronic obstructive pulmonary disease (COPD). Nevertheless, also in HFpEF, PH exerts additional adverse prognostic impact on the prognosis in such population (Lam et al., 2009; Gerges et al., 2015). Also in cohorts of patients with valve diseases the presence of PH seems to be an independent predictor of the worst prognosis (Mentias et al., 2016).
Pulmonary hypertension in left heart disease – treatment
Left sided ventricle failure issue
The main approach in PH-LHD should be aggressive treatment or optimization of the management of the underlying heart disease. Simultaneously, adequate HF treatment consisting of diuretics, beta-blockers, neurohormonal antagonists, and vasodilators, should be introduced and modified until the target dosages are achieved. If needed implantable device therapies should be applied (e.g. cardiac resynchronization therapy (CRT), or implantable cardiac defibrillator (ICD)). All the treatment mentioned above, usually help to lower left-sided filling together with pulmonary pressures (Capomolla et al., 2000; Stolfo et al., 2015b; Stolfo et al., 2015a; Nasser et al., 2017; Martens et al., 2018; Tigges et al., 2018).
Unloading and heart rhythm or rate control are the basic approaches in HF management. Previous studies reported that inhibition of the renin angiotensin-aldosterone system improved LVEF and antagonized cardiac remodeling, as well as reduced the risk of cardiovascular death in HFrEF patients (Ponikowski et al., 2016). Also diuretics play an important role in the appropriate load correction and pulmonary pressure reduction. As it was shown in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial the consideration of the PAP values (assessed by an implantable device) optimized HF treatment including adjustment of diuretics. The properly targeted treatment importantly reduced the PAP together with HF-associated hospitalizations in both HFpEF and HFrEF (Abraham et al., 2011; Adamson et al., 2014). All comorbidities that may contribute to PH such as COPD, pulmonary embolism, or sleep apnea should be diagnosed and optimally treated.
Mitral regurgitation
Functional mitral regurgitation (MR) is present to varying degrees in most patients with chronic HF and LV systolic dysfunction. In 30% of cases its magnitude is hemodynamically meaningful. Moreover, MR may represent the main cause of PH and leads to increased mortality (Mentias et al., 2016). Even in patients with asymptomatic MR, the exercise induced PH together with RV dysfunction is associated with adverse outcome (Asgar et al., 2015). There is a good evidence that HF patients with functional MR, who received optimal medical therapy consisting of angiotensin-converting enzyme (ACE) Inhibitors or angiotensin receptor-neprilysin inhibitors might undergo reversal of left ventricle remodeling and reduce functional MR. This benefit often requires high doses of these agents. Furthermore, a randomized trial with an angiotensin receptor - neprilysin inhibitor showed more pronounced reduction in MR than an angiotensin receptor inhibitor alone (Kang et al., 2019). Similarly, beta-blockers are effective in ameliorating functional MR in patients with both an ischemic and nonischemic HF. However, it should be noted that the absence of left bundle branch block was the primary predictor of a favorable response to such drug therapy. Patients presenting with marked QRS prolongation often showed worsening MR even with optimal medical management (Asgar et al., 2015).
Independently of LV end-diastolic volume, left ventricular dyssynchrony contributes significantly to functional MR. Important QRS prolongation, and related left ventricular contractile dyssynchrony, causes unequal contraction of papillary muscle bearing walls, preventing coordinated closure of the mitral valve (MV) leaflets. In two-thirds of HF patients with functional MR, cardiac resynchronization, by increasing LV closure forces resulting from improved myocardial contractility, and by restoring papillary muscle coordination, markedly reduces MR. MR reduction, not only improves the prognosis of HF patients but also reduces PAP (Cleland et al., 2012). As MR increases PH in LV HF, in patients who are on optimized medical/resynchronization (if appropriate) treatments, further appropriate MV correction should be considered. At this stage, the effective treatment of the MV, including catheter-based interventions, leads to substantial functional improvement. It seems that in a properly selected group of patients successful percutaneous MitraClip functional MR repair decreases all-cause mortality as well as the combined risk of death or hospitalization for HF. Moreover MR repair improves pulmonary hemodynamics, including reduction of the mean PAP and PAWP (mainly via reduction of the v-wave), and also profoundly improves the cardiac index (Grayburn et al., 2019).
There are also surgical options for functional MR correction. They include surgical MV repair or replacement. MV surgery has never clearly been demonstrated to alter the natural history of the primary disease and LV dilatation, or improve survival. Also the response to surgery in functional MR differs between ischemic versus nonischemic etiology (Asgar et al., 2015).
Atrial fibrillation (AF)
In patients with LV dysfunction, left atrial enlargement gradually appears due to elevated ventricular volume, pressure overload, and other factors that may develop, including functional mitral regurgitation. Left atrium chamber enlargement and atrial wall remodeling lead to its dysfunction. Development of AF has been shown to have a linear relationship with increasing left atrial volumes and further atrial dysfunction. Moreover, AF results in a loss of atrial systole (or atrial “kick”), which may impair cardiac output by up to 25%. Both atrial remodeling and also AF are associated with a reduction in conduit and reservoir atrial function (both of which facilitate drainage of pulmonary venous blood and passively fill the left ventricle), further compromising cardiac output (Rossi et al., 2014). Rhythm control of AF, either electrically or pharmacologically, should be attempted in HF patients, particularly following an initial presentation of AF with HF-rEF or HFpEF.
In HFrEF, the benefits of a rhythm-control strategy achieved via catheter ablation were proved in several multicenter studies (Marrouche et al., 2018). In almost all trials, AF catheter ablation was associated with decreased all-cause mortality, improved ejection fraction, and freedom of AF compared with medical treatment. There was no significant difference in the complication rates between catheter ablation and other medical treatments (Marrouche et al., 2018). In HFpEF, there is a paucity of well-designed large clinical studies assessing the effectiveness of catheter ablation for AF. Whether successful catheter ablation can improve symptoms and mortality in this group is an area that requires further research.
Targeted pharmacology for pulmonary hypertension in left ventricle failure
So far, targeted PH therapies in PH-LHD have never been investigated properly. There is need for controlled randomized trials in larger populations separately for PH-HFrEF and PH- HFpEF. The study population should present with PH and be precisely characterized, with longer observation times, and diverse endpoints related to PAP changes, and also definitive ones such as death, HF hospitalization etc. The included patients should be on optimized regimens of HF therapy and fluid balance achieved before randomization and initial baseline assessment. Only a limited number of clinical trials have evaluated the safety of specific PAH-like therapies in PH-LHD. These trials are either neutral or small single-center studies. Therefore, PAH-specific therapies are currently not approved for the treatment of PH-LHD. Targeted therapies approved for the treatment of PAH include prostanoids, endothelin receptor antagonists (ERAs), phosphodiesterase type 5 inhibitors (PDE5i), and stimulators of soluble guanylate cyclase (sGC) (Table 3).
Table 3: Studies on pulmonary hypertension (PH) vasodilators in pulmonary hypertension associated with left heart disease (PH-LHD)
|
Drug |
n |
Population |
Final endpoint |
|
|
FIRST(Califf et al., 1997) |
IV epoprostenol vs. standard care |
47 |
LVEF < 35%, NYHA IIIB/IV |
Acute: CI increase, PCWP reduction, Early termination; trend towards in- creased mortality in treatment group |
|
ENDOTHELIN RECEPTOR ANTAGONISTS (ERA) |
||||
|
HFrEF |
||||
|
REACH-1 (Mylona and Cleland, 1999) |
Bosentan (non-selective ERA) vs. placebo - 26 weeks |
370 |
LVEF < 35%, NYHA III/IV |
Early termination due to AE-affects dizziness, blurred vision, worsening liver function, and early HF worsening |
|
ENABLE (Kalra et al., 2002) |
Bosentan (non-selective ERA) vs. placebo - 1.5 years |
1613 |
LVEF < 35%, NYHA III/IV |
Early risk of HF worsening, fluid reten- tion and hospitalization |
|
ENCOR (Kelland and Webb, 2007) |
Enrasentan (non-selective ERA) vs. place- bo - UNK |
369 |
LV < 35%, NYHA II/III |
Results not fully published. Failed |
|
HEAT (Lüscher et al., 2002) |
Darusentan (selective ERA) vs. placebo - 3 weeks |
157 |
LVEF < 35%, NYHA III, PCWP > 12, CI < 26 L/min/m2 |
Cardiac index increase, no effect on PCWP, PVR, HR, BP, plasma catechol- amines, higher dosages group a trend to AE (including death) |
|
EARTH (Anand et al., 2004) |
Darusentan (selective ERA) vs. placebo - 24 weeks |
642 |
LVEF < 35%, NYHA II-IV |
No effect on change in LV end systolic volume, no effect on symptoms |
|
HFmrEF |
||||
|
NCT00840463 |
Ambrisentan (selective ERA) vs placebo – 16 weeks |
UNK |
LVEF > 40%, NYHA II/III, PA mean >25mmHg< PVR >3 WU or TPG >12 mmHg |
Results not published |
|
SERENADE NCT03153111 |
Macitentan (non-selective ERA) vs. place- bo - 24-52 weeks |
143 |
LVEF ≥ 40%, NYHA I/II, elevated NT-proBNP, |
Results not published |
|
HFpEF |
||||
|
BADDHY (Koller et al., 2017) |
Bosentan (non-selective ERA) vs. placebo - 12 weeks |
20 |
LVEF > 50%, NYHA II/III, mPAP > 25 mmHg, PAWP > 15 mmHg |
The study was aborted early, interim analysis favored the placebo |
|
HFrEF / HFpEF |
||||
|
MELODY-1 (Vachiéry et al., 2018) NCT02070991 |
Macitentan (non-selective ERA) vs. place- bo – 12 weeks |
63 |
LVEF ⩾30%, and stratified (LVEF <50% vs ⩾ 50%), NYHA II/III, mPAP ⩾25 mmHg, PAWP >15 - <25 mmHg, PVR ⩾3 WU and DPG ⩾7 mmHg |
Macitentan group resulted in no significant changes in any exploratory end-points and experience significant fluid retention versus placebo |
|
Phosphodiesterase type 5 (PDE-5) inhibitors |
||||
|
HFrEF |
||||
|
Guazzi et al. (Guazzi et al., 2011b) NCT00975494 |
Sildenafil vs. placebo – 1 year |
45 |
LVEF < 40%, NYHA II/Ill |
Sildenafil treatment reversed LV and left atrium remodeling, improved exercise performance (peak VO2), ventilation efficiency (ventilation to CO2 production slope), and quality of life |
|
Lewis et al. (Lewis et al., 2007) |
Sildenafil vs. placebo - 12 weeks |
34 |
LVEF < 35%, NYHA II-IV, mPA > 25mmHg |
Sildenafil reduced PVR, increased CO without altering PAWP, BP, HR, and SVR. Also sildenafil augmented the peak V ̇ O2, improved 6-minute walk distance and Minnesota Living With Heart Failure score. Subjects in the sildenafil group experienced fewer hospitalizations for HF and a higher incidence of headache than those in the placebo group without incurring excess serious adverse events |
|
SILF-HF (Cooper et al., 2013) NCT01616381 |
Sildenafil vs. placebo – 24 weeks |
210 |
LVEF ≤ 40%, NYHA II/III, syst PA > 40 mmHg |
Still ongoing, results not available yet |
|
PITCH-HF NCT01910389 |
Tadalafil vs placebo – up to 54 months |
2102 |
LVEF ≤ 40%, NYHA II-IV, mPA ≥ 25mmHg |
Study terminated early, results not available yet |
|
HFpEF |
||||
|
Guazzi et al (Guazzi et al., 2011a) NCT01156636 |
Sildenafil vs. placebo - - 6 months |
44 |
LVEF ≥ 50%, NYHA II/III, systolic PA > 40 mmHg |
Improvement in pulmonary pressure and vasomotility, RV function and dimension, left ventricular relaxation |
|
RELAX (Redfield et al., 2013) |
Sildenafil vs. placebo - 24 weeks |
216 |
LVEF ≥ 50% NYHA II-IV and el- evated NT-proBNP or elevated filling pressures |
Chronic therapy with sildenafil was not associated with clinical benefit in HFpEF (no effect on peak V02, 6 min walking distance etc.) |
|
Hoendermis et al (Hoendermis et al., 2015) NCT01726049 |
Sildenafil vs. placebo - 12 weeks |
52 |
LVEF ≥ 45%, NYHA II-IV, mPA ≥ 25 mmHg, PCWP >15mmHg |
Sildenafil did not have a favourable effect on PAWP, cardiac output, and peak VO2 in HFpEF, adverse events were overall comparable with placebo |
|
Valvular Heart Disease |
||||
|
SIOVAC (Bermejo et al., 2018) NCT00862043 |
Sildenafil vs. placebo – 6 months |
200 |
Successful valve replacement or repair at least 1 year before inclusion / mPAP ≥ 30 mmHg |
Treatment with sildenafil in patients with persistent PH after successful correction of valvular heart disease is associated to unfavorable clinical outcomes as compared to placebo |
|
Soluble guanylate cyclase (sGC) agonists |
||||
|
HFrEF |
||||
|
LEPHT (Bonderman et al., 2013) NCT01065454 |
Riociguat vs. placebo - 16 weeks |
201 |
LVEF ≤ 40%, mPA ≥ 25 mmHg |
Riociguat was well tolerated in PH due to HFrEF patients and improved cardiac index and pulmonary and systemic vascular resistance |
|
SOCRATES-HFrEF (Gheorghiade et al., 2015) NCT01951625 |
Vericiguat vs. placebo –12 weeks |
351 |
LVEF < 45%, 45% within 4 weeks of a worsening chronic HF event |
Vericiguat did not have a statisti- cally significant effect on change in NT-proBNP level at 12 weeks but was well-tolerated |
|
VICTORIA (Armstrong et al., 2020) NCT02861534 |
Vericiguat vs. placebo – mean 10.8 months |
5050 |
LVEF < 45%, NYHA II-IV |
The incidence of the primary outcome of death from cardiovascular causes or first hospitalization for heart failure was significantly lower with vericiguat than with placebo |
|
HFpEF |
||||
|
DILATE (Bonderman et al., 2014) NCT01172756 |
Riociguat vs placebo – 16 week |
48 |
LVEF ≥ 50%, mPA ≥ 25 mmHg, PCWP >15mmHg |
In patients with HFpEF and PH, riocig- uat was well tolerated, had no signifi- cant effect mPAP and hemodynamic / echocardiographic parameters |
|
VITALITY-HFpEF (Butler et al., 2019) NCT03547583 |
Vericiguat vs. placebo – 24 weeks |
735 |
LVEF≥45% NYHA II-III |
Terminated, the results are still not available yet |
|
SOCRATES-HFpEF (Pieske et al., 2017) NCT01951638 |
Vericiguat vs. placebo –12 weeks |
477 |
LVEF>_ 45%, NYHA II–IV |
Vericiguat was well tolerated, did not change NT-proBNP, LAV at 12 weeks compared with placebo, was associ- ated with improvements in quality of life |
Prostacyclin
Epoprostenol, a synthetic prostacyclin, provided the rationale for the Flolan International Randomized Survival Trial (FIRST). In the acute setting epoprostenol produced arterial vasodilatation both in pulmonary and systemic circulation with acute hemodynamic improvements including reduced PAWP, PVR, systemic vascular resistance (SVR), and increased cardiac output. However, the FIRST trial, which investigated chronic treatment with intravenous epoprostenol in patients with PH- HFrEF, was prematurely terminated due to neutral influence on exercise tolerance and a trend for increased mortality in the treatment group when compared with placebo (Califf et al., 1997).
Endothelin receptor antagonists (ERA)
ERAs block endothelin receptor in a non-selective (ET receptor A/B) or selective (ET receptor A) way. ET-A receptors are located on smooth muscle cells of the vascular wall and are responsible for endothelin-induced vasoconstriction while ET-B receptors are located on endothelial cells and induce these cells to release nitric oxide (NO) and prostacyclin. Multiple trials of endothelin receptor antagonists for the treatment of HF patients have been performed, however several of these negative studies have never been published. Notably, ERAs have mainly been investigated in patients with HF in general without considering the presence of PH. Therefore the impact of ERA’s on PH-LHD remains unknown.
The HFrEF trials of the non-selective ERA – bosentan (REACH-1, ENABLE - 1 and -2) were stopped prematurely due to the increased number of hospitalization for HF, fluid retention, edema, and liver function abnormalities (Mylona and Cleland, 1999; Kalra et al., 2002). Also the complete results of the Enrasentan Cooperative Randomized Evaluation (ENCOR) trial of the non-selective ERA enrasentan have never been published. The treatment with enrasentan was associated with increased hospitalization rate, higher mortality, and progressive LV dysfunction (Kelland and Webb, 2007).
The results of the selective ET-A receptor antagonist darusentan was assessed in two trials – Heart Failure ET(A) Receptor Blockade Trial (HEAT) and Endothelin A Receptor Antagonist trial in Heart Failure (EARTH). Summing up, dorusentan administration resulted in a significant cardiac index increase and non-significant changes in PAP, PAWP, right atrial pressure, and PVR. Left ventricular end systolic volume remained unchanged. Moreover, there was a trend toward an increased number of HF exacerbations and death (Lüscher et al., 2002; Anand et al., 2004). Despite these negative trials, in HFrEF patients, a potential benefit of ERAs in PH and HFmrEF or HFpEF led to additional trials. The trial of bosentan (non- selective ERA) in PH and HFpEF patients (BADDHY) was prematurely aborted due to analysis favoring the placebo arm versus bosentan (Koller et al., 2017).
The assessment of selective ET-A receptor antagonist ambrisentan in HFmrEF patients (Trial to treat Diastolic Heart Failure - NCT00840463) with PH has been terminated early for poor enrollment. The non-selective ERA – macitentan is currently under evaluation in the Study to Evaluate Whether Macitentan is an Effective and Safe Treatment for Patients With Heart Failure With Preserved Ejection Fraction and Pulmonary Vascular Disease (SERENADE) trial (NCT03153111) as a treatment for patients with HFmrEF and confirmed pulmonary vascular disease.
Recently the negative results of the phase II double-blind, randomized trial (MELODY) of macitentan in patients with HFrEF/HFpEF and PH were published. The treatment with macitentan vs. placebo was associated with a main endpoint of significant fluid retention (weight gain ≥ 5 kg or ≥ 5% due to fluid overload or the need for parenteral administration of diuretics) or worsening in New York Heart Association functional class from baseline to end of treatment (Vachiéry et al., 2018).
Phosphodiesterase type 5 inhibitors
NO contributes to guanylate cyclase (GC) activity and production of cyclic guanosine monophosphate (cGMP), which activates protein kinase G leading to vasodilatation and inhibition of smooth muscle cell proliferation. The clinical role of NO in PH was shown by Stamler et al. (1994) who reported that inhalation of NO gas causes pulmonary vasodilation and PVR reduction in patients with primary and secondary forms of PH. It is important to point out that it may cause adverse effects due to increase in PAWP in HF patients (Kumar et al., 2009).
Sildenafil, and longer acting tadalafil are phosphodiesterase type 5 (PDE5) inhibitors, which prevent degradation of cGMP in smooth muscle cells and prolong the vasodilation provided by NO. After recognition and confirmation of the potential benefits of sildenafil in patients with PAH in Sildenafil Use in Pulmonary Arterial Hypertension trial, the drug was assessed for the treatment of HFrEF and HFpEF patients (Goldsmith, 2007; Kumar et al., 2009). Several trials have demonstrated a favorable acute hemodynamic response to sildenafil in PH HFrEF patients. In studies with small samples sizes, it has been shown that sildenafil has beneficial acute hemodynamic effects, as there was significant reduction in the mean PAP, PVR, PAWP (but not in all trials), and a diastolic RV function improvement. Additionally, in patients with HFrEF and PH sildenafil also significantly improved exercise tolerance, gas exchange, ventilatory efficiency, and skeletal muscle function (Cooper et al., 2013).
In the long-term treatment with sildenafil compared to the placebo in patients with HFrEF on a stable medical regimen Guazzi et al. (2007) reported improvement in LVEF, diastolic function, left atrial size index, left mass index, and reduction in PA pressures. Moreover, it significantly improved endothelial function, exercise tolerance, peak exercise oxygen consumption, ventilation efficiency, and quality of life without significant adverse effects (Guazzi et al., 2007). These positive results were confirmed by Lewis et al. (2007). Meta-analysis of six randomized trials investigating PDE5 inhibitors in patients with HFrEF, however each in a small number of patients, confirmed that in comparison to placebo the use of a PDE5 inhibitor improved hemodynamics and exercise capacity, reduced symptoms and the number of hospitalizations (Wu et al., 2014). Two other studies were terminated but the results are still unavailable (NCT01616381; NCT01910389).
Studies evaluating the use of sildenafil in HFpEF patients provide confusing data. The multicenter randomized controlled Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial with sildenafil has shown its neutral effects on peak oxygen consumption or exercise capacity in patients with HFpEF in the absence of PH. However PH was not required for trial entry, and the authors did not specifically investigate pulmonary hemodynamics and RV function (Redfield et al., 2013). These neutral results were confirmed by Hoendermis et al. (2015). In contrast, Guazzi et al. (2011b) in a small single-center trial with HFpEF patients, reported that sildenafil improved left ventricular relaxation and distensibility, pulmonary pressure, RV function and dimension.
Recently, the Sildenafil for Improving Outcomes after Valvular Correction (SIOVAC) study, included patients after successful valvular heart disease repair with mean PAP > 30 mmHg. Randomized treatment with sildenafil was associated with unfavorable clinical outcomes as compared to placebo (Bermejo et al., 2018). In this trial many patients presented normal or near normal values of pulmonary vascular resistance. All data mentioned above, should be interpreted with great caution. They were obtained in small studies, often single centers, in highly selected patients, and data from larger randomized multicenter trials separately for HFrEF and HFpEF with PH are still lacking.
Soluble guanylate cyclase (sGC) agonists
Rather than inhibiting PDE-5 to block the break down of cGMP, an alternative way is via stimulation or activation of sGC. The sensitization of sGC to endogenous NO stabilizes NO binding to the binding site, thereby enhancing activation of the cGMP pathway. Typical for HF, deficiency in sGC-derived cGMP causes both myocardial dysfunction and impaired endothelium- dependent vasomotor regulation that includes myocardial microcirculation. Hence, restoration of sufficient NO -> sGC -> cGMP signaling has been proposed as a promising research direction in HF, especially HF with PH. Several experimental studies have suggested multiple potential benefits of sGC stimulators including left ventricular function, as well as reduction of pulmonary resistance via pulmonary vasodilation (Stasch et al., 2011).
In patients with PH-HFrEF the sGC stimulator riociguat was investigated in the randomized Left Ventricular Systolic Dysfunction Associated with Pulmonary Hypertension Riociguat (LEPTH) trial. Riociuat failed to reach the primary endpoint of lowering PAP compared to placebo, but due to a substantial increase in cardiac index, it significantly reduced PVR (Bonderman et al., 2013). Recently, the new sGC compound vericiguat was assessed initially in the (Soluble Guanylate Cyclase Stimulator in Heart Failure (SOCRATES)- HFrEF randomized trial. Vericiguat was well tolerated but did not have a significant effect on change in the N-terminal- probrain natriuretic peptide (NT-proBNP) level at 12 weeks (Gheorghiade et al., 2015). In the Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction (VICTORIA) trial HFrEF patients were assigned to receive vericiguat or placebo, in addition to guideline-based medical therapy. However, PH was not required for trial entry, and the trial did not specifically investigate pulmonary hemodynamics and RV function. The VICTORIA trial showed promising results as the incidence of death from cardiovascular causes or hospitalization for HF was lower in the vericiguat vs. placebo groups (Armstrong et al., 2020). Likewise, a proof-of-concept clinical trial testing the acute effects of riociguat in patients with PH-HFpEF (Acute hemodynamic effects of riociguat (BAY63-2521) in patients with pulmonary hypertension associated with diastolic heart failure or DILATE trial) showed no difference in the change in mean PA pressure between baseline and 6 h after the administration of riociguat (Bonderman et al., 2014). Also Vericiguat, had been evaluated for HFpEF in the randomized VITALITY-HFpEF phase II trial to improve the Kansas City Cardiomyopathy Questionnaire Physical limitation score (KCCQ PLS). The trial was designed to evaluate the efficacy and safety of vericiguat to improve physical functioning in activities of daily living in HFpEF patients. Although the trial was terminated on Nov 04, 2019, the results are still not available (Butler et al., 2019). Unlike the previous study in the SOCRATES-HFpEF randomized trial, vericiguat was well tolerated and associated with improvements in health-related quality of life. However, it did not show NT-proBNP and left atrial volume reduction (Pieske et al., 2017). It is important to mention that the goal of all the trials with vericiguat was not to improve PH in HFpEF populations.
Clinical conclusion
Despite significant achievements in the diagnosis and treatment of PH-LHD made recently, important evidence gaps still exist. Based on the current evidence, the use of targeted PAH therapies in a wide PH-LHD patient population is discouraged, and only selected patients with Cpc-PH and/or RV phenotypes should be referred to centers with expertise in treating both HF and PH. In such centers, depending on the phenotype, treatment decisions can be individualized.
Surgical interventions for PH in left ventricular failure
Fixed PH with elevated PVR was an established contraindication that had potentially prevented patients from being considered for heart transplantation. Nowadays, even in patents with HFrEF and severe ‘fixed’ PH the unloading of the LV by implantation of a LVAD may substantially lower or even normalize PAP over time, indicating that the alterations in the pulmonary circulation are partly reversible at least in some patients (Tsukashita et al., 2015). However, the severely compromised RV function negatively determines the LVAD implantations results. Long-term, durable RV assist device for irrecoverable forms of RV dysfunction are limited, and destination therapy for chronic advanced right HF is not well studied. In patients with HFrEF, PH, and RV dysfunction, durable devices used for long-term or permanent RV support have been designed for LV support, and their use for the RV represents an off-label or unapproved indication. The total artificial heart (TAH) represents an alternative therapy for biventricular support for the failing RV and LV. Although the use of the TAH may be advantageous over the biventricular assist device support options in strictly limited clinical situations (Copeland et al., 2004; Cook et al., 2015).
Left ventricular failure vs PH and right ventricular failure phenotype
Among patients with LV HF there is a great variation with regard to PAP. On the current state of knowledge it is impossible to explain the heterogeneity of PH. The reasons why some patients develop severe PH and RV dysfunction whereas others do not are not clear. In many cases it could be explained by the time factor, as a long lasting left ventricle failure may finally lead to a gradual increase in pulmonary pressure and PH. So longitudinal studies should be performed that investigate the development of PH and further RV dysfunction in patients with LV HF over time. However it is not the case in all patients. In a subset of patients with LV HF, potential susceptibility for PH and RV dysfunction may exist. Moreover the mortality increases as the RV phenotype develops, but we are not able to elucidate the factors (genetic factors, environmental stressors, and comorbidities) predisposing and accelerating evolution from an LV phenotype to a PH and RV failure phenotype over the time. This subject requires further investigation.
Final conclusion
PH secondary to left-sided heart disease (Group 2 PH) is a heterogeneous phenotypic disorder that worsens survival independent of LV function. In this review, an update of the current knowledge and some potential challenges about the pathophysiology and treatments of group 2 PH in patients with HF are provided. Despite significant achievements that were made recently, important evidence gaps remain that need to be addressed in future studies.
References
Przemysław Leszek1
1Heart Failure and Transplantology Department, The Cardinal Stefan Wyszyński National Institute of Cardiology, Warsaw, Poland.
Marcin Kurzyna2
2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education in Warsaw, European Health Center Otwock, Poland.
Michał Mączewski3
3Department of Clinical Physiology, Centre of Postgraduate Medical Education, Warsaw, Poland.
Corresponding author:
Przemysław Leszek
Email: przemyslaw.leszek@ikard.pl

In a new window | Download PPT
Figure 1: Postulated pathophysiology of pulmonary hypertension related to left heart disease (PH-LHD)
Backward transmission of elevated left atrial pressure results in isolated postcapillary PH (IpcPH). Time and additional factors, such as oxidative stress, pressure induced pulmonary vascular injury, endothelial dysfunction, and intracellular molecular abnormalities, such as reduced activation of PTEN (phosphatase-and-tensin homolog on chromosome 10) result in pulmonary vasculopathy, increased pulmonary vascular resistance (PVR), and eventually combined post- and precapillary PH (CpcPH).

In a new window | Download PPT
Figure 2: Treatment possibilities in pulmonary hypertension related to left heart disease. In pulmonary hypertension due to left heart disease (PH-LHD) the treatment possibilities are related to the cause of PH-LHD. Etiology dependent correction, heart failure treatment optimization and appropriate rhythm control are independent from the initial cause of PH-LHD. However further steps as mitral / tricuspid regurgitation correction (if appropriate and possible) and referral for left ventricle assist device (LVAD) implantation or orthotropic heart transplantation (OHT) should be individualized but are also related to the initial cause of PH-LH.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7525 | 10 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA