Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Stroke: Gut to be Wild
Time:2021-06-08
Number:6720
Alexa Moscatello1, Beverly Brooks1, Chase Kingsbury1, Blaise Cozene2, Justin Cho1, Zhen-Jie Wang1, Alma R. Lezama3, Felipe Esparza3, German Rivera Monroy3, Madeline Saft4, Alex Shear5, Henry Q. Zhang5, Nadia Sadandan6, Reed Berlet7, Bella Gonzales-Portillo8, Jea-Young Lee1, Cesario V. Borlongan1
Author Affiliations


- 1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA
- 2Tulane University, 6823 St. Charles Ave, New Orleans, LA 70118, USA.
- 3Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
- 4University of Michigan, 701 E. University Ave, Ann Arbor, MI 48109, USA.
- 5University of Florida, 205 Fletcher Drive, Gainesville, FL 32611, USA.
- 6Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
- 7Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064 USA.
- 8Northwestern University, 633 Clark St, Evanston, IL 60208, USA.
Conditioning Medicine 2021. 4(2): 119-123.
Abstract
Stroke manifests an aberrant inflammation associated with related cognitive impairments. While traditionally rampant in the brain, upregulated inflammation also arises in the periphery, in particular the gut, supporting the concept of the gut-brain axis as a key secondary cell death mechanism, as well as a therapeutic target to augment cognitive deficits. Gut dysbiosis reflected in inflammatory microbiomes may represent as biomarkers and therapeutic indices. Here, we discuss the gut-brain axis as a critical signaling pathway in our understanding of stroke pathology and treatment, with an overview of the potential of stem cell transplantation to treat both gut and brain dysfunction.
Keywords: Cerebral ischemia; Gut microbiome; Inflammation; Stem cells; Transplantation
Abstract
Stroke manifests an aberrant inflammation associated with related cognitive impairments. While traditionally rampant in the brain, upregulated inflammation also arises in the periphery, in particular the gut, supporting the concept of the gut-brain axis as a key secondary cell death mechanism, as well as a therapeutic target to augment cognitive deficits. Gut dysbiosis reflected in inflammatory microbiomes may represent as biomarkers and therapeutic indices. Here, we discuss the gut-brain axis as a critical signaling pathway in our understanding of stroke pathology and treatment, with an overview of the potential of stem cell transplantation to treat both gut and brain dysfunction.
Keywords: Cerebral ischemia; Gut microbiome; Inflammation; Stem cells; Transplantation
Stroke: A significant unmet clinical need
Stroke presents as the fifth leading cause of death in the United States. Minimal treatment options are at the disposal of acute and chronic stroke patients. Lack of oxygen and nutrients result in primary cell death, however inflammation-induced secondary cell death is responsible for debilitating neurological deficits prevalent in stroke patients. Targeting the inflammation associated with secondary cell death serves as a promising therapeutic mechanism to decrease neurologic deficits post-stroke.
Initially, the central nervous system (CNS) was believed to solely play an integral role in stroke progression, however accumulating evidence indicates a major role of the peripheral nervous system in stroke-associated secondary cell death. Furthermore, the abnormal increase in inflammation in the gut and brain post-stroke indicates that the gut-brain axis may mediate stroke progression (Venkat et al., 2018; Singh et al., 2018; Arumugam et al., 2017). Microorganisms and bacteria maintain normal gut functions as well as anti-inflammatory microbiomes. Peripheral microbiomes have been identified in the skin, oral cavity, vagina, and the gut (Sharma et al., 2018). Altered metabolism and dysfunction of the immune system often result in gut dysbiosis, an imbalance of microbiota populations, which may influence the brain during the onset and progression of stroke via the gut-brain axis.
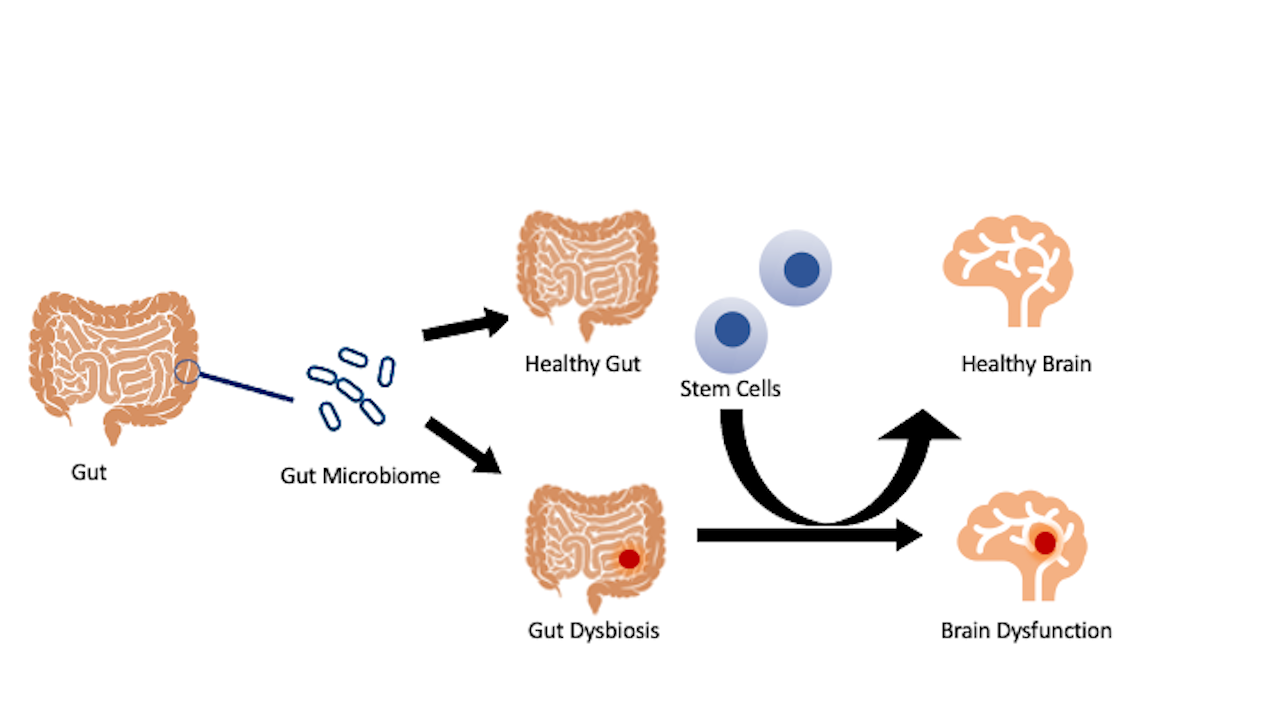
Although the enteric nervous system, or gastrointestinal (GI) tract, independently functions from the CNS, the GI tract can connect to the CNS through digestive activities that involve both parasympathetic and sympathetic controls. The CNS regulates functions of the GI via neural fibers that transmit sensory information. The gut-brain axis has been implicated in the pathology of stroke and other neurological disorders. Interrogating the processes involved in the homeostasis and dysfunctional gut-brain axis may uncover novel insights into the disease and treatment of the disease. Dysregulation in the gut microbiota, resulting from a stroke, may trigger a similar pathological brain microbiome. Conversely, transplanting stem cells to the gut may restore its healthy microbiome, thus protecting the brain from the damaging effects seen from a deviation in the gut microbiota (Figure 1). Investigations designed to unravel gut dysbiosis with a focus on inflammatory microbiota may serve as a fertile ground for deciphering innovative pathways of secondary cell death, as well as novel therapeutic targets for stroke.
In a new window | Download PPT
Figure 1: Stroke gut and brain pathology. Stroke manifests altered gut microbiota and inflammation that may extend to the brain and result in a dysregulated brain microbiome. Accordingly, treating the gut with stem cells may confer brain protection by transforming gut dysbiosis to one that fosters a healthy microbiome population that can consequently be seen in the brain.
Stroke-induced cognitive impairments and inflammation
Approximately 25-30% of patients develop cognitive impairment immediately or after a delay post-stroke (Kalaria et al., 2016). Both cognitive impairment and dementia occur about three months after a stroke episode. Advanced age, family history, genetic variants, vascular comorbidities and having had a previous or recurrent ischemic stroke are the main risk factors for developing dementia. Additional comorbidities such as hypertension, diabetes, obesity, and dyslipidemia may increase the risk of presenting this cognitive deficit after stroke, so it is essential to understand the mechanisms that accompany a stroke injury (Kalaria et al.,2016). Hippocampal injuries and white matter injuries caused by stroke and cerebral microbleeds contribute to the pathophysiology of cognitive decline after a stroke (Lo Coco et al., 2016; Sun et al., 2014).
Ischemic stroke-induced dementia is accompanied by neuroanatomic lesions. Infarctions in specific areas of the brain, such as the entorhinal cortex and the hippocampus, are key factors mediating cognitive impairment and are associated with the severity of dementia (Tomlinson et al., 1970). White matter lesions are common features of brain parenchymal damage from small cerebrovascular disease (Sun et al., 2014). Stroke survivors exhibit hyperintensity volumes of white matter coincident with the onset of dementia and damage to the blood-brain barrier (BBB) (Kalaria et al., 2016). Furthermore, small vessel disease closely accompanies the pathophysiology of cerebrovascular accidents, suggesting its contribution to cognitive impairment and functional loss, mainly in elderly patients (Lo Coco et al., 2016). Understanding the pathology of stroke, with an emphasis on cognitive behaviors, can help us develop new therapeutic approaches for patients with this pathology.
Multivariate lesion-mapping can be used on ischemic stroke patients in order to identify strategic regions of the brain for post-stroke cognitive impairment. There are specific neuronal structures, such as the left angular gyrus, left basal ganglia, and the white matter around the left basal ganglia that display pathological alterations associated with cognitive impairment (Zhao et al., 2018). With this information on brain organ-specific behavioral outcomes, comprehensive models are warranted to recapitulate post-stroke cognitive impairment towards a better understanding of the brain pathology and its treatment.
Mechanistic consequences of stroke-induced cognitive dysfunction may involve cellular damage to specific regions of the brain (Kalaria et al., 2016). Brain insult to the hippocampus causes stroke survivors to be at a higher risk for cognitive impairment (Allan et al., 2011; Kokmen et al., 1996). In tandem, neuroinflammation and immune system dysregulation can lead to cell death, which promotes further detrimental effects on the physiological structure and cognitive functions of the brain (McColl et al., 2007; Meisel and Meisel, 2011; Gold et al., 2011; Swardfager et al., 2013; Brown et al., 2020). Pathophysiological pathways leading to dementia may be influenced by these proinflammatory mechanisms (Kinney et al., 2018). Specifically, microglia and astrocytes exhibit a dampened cytokine response caused by cerebral atrophy in stroke-induced dementia patients (Chen et al., 2016; Li et al., 2020; Wen et al., 2020).
Primary stroke lesions induce secondary cell death via neuroinflammatory pathways and dysregulation of the immune response, leading to worsened cognitive deficits and physiological damage within the brain. A possible link exists between post-stroke neuroinflammation and the associated cognitive deficits with GI microbiota via bidirectional communication between the gut and the brain. Such gut-brain axis crosstalk manifests in germ-free mice colonized with microbiota acquired from stroke mice. After the recipient mice experienced cortical stroke injury, they displayed greater infarct volumes when compared to the nonrecipient mice. The recipient mice also expressed elevated levels of the inflammatory T-cells Th1 and Th17; these specific T-cells may be involved in the pathogenesis of stroke (Yamashiro et al., 2017). The elevated levels of Th1 and Th17 suggest not only a relationship between stroke-induced neuroinflammation and gut microbiota, but more specifically that altered microbiota may worsen post-stroke inflammation. This gut dysbiosis exacerbates physiological and cognitive dysfunction. In parallel, evaluation of the anti-inflammatory neuroprotective effects of administering antibiotics prior to stroke revealed a therapeutic link between neuroinflammation and microbiota modifications. The mice that received antibiotic treatment prior to stroke injury, demonstrated a 60% decreased infarct volume post-stroke, when compared to the control group (Benakis et al., 2016). Furthermore, the promotion of immune and neuroinflammation modulation was demonstrated by increased levels of regulatory T-cells and decreased levels of cells inhibiting effector T-cells interleukin-17+ γδ (Benakis et al., 2016). Approaches that restore GI microbiota to normal levels, while also enhancing anti-inflammatory cytokines, should be further explored due to the possible beneficial effects this would have in preventing cognitive dysfunction related to stroke and other neurological diseases.
Cell therapy-mediated sequestration of inflammation in gut and brain
Therapeutic techniques targeting the gut microbiome show major promise in their abilities to treat the damaging effects of stroke. Essential bacteria within the gut may assist in mitigating neuroinflammation, thus slowing neurodegeneration and the resulting psychological deficits fundamental in stroke patients. Microbiota-based treatments, such as prebiotics and probiotics, have demonstrated positive outcomes in decreasing modifications of the hippocampus and enhancing cognition in mouse models (Romo-Araiza et al., 2018; Abraham et al., 2019; Chen et al., 2017; Zhou et al., 2019; MahmoudianDehkordi et al., 2019). These therapeutic strategies hold great potential in their application to stroke models. Various probiotic regimens, such as Clostridium butyricum, Lactobacillus Plantarum ZDY2013, certain beneficial bacterial mixtures, and bacterial metabolites have all appeared to alleviate the symptoms of stroke-related disorders (Xie et al., 2016; Sun et al., 2016). Lactobacillus is a highly influential type of probiotic bacteria found within the host gut that may enhance cognitive function and mood, as well as to mitigate inflammation associated with aging (Pluta et al., 2021). After a brain infarction in monkeys, there was a relative decrease in the level of Lactobacillus, further suggesting a connection between these beneficial bacterial metabolites in the gut that appears to be associated with brain health (Pluta et al., 2021).
The overproduction of free radicals and pro-inflammatory cytokines plays a critical role in the advancement of ischemic-related damage post-stroke. Accordingly, the suppression of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), and free radicals may help to attenuate neuronal damage after ischemic injury. A significant reduction in TNF-α and malondialdehyde levels in the brains of stroke animals that received two weeks of probiotic treatment prior to middle cerebral artery occlusion (Akhoundzadeh et al., 2018). These findings demonstrate the antioxidant activity and pro-inflammatory cytokine suppression capabilities of probiotic treatment. For some cases involving acute stroke-caused deficiencies, the GI tract might necessitate a mass alteration or an entire repopulation of gut microbiota through fecal transplantation (Spychala et al., 2018).
Stem cell-based regeneration has recently become a popular target for stroke treatment. Stem cell transplantation has broadened the range of therapeutic stroke targets from central to peripheral organs (Otero-Ortega et al., 2018; Sun et al., 2018). Peripherally administered stem cells after a neurovascular insult tend to migrate heavily to the spleen (Crowley et al., 2017). This preferential migration of stem cells towards the spleen further advances the concept that stem cells are inflammation-homing biologics that may exert neuroprotection via sequestration of systemic inflammatory response (Crowley et al., 2017). Further research regarding cell-based regeneration may reveal optimal cell-based treatment for stroke and other neurological disorders (Otero-Ortega et al., 2018). Profiling of different microbiomes in the brain and gut may provide novel insights in the pathology of PD (Thomson-Luque et al., 2018; Cheemalapati et al., 2016). The intestinal tract of PD patients is decorated by pro-inflammatory microbiota that stimulate the pathology known as “leaky gut”, a condition characterized by inflammatory mediators and bacteria moving from the gut mucosa into the hosts blood (Castelli et al., 2020). Additionally, the GI tract of PD patients contains a dissimilar gut microbiota when compared to healthy individuals (Castelli et al., 2020). Our studies on PD demonstrate the crucial function of the microbiome as a biomarker and a potential therapeutic target (Lee et al., 2019; Lee et al., 2019). PD treatment and diagnosis primarily focus on the depletion of nigrostriatal dopamine in the brain, however, abnormal GI function precedes the onset of common symptoms seen in the brain of PD patients (Lee et al., 2019; Lee et al., 2019). These findings suggest that the gut microbiome could reveal further insights into PD pathology and its treatment. Our most recent studies revealed upregulation of three specific gut microbes: LAB158, BAC303, and EREC482 in PD animal models. These specific microbes play a pivotal role in gut-to-brain pro-inflammation in response to injury. Moreover, these same microbiota were downregulated after stem cell treatment (Lee et al., 2019; Lee et al., 2019). Based on these findings, gut inflammatory microbiota may serve as biomarkers for neurodegeneration, as well as potent therapeutic indices of cell in PD.
Conclusion
The gut microbiome plays a key role in stroke pathology. Targeting the gut and its inflammatory microbiome may serve as a robust stroke therapeutic. Cognizant that gut dysbiosis closely accompanies stroke provides the impetus to probe the gut and its microbiome in an effort to develop innovative concepts on stroke pathology and its treatment.
Acknowledgments
The authors thank the entire staff of Dr. Borlongan’s Neural Transplant Laboratory for critical discussions of this manuscript. C.V.B. is funded by National Institutes of Health (NIH) R01NS090962, NIH R01NS102395, and NIH R21NS109575.
Declaration of competing interests
The authors declare no competing interests.
References
Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M, Juhasz J, Ligeti B, Pongor S, Gomez-Cabrera MC, Vina J, Higuchi M, Suzuki K, Boldogh I, Radak Z (2019) Exercise and probiotics attenuate the development of Alzheimer's disease in transgenic mice: role of microbiome. Exp Gerontol 115:122-131.
Akhoundzadeh K, Vakili A, Shadnoush M, Sadeghzadeh J (2018) Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci 43:32-40.
Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, O'Brien JT, Kalaria RN (2011) Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain J Neurol 134:3716-3727.
Arumugam TV, Manzanero S, Furtado M, Biggins PJ, Hsieh Y, Gelderblom M, MacDonald KP, Salimova E, Li Y, Korn O, Dewar D, Macrae IM, Ashman RB, Tang S, Rosenthal NA, Ruitenberg MJ, Magnus T, Wells CA (2017) An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab 37:2098-2111.
Barbosa PM, Barbosa ER (2020) The gut brain-axis in neurological diseases. Int J Cardiovasc Sci 33:ePub.
Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nature medicine 22(5):516-23.
Brown J, Kingsbury C, Lee JY, Vandenbark AA, Meza-Romero R, Offner H, Borlongan CV (2020) Spleen participation in partial MHC class II construct neuroprotection in stroke. CNS Neurosci Ther 26:663–9.
Castelli V, d'Angelo M, Lombardi F, Alfonsetti M, Antonosante A, Catanesi M, Benedetti E, Palumbo P, Cifone MG, Giordano A, Desideri G, Cimini A (2020) Effects of the probiotic formulation SLAB51 in in vitro and in vivo parkinson's disease models. Aging (Albany NY) 12:4641-4659.
Cheemalapati SV, Winskas J, Wang H, Konnaiyan K, Zhdanov A, Roth A, Adapa SR, Deonarine A, Noble M, Das T, Gatenby R, Westerheide SD, Jiang RHY, Pyayt A (2016) Subcellular and in-vivo nano-endoscopy. Sci Rep 6:34400.
Chen A, Oakley AE, Monteiro M, Tuomela K, Allan LM, Mukaetova-Ladinska EB, O'Brien JT, Kalaria RN (2016) Multiplex analyte assays to characterise different dementias: brain inflammatory cytokines in post-stroke and other dementias. Neurobiol Aging 38:56-67.
Chen D, Yang X, Yang J, Lai G, Yong T, Tang X, Shuai O, Zhou G, Xie Y, Wu Q (2017) Prebiotic effect of fructooligosaccharides from morinda officinalis on Alzheimer's disease in rodent models by targeting the microbiota-gut-brain axis. Front Aging Neurosci 9:403.
Chi L, Du K, Liu D, Bo Y, Li W (2018) Electroacupuncture brain protection during ischemic stroke: A role for the parasympathetic nervous system. J Cereb Blood Flow Metab 38:479-491.
Crowley MG, Liska MG, Borlongan CV (2017) Stem cell therapy for sequestering neuroinflammation in traumatic brain injury: an update on exosome-targeting to the spleen. J Neurosurg Sci 61:291-302.
Gold AB, Herrmann N, Swardfager W, Black SE, Aviv RI, Tennen G, Kiss A, Lanctôt KL (2011) The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflammation 8:17.
Kalaria RN, Akinyemi R, Ihara M (2016) Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 1862(5):915-925.
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's & Dementia: Translational Research & Clinical Interventions 4(1):575–90.
Kokmen E, Whisnant JP, O'Fallon WM, Chu CP, Beard CM (1996) Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984). Neurology 46:154-159.
Lee JY, Tuazon JP, Corey S, Bonsack B, Acosta S, Ehrhart J, Sanberg PR, Borlongan CV (2019) A gutsy move for cell-based regenerative medicine in Parkinson’s disease: targeting the gut microbiome to sequester inflammation and neurotoxicity. Stem Cell Rev Rep 15:690–702.
Lee JY, Tuazon JP, Ehrhart J, Sanberg PR, Borlongan CV (2019) Gutting the brain of inflammation: a key role of gut microbiome in human umbilical cord blood plasma therapy in Parkinson's disease model. J Cell Mol Med 23:5466-5474.
Li LZ, Huang YY, Yang ZH, Zhang SJ, Han ZP, Luo YM (2020) Potential microglia-based interventions for stroke. CNS Neurosci Ther 26(3):288–96.
Lo Coco D, Lopez G, Corrao S (2016) Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag 12:105–16.
MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, Louie G, Kueider-Paisley A, Moseley MA, Thompson JW, Williams LS (2019) Altered bile acid profile associates with cognitive impairment in Alzheimer's disease—an emerging role for gut microbiome. Alzheimer's & Dementia 15:76-92.
McColl BW, Allan SM, Rothwell NJ (2007) Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans 35:1163-1165.
Meisel C, Meisel A (2011) Suppressing immunosuppression after stroke. N Engl J Med 365:2134-2136.
Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vazquez J, Diez-Tejedor E, Gutierrez-Fernandez M (2018) Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab 38:767-779.
Pluta R, Januszewski S, Czuczwar SJ (2021) The role of gut microbiota in an ischemic stroke. Int J Mol Sci 22:915.
Roberts RC, Farmer CB, Walker CK (2018) The human brain microbiome; there are bacteria in our brains! Society of Neuroscience 2018 Program No. 594:08.
Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H, García-Contreras V, Fernández-Presas AM, Jasso-Chávez R, Borlongan CV, Ibarra A (2018) Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci 10:416.
Salehi MS, Pandamooz S, Safari A, Jurek B, Tamadon A, Namavar MR, Dianatpour M, Dargahi L, Azarpira N, Fattahi S, Moosavi SMS, Keshavarz S, Khodabandeh Z, Zare S, Nazari S, Heidari M, Izadi S, Poursadeghfard M, Borhani-Haghighi A (2020) Epidermal neural crest stem cell transplantation as a promising therapeutic strategy for ischemic stroke. CNS Neurosci Ther 26:670-681.
Sharma N, Bhatia S, Sodhi AS, Batra N (2018) Oral microbiome and health. AIMS Microbiol 4:42-66.
Singh V, Sadler R, Heindl S, Llovera G, Roth S, Benakis C, Liesz A (2018) The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab 38:1293-1298.
Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD (2018) Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 84:23-26.
Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J (2016) Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res 1642:180-188.
Sun JH, Tan L, Yu JT (2014) Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med 2(8):80.
Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ (2018) MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab 38:1125-1148.
Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ (2018) MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab 38:1125–1148.
Sun Z, Gu L, Wu K, Wang K, Ru J, Yang S, Wang Z, Zhuge Q, Huang L, Huang S (2020) VX-765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke-induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neurosci Ther 26:952-961.
Swardfager W, Winer DA, Herrmann N, Winer S, Lanctot KL (2013) Interleukin-17 in post-stroke neurodegeneration. Neurosci Biobehav 37:436–447.
Thomson-Luque R, Wang C, Ntumngia FB, Xu S, Szekeres K, Conway A, Adapa SR, Barnes SJ, Adams JH, Jiang RHY (2018) In-depth phenotypic characterization of reticulocyte maturation using mass cytometry. Blood Cells Mol Dis 72:22.
Thomson-Luque R, Wang C, Ntumngia FB, Xu S, Szekeres K, Conway A, Adapa SR, Barnes SJ, Adams JH, Jiang RHY (2018) In-depth phenotypic characterization of reticulocyte maturation using mass cytometry. Blood Cells Mol Dis 72:22-33.
Tomlinson BE, Blessed G, Roth M (1970) Observations on the brains of demented old people. J Neurol Sci 11(3):205–42.
Venkat P, Chen J, Chopp M (2018) Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab 38:2165-2178.
Wen RX, Shen H, Huang SX, Wang LP, Li ZW, Peng P, Mamtilahun M, Tang YH, Shen FX, Tian HL, Yang GY (2020) P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther 26(4):416–29.
Xie Q, Pan M, Huang R, Tian X, Tao X, Shah NP, Wei H, Wan C (2016) Short communication: modulation of the small intestinal microbial community composition over short-term or long-term administration with Lactobacillus plantarum ZDY2013. J Dairy Sci 99(9):6913-6921.
Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N (2017) Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One 12(2):e0171521. doi:10.1371/journal.pone.0171521
Zhao L, Biesbroek JM, Shi L, Liu W, Kuijf HJ, Chu WW, Abrigo JM, Lee RK, Leung TW, Lau AY, Biessels GJ, Mok V, Wong A (2018) Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab 38(8):1299–311.
Zhou H, Tai J, Xu H, Lu X, Meng D (2019) Xanthoceraside could ameliorate Alzheimer's disease symptoms of rats by affecting the gut microbiota composition and modulating the endogenous metabolite levels. Front Pharmacol 10:1035.
References
Alexa Moscatello1*
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Beverly Brooks1*
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Chase Kingsbury1*
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Blaise Cozene2*
2Tulane University, 6823 St. Charles Ave, New Orleans, LA 70118, USA.
Justin Cho1
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Zhen-Jie Wang1
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Alma R. Lezama3
3Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
Felipe Esparza3
3Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
German Rivera Monroy3
3Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
Madeline Saft4
4University of Michigan, 701 E. University Ave, Ann Arbor, MI 48109, USA.
Alex Shear5
5University of Florida, 205 Fletcher Drive, Gainesville, FL 32611, USA.
Henry Q. Zhang5
5University of Florida, 205 Fletcher Drive, Gainesville, FL 32611, USA.
Nadia Sadandan6
6Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
Reed Berlet7
7Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064 USA.
Bella Gonzales-Portillo8
8Northwestern University, 633 Clark St, Evanston, IL 60208, USA.
Jea-Young Lee1
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Cesario V. Borlongan1#
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Corresponding author:
Prof. Cesar V. Borlongan
Email: cborlong@usf.edu)

In a new window | Download PPT
Figure 1: Stroke gut and brain pathology. Stroke manifests altered gut microbiota and inflammation that may extend to the brain and result in a dysregulated brain microbiome. Accordingly, treating the gut with stem cells may confer brain protection by transforming gut dysbiosis to one that fosters a healthy microbiome population that can consequently be seen in the brain.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6720 | 33 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA