Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Cell therapy and conditioning medicine for stroke
Time:2021-06-08
Number:6199
Chase Kingsbury1, Blaise Cozene2, Justin Cho1, Zhen-Jie Wang1, Alma R. Lezama3, Felipe Esparza3, German Rivera Monroy3, Madeline Saft4, Alex Shear5, Nadia Sadanandan6, Reed Berlet7, Jea-Young Lee1, Cesario V. Borlongan1
Author Affiliations


- 1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
- 2Tulane University, 6823 St. Charles Ave, New Orleans, LA 70118, USA.
- 3Centro de Investigación en Ciencias de la Salud (CICSA); FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
- 4University of Michigan, 701 E. University Ave, Ann Arbor, MI 48109, USA.
- 5University of Florida, 205 Fletcher Drive, Gainesville, FL 32611, USA.
- 6Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
- 7Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064 USA.
Conditioning Medicine 2021. 4(2): 113-118.
Abstract
Transplanted stem cells exhibit modest engraftment in the stroke brain. Despite this restricted graft survival, transplanted stroke animals display functional recovery owing in part to stem cells’ secretion of therapeutic molecules. Clinical trials of cell therapy in stroke patients commenced in 1998 generating a solid safety profile but efficacy remains elusive. Indeed, two recent stroke clinical trials while demonstrating safety, failed to produce significant clinical functional recovery. Here, we discuss the potential of conditioning stem cells to prepare them for the harsh microenvironment of the ischemic brain. In particular, we present hypoxic conditioning or oxygen deprivation as a conditioning strategy for generating a hi-breed of stem cells with enhanced survival and function in the stroke brain. We advance here the concept of combining cell transplantation and conditioning medicine towards developing an improved stem cell-based stroke therapeutic.
Keywords: Stroke, Stem cell therapy, Regenerative medicine, Hypoxia, Microenvironment
Abstract
Transplanted stem cells exhibit modest engraftment in the stroke brain. Despite this restricted graft survival, transplanted stroke animals display functional recovery owing in part to stem cells’ secretion of therapeutic molecules. Clinical trials of cell therapy in stroke patients commenced in 1998 generating a solid safety profile but efficacy remains elusive. Indeed, two recent stroke clinical trials while demonstrating safety, failed to produce significant clinical functional recovery. Here, we discuss the potential of conditioning stem cells to prepare them for the harsh microenvironment of the ischemic brain. In particular, we present hypoxic conditioning or oxygen deprivation as a conditioning strategy for generating a hi-breed of stem cells with enhanced survival and function in the stroke brain. We advance here the concept of combining cell transplantation and conditioning medicine towards developing an improved stem cell-based stroke therapeutic.
Keywords: Stroke, Stem cell therapy, Regenerative medicine, Hypoxia, Microenvironment
Cell transplantation and stroke
Pioneering investigations of stroke cell therapy initiated in the late 1980s show the survival of mouse fetal neocortical grafts in the ischemic cortex of aged rats (Mampalam et al., 1988; Sharp, 1993). These grafted fetal cells integrated with ischemic tissues by receiving afferent fibers as well as the vascularization of the healthy tissue of the host, thus aiding the brain in responding to sensory stimulation and increasing its metabolic activity Grabowski et al., 1992a; 1992b; 1993). Equally a milestone discovery, stroke animals transplanted with fetal striatal cells displayed improvements in cognitive tasks (Nishino et al., 1993; Aihara et al., 1994).
Over the last forty years, preclinical and clinical evidence shows survival, migration, differentiation, and functional integration of transplanted cells in the ischemic brain. Such graft persistence coincides with brain circuitry remodeling, anatomical reconstruction, and neurochemical, physiological, and behavioral recovery has been demonstrated (Sharp, 1993; Borlongan et al., 1997). Therapeutic effects of cell transplants in stoke may entail transplanted cells replacing ischemic or dead cells. Compelling evidence also demonstrates that therapeutic substances secreted by the grafted cells may promote angiogenesis, vasculogenesis, neurogenesis, and anti-inflammation, among other regenerative processes (Bliss et al., 2007; Liu et al., 2014; Zhang et al., 2016; Stonesifer et al., 2017). Altogether, these brain repair processes served as the basis for the introduction of cell transplantation therapy for stroke in the clinic. Allogeneic transplantation of fetal cells paved the way for the initial clinical trials of cell therapy in Parkinson’s disease but these cells encountered logistical and ethical challenges. Circumventing these lab-to-clinical hurdles led to the development of human teratocarcinoma cells modified to differentiate into postmitotic neurons, eventually transplanted into stroke patients (Borlongan et al., 1998; Kondziolka et al., 2000). Subsequently, along the lines of finding a non-fetal and non-cancerous transplantable cells, embryonic stem cells, induced pluripotent stem cells, and cells derived from adult tissues, including those from bone marrow, umbilical cord and adipose tissue, represent viable donor cells for transplantation therapy in stroke (Sun and Kurtzberg, 2015; Napoli and Borlongan, 2017; Bateman et al., 2018).
Focusing on adult tissue-derived stem cells
Bone marrow has become the preferred adult tissue stem cell source due to its solid safety profile (Tang et al., 2007; Borlongan 2016a; 2016b; Napoli and Borlongan, 2016; 2017; Napoli et al., 2018). Mesenchymal stem or stromal cells (MSCs), mononuclear cells (MNCs), endothelial progenitor cells (EPCs), SB623, multipotent adult progenitor cells (MAPCs), and multilineage-differentiating stress-enduring cells (MUSE), are some characterized cell populations derived from the bone marrow and engineered stem/progenitor cells (Yasuhara et al., 2008; 2009; Gnecchi and Melo, 2009; Li et al., 2015; Uchida et al., 2015). These bone marrow-derived stem cells display multipotent cell capacities in preclinical models of stroke (Joyce et al., 2010; Borlongan 2011; Borlongan et al., 2011; Kocsis and Honmou, 2012; Eckert et al., 2013; Rowart et al., 2015). Furthermore, after transplantation, grafted stem cells attenuate histological and behavioral deficits in vivo (van Velthoven et al., 2014). Preclinical data demonstrate bone marrow-derived stem cells’ potential as a powerful therapeutic, paving the way for clinical trials of cell therapy. A study intravenously administered autologous bone marrow MSCs to patients 4-weeks post-stroke resulting in improved neurologic outcomes that were initially observed alongside no adverse effects, but these benefits diminished by 12-months post-transplant (Bang et al., 2005). An open-labeled trial administered autologous bone marrow MNCs intravenously 24-72 hours after stroke onset also showed safety as well as improved functional recovery that lasted six months after transplantation (Savitz et al., 2011). A phase II, multicenter, parallel group, randomized, and blinded trial intravenously transplanted autologous bone marrow MNCs at an average of 18.5 days post-stroke. Compared to the previous study, data indicated that this treatment was safe, however no functional improvements were observed (Prasad et al., 2014). Furthermore, an open-labeled trial administered a subset of cluster of differentiation (CD)34+ bone marrow MNCs intra arterially in a 7-day window post stroke. This also demonstrated safe application as well as increased functional improvements throughout a six-month period (Banerjee et al., 2014). More recent clinical trials have reported consistent safety among cell transplantation, however mixed outcomes in terms of functional recovery. Data indicate a lack of efficacy in intravenous transplantation of MAPCs in acute stroke patients (Hess et al., 2017) and in chronic stroke patients after intracerebral administration of SB623 (Steinberg et al., 2016a; 2019b; SanBio).
Based on the previously discussed interim clinical trials, transplantation of bone marrow stem cell derivatives, especially MSCs and MNCs, in stroke is safe. However, the efficacy of the treatment remains elusive. Conclusive interpretations drawn from clinical data are hindered by a small number of patients enrolled in the trials in addition to the open-labeled approach. Vis-à-vis comparisons between hindered trials are difficult due to the difference in donor cells and varied clinical transplant protocol, such as cell dose, timing, and delivery routes. MSC phenotypic markers include SH-2 and SH-4 (Bang et al., 2005) specific flow cytometric antibodies comprise of CD3, CD14, CD16, CD19, CD20, CD34, CD45, CD56, Lin 1, and CD133-2 (Savitz et al., 2011) although some studies are limited to CD34 and CD45 (Prasad et al., 2014) or focus on CD34+ cells in magnetic cell isolation procedures (Banerjee et al., 2014). MAPCs are defined as c-Kit+, CD0+, CD13+, CD31+, CD44-, MHC-I--, Thy1- (Sohni and Verfaillie 2011; Hess et al., 2017), while SB623 are notched-induced MSCs (Steinberg et al., 2016; 2019; Dezawa et al., 2004; Tajiri et al., 2013; 2014). In addition to various administration routes across the trials (Bang et al., 2005; Savitz et al., 2011; Banerjee et al., 2014; Prasad et al., 2014; Steinberg et al., 2016; 2019; Hess et al., 2017), therapeutic windows span from acute to chronic stroke stages.
Safe, but not effective in clinical trials
A primary reason for the lack of success of clinical trials to achieve efficacy standards is the inconsistency in the laboratory and clinical stem cell transplant protocols. The efficacy seen in the laboratory, which follows specific dosing and timing of transplants, is not strictly adhered in the clinic. In preclinical studies, an effective dose administered intravenously is approximately 4 million cells in stroke rats weighing 250 g and 840 million cells in a stroke patient weighing 75 kg (Diamandis and Borlongan, 2015). However, most clinical studies use cell counts far below the demonstrated effective dose (Bang et al., 2005; Banerjee et al., 2014; Prasad et al., 2014). Notably, clinical trials that gave stroke patients the preclinical cell dose showed improvements. In intracerebral transplants, the effective dose in stroke rats is 200,000 cells, which equates to about 56 million cells in stroke patients, yet clinical doses (2.5 million and 5 million) administered were far below the recommended dose, potentially explaining the lack of efficacy (Steinberg et al., 2016; 2019). The efficacious cell dose (400 million to 1200 million cells) indicated in stroke rats was maintained in the MAPC trial (Hess et al., 2017), but efficacy was not attained (Kenmuir and Wechsler, 2017). Posthoc analysis uncovered that patients who received the MAPC transplant within 36 hours displayed functional improvements (Athersys 2015), which resembled the preclinical study (Mays et al., 2010) and are now being pursued in another MAPC trial (Doeppner and Hermann, 2010). Clinical trials should strictly follow previous preclinical guidelines for optimal dosing, timing, and route to achieve maximum efficacy.
Strategies to enhance stem cell survival and function in stroke
The clinical trial design of stem cell therapy has highlighted the logistics of the transplant treatment, yet it may have disregarded basic discoveries about the properties of stem cells. The standard thought process when visualizing a stem cell clinical product always involves a clear set of respective phenotypic markers plus a firm grasp of the functioning of the stem cells. Acquiring clinical grade stem cells should prevent conventional product release rules of either homogeneous populations of cells or a reliable generation of the same stem cell population. Additionally, the clinical transplant regimen should expand upon the postulated methods from the laboratory, involving growth factor secretion, cell replacement, and promotion of endogenous brain repair processes (Duffy et al., 2009; Ning et al., 2011; Ishikawa et al., 2013; Anderson et al., 2016; US National Library of Medicine, 2019), as these methods can simultaneously function to contest cell death pathways seen in stroke (Sozmen et al., 2012; Puyal et al., 2013; Schweizer et al., 2013). Overall, stem cell therapy can enhance its therapeutic potential when it does not act independently but rather combined with tissue plasminogen activator (tPA), other neuroprotective drugs, biomaterials (Incontri et al., 2018), or thrombectomy (see below), along with standard stroke procedures for rehabilitation (Polgar et al., 1997; Borlongan et al., 2015).
Additionally, it is important to analyze both the safety and efficacy of the stem cells in preclinical stroke models. This includes the analysis of their tissue formation capacity, persistence, and cell fate after transplantation. When a stem cell differentiates, it may induce a damaging immunomodulatory or inflammatory response that is toxic or cause tumorigenesis. Thus, extra precautions should be taken when using genetically engineered stem cells such as SB623 and immortalized cells such as CTX03 (Borlongan, 2016; Napoli and Borlongan 2017). Additional safety precautions are also warranted when using cryopreserved cells or extracellular matrix-loaded cells. The use of delivery devices including nanoparticles, exosomes, extracellular vesicles, microRNAs, mitochondria, and other cellular components also requires further safety deliberations (Chen et al., 2017; Lamanna et al., 2017; Labriola et al., 2018; Liebeskind et al., 2018; Nguyen et al., 2018; Venkat et al., 2018). Further advancements have paved the way for the use of cell-derivative and cell-free compositions over cell-directed transplantation for stroke treatment that requires successful safety outcome evaluations.
Conditioning medicine as a novel approach to improve cell therapy outcomes
Conditioning medicine is defined as a strategy designed to increase the tolerance level of an organ against injury (Yang et al., 2016). In the case of stroke, brain exposure to a conditioning paradigm designed to enhance ischemic tolerance results in brain protection against a subsequent stroke. There are several forms of conditioning, including physical (exercise), pharmacologic (drugs), biologics (stem cells), and a sublethal form of the injury itself. The timing of conditioning can be initiated prior to (pre), during (per), or after (post) the injury. The target site of conditioning can be direct to the organ or remotely. Exposure of cultured stem cells to a hypoxic environment or oxygen deprivation prior to transplantation represents a robust strategy in conditioning the cells to the non-conductive tissue environment of the stroke brain (Figure 1). Hypoxic preconditioning increases cell viability and paracrine activity, specifically vascular endothelial growth factor-A, stromal cell-derived factor-A, interleukins, and tumor necrosis factor (Li et al., 2010; Andreeva et al., 2015; Bader et al., 2015; Veighey, 2020; Zhao et al., 2020). A similar stroke-like stressful event upregulates trophic factor secretion in stem cells (Hou et al., 2017; Bachmann et al., 2020). Moreover, hypoxia preconditioning increases the survival of transplanted MSCs while upregulating the therapeutic paracrine effects of injured cells (Huang et al., 2020). In parallel, hypoxic preconditioning of MSC-derived conditioned medium drives microglial cells to polarize into an anti-inflammatory phenotype thereby protecting against stroke (Zhang et al., 2019; Yu et al., 2021). The participation of exosome in such preconditioning has been suggested (Deng et al, 2018). Altogether, these results suggest that hypoxia preconditioning improves stem cell therapy in ischemic stroke.
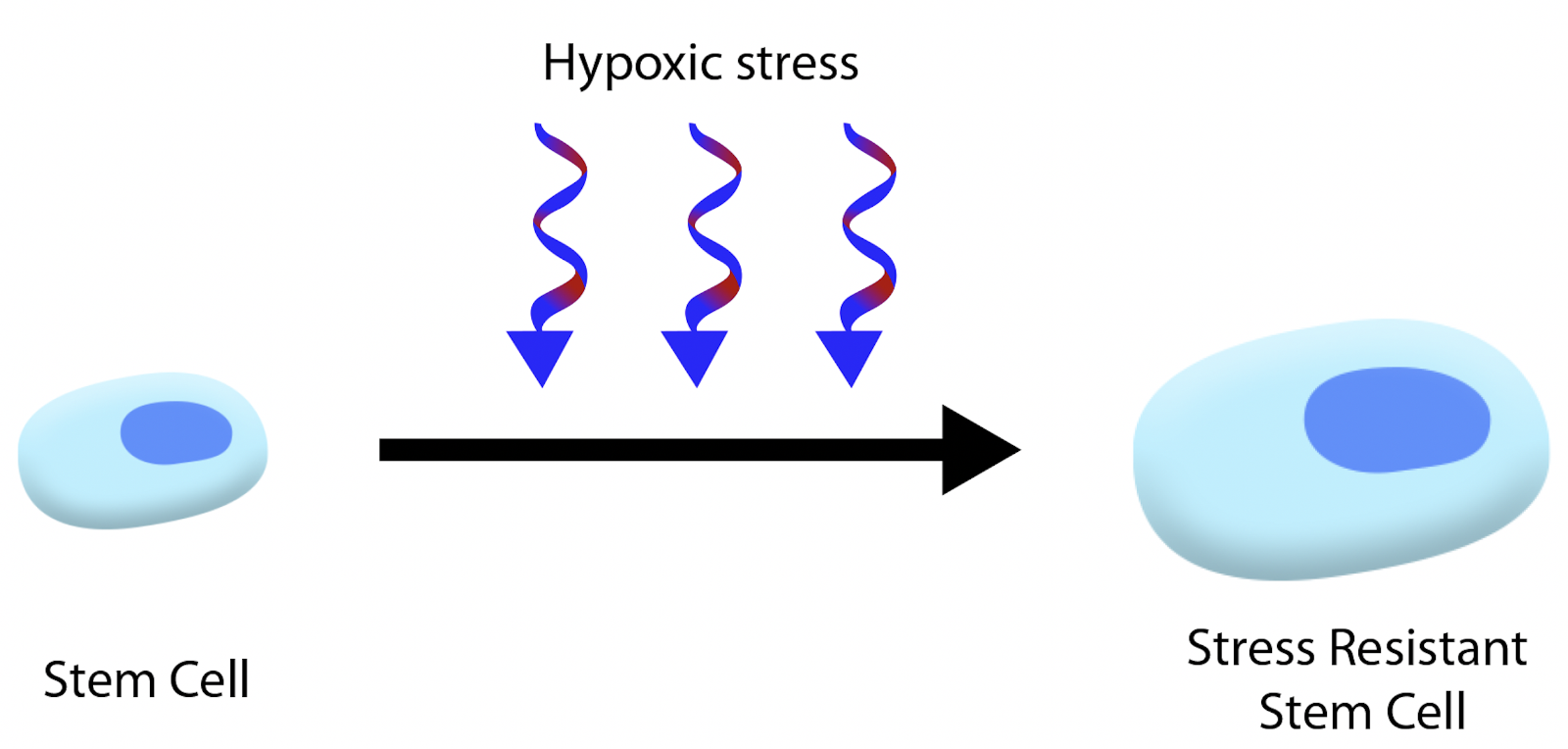
In a new window | Download PPT
Figure 1: Conditioning of stem cells. Exposing cultured stem cells to hypoxic stress and oxygen deprivation prior to transplantation enhances ischemic tolerance. These conditioned stem cells may be more tolerant to the harsh environment of the ischemic brain, allowing them to survive and function better than non-conditioned stem cells.
Conclusion
Stem cell transplantation for neurological disorders is safe, but its efficacy remains in question. Several types of stem cells have been explored in the laboratory, and a few of these cells have advanced to clinical trials, with varying modest results. Strategies designed to improve the clinical outcomes of stem cell transplantation have entailed strict adherence to stem cell protocols outlined in preclinical studies to achieve consistent results. Adjunctive application with other available treatments, such as neuroprotective drugs, biomaterials, tPA, or mechanical thrombectomy, may enhance the therapeutic effects of stem cells. With this in mind, exposure of stem cells to a hypoxic environment or oxygen deprivation may improve transplanted stem cell survival and function in the stroke brain. Combination of cell transplantation and conditioning medicine represent a new field that will likely benefit the field of regenerative medicine for stroke and other related neurological disorders.
References
Aihara N, Mizukawa K, Koide K, Mabe H, Nishino H (1994) Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABAA receptor and improve water-maze learning in the rat. Br Res Bull 33:483-8.
Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. (2016) Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells 34:601-13.
Andreeva ER, Lobanova MV, Udartseva OO, Buravkova LB (2015) Response of adipose tissue-derived stromal cells in tissue-related O2 microenvironment to short-term hypoxic stress. Cells Tissues Organs. 200:307–15.
Athersys (2015) The SAGE Encyclopedia of Stem Cell Research: SAGE Publications, Inc.
Bachmann J, Ehlert E, Becker M, Otto C, Radeloff K, Blunk T, et al (2020) Ischemia-like stress conditions stimulate trophic activities of adipose-derived stromal/stem cells. Cells 9:1935.
Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, et al (2015) Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS One 10(9):e0138477.
Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A, et al. (2014) Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 3:1322-30.
Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874-82.
Bateman ME, Strong AL, Gimble JM, Bunnell BA (2018) Concise review: Using fat to fight disease: a systematic review of nonhomologous adipose-derived stromal/stem cell therapies. Stem Cells 36:1311-28.
Bliss T, Guzman R, Daadi M, Steinberg GK (2007) Cell transplantation therapy for stroke. Stroke 38:817-26.
Borlongan CV (2011) Bone marrow stem cell mobilization in stroke: a 'bonehead' may be good after all! Leukemia 25:1674-86.
Borlongan CV (2016a) Age of PISCES: stem-cell clinical trials in stroke. The Lancet 388:736-8.
Borlongan CV (2016b) Preliminary reports of stereotaxic stem cell transplants in chronic stroke patients. Mol Ther 24:1710-1.
Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB (2011) The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 95:213-28.
Borlongan CV, Koutouzis TK, Jorden JR, Martinez R, Rodriguez AI, Poulos SG, et al. (1997) Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci Biobehav Rev 21:79-90.
Borlongan CV, Jolkkonen J, Detante O (2015) The future of stem cell therapy for stroke rehabilitation. Future Neurol. 10:313-9.
Borlongan CV, Nguyen H, Lippert T, Russo E, Tuazon J, Xu K, et al. (2019) May the force be with you: Transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab 39:367-70
Borlongan CV, Tajima Y, Trojanowski JQ, Lee VMY, Sanberg PR (1998) Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N Cells) promotes functional recovery in ischemic rats. Exp Neuro 149:310-21.
Chen B, Li Q, Zhao B, Wang Y (2017) Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Transl Med. 6:1753-8.
Deng M, Xiao H, Peng H, Yuan H, Xu Y, Zhang G, et al. (2018) Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions. Eur J Neurosci 47:150-7.
Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. (2004) Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 113:1701-10.
Diamandis T, Borlongan CV (2015) One, two, three steps toward cell therapy for stroke. Stroke. 46:588-91.
Doeppner TR, Hermann DM (2010) Mesenchymal stem cells in the treatment of ischemic stroke: progress and possibilities. Stem Cells Cloning 3:157-63.
Duffy GP, Ahsan T, O'Brien T, Barry F, Nerem RM (2009) Bone marrow–derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 15:2459-70.
Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC, et al. (2013) Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab 33:1322-34.
Gnecchi M, Melo LG (2009) Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods in Molecular Biology: Humana Press p. 281-94.
Grabowski M, Brundin P, Johansson BB (1992) Fetal neocortical grafts implanted in adult hypertensive rats with cortical infarcts following a middle cerebral artery occlusion: Ingrowth of afferent fibers from the host brain. Exp Neurol. 116(2):105-21.
Grabowski M, Christofferson RH, Brundin P, Johansson B (1992) Vascularization of fetal neocortical grafts implanted in brain infarcts in spontaneously hypertensive rats. Neuroscience 51:673-82.
Grabowski M, Brundin P, Johansson B (1993) Functional integration of cortical grafts placed in brain infarcts of rats. Ann Neurol 34(3):362-8.
Nishino H, Aihara N, Czurko A, Hashitani T, Isobe Y, Ichikawa O, Watari H (1993) Reconstruction of GABAergic transmission and behavior by striatal cell grafts in rats with ischemic infarcts in the middle cerebral artery. J Neural Transplant Plast 4:147-55.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, Sen S, Sila CA, Vest JD, Mays RW (2017) Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 16:360-8.
Hou J, Zhong T, Guo T, Miao C, Zhou C, Long H, et al (2017) Apelin promotes mesenchymal stem cells survival and vascularization under hypoxic-ischemic condition in vitro involving the upregulation of vascular endothelial growth factor. Exp Mol Pathol 102:203–9.
Huang Y, Liu Z, Tan F, Hu Z, Lu M (2020) Effects of the insulted neuronal cells-derived extracellular vesicles on the survival of umbilical cord-derived mesenchymal stem cells following cerebral ischemia/reperfusion injury. Oxid Med Cell Longev :9768713.
Incontri Abraham D, Gonzales M, Ibarra A, Borlongan CV (2018) Stand alone or join forces? Stem cell therapy for stroke. Expert Opin Biol Ther 19:25-33.
Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, Mimura O, Dezawa M, Kim SU, Borlongan CV (2013) Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke 44:3473-81.
Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 5:933-46.
Kenmuir CL, Wechsler LR (2017) Update on cell therapy for stroke. Stroke Vasc Neurol. 2:59-64.
Kocsis JD, Honmou O (2012) Bone marrow stem cells in experimental stroke. Functional Neural Transplantation III - Primary and Stem Cell Therapies for Brain Repair, Part II: Elsevier; p. 79-98.
Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, et al. (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55:565-9.
Labriola NR, Azagury A, Gutierrez R, Mathiowitz E, Darling EM (2018) Concise review: Fabrication, customization, and application of cell mimicking microparticles in stem cell science. Stem Cells Transl Med 7(2):232-40.
Lamanna JJ, Gutierrez J, Urquia LN, Hurtig CV, Amador E, Grin N, Svendsen CN, Federici T, Oshinski JN, Boulis NM (2017) Ferumoxytol labeling of human neural progenitor cells for diagnostic cellular tracking in the porcine spinal cord with magnetic resonance imaging. Stem Cells Transl Med 6:139-50.
Li Y-F, Ren L-N, Guo G, Cannella LA, Chernaya V, Samuel S, Liu SX, Wang H, Yang XF (2015) Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy. J Hematol Oncol. 8:33.
Li Z, Wei H, Deng L, Cong X, Chen X (2010) Expression and secretion of interleukin-1β, tumour necrosis factor-α and interleukin-10 by hypoxia- and serum-deprivation-stimulated mesenchymal stem cells: Paracrine anti-fibrotic effects of MSCs in vitro. FEBS J 277:3688–98.
Liebeskind DS, Derdeyn CP, Wechsler LR Stair X Consortium (2018) Emerging considerations in developing and evaluating new stroke therapies. Stroke 49:2241-2247.
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J (2014) Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol 115:92-115.
Mampalam TJ, Gonzalez MF, Weinstein P, Sharp FR (1988) Neuronal changes in fetal cortex transplanted to ischemic adult rat cortex. J Neurosurg 69:904-12.
Mays RW, Borlongan CV, Yasuhara T, Hara K, Maki M, Carroll JE, Deans RJ, Hess DC (2010) Development of an allogeneic adherent stem cell therapy for treatment of ischemic stroke. J Exp Stroke Transl Med 3:34-46.
Napoli E, Borlongan CV (2016) Recent advances in stem cell-based therapeutics for stroke. Transl Stroke Res 7:452-7.
Napoli E, Borlongan CV (2017) Stem cell recipes of bone marrow and fish: just what the stroke doctors ordered. Stem Cell Reviews and Reports. 13:192-7.
Napoli E, Lippert T, Borlongan CV (2018) Stem cell therapy: repurposing cell-based regenerative medicine beyond cell replacement. Advances in Experimental Medicine and Biology: Springer International Publishing. p. 87-91.
Nguyen H, Zarriello S, Rajani M, Tuazon J, Napoli E, Borlongan CV (2018) Understanding the role of dysfunctional and healthy mitochondria in stroke pathology and its treatment. Int J Mol Sci 19:2127.
Ning R, Xiong Y, Mahmood A, Zhang Y, Meng Y, Qu C, Chopp M (2011) Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Br Res 1384:140-50.
Polgar S, Borlongan CV, Koutouzis TK, Todd SL, Cahill DW, Sanberg PR (1997) Implications of neurological rehabilitation for advancing intracerebral transplantation. Br Res Bull 44:229-32.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S, InveST Study Group (2014) Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. 45:3618-24.
Puyal J, Ginet V, Clarke PGH (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog Neurobiol 105:24-48.
Rowart P, Erpicum P, Detry O, Weekers L, Grégoire C, Lechanteur C, Briquer A, Beguin Y, Krzesinski JM, Jouret F (2015) Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J Immunol Res :602597.
SanBio Co. Ltd. SanBio and Sumitomo Dainippon Pharma Announce Topline Results from a Phase 2b Study in the U.S. Evaluating SB623, a Regenerative Cell Medicine for the Treatment of Patients with Chronic Stroke.
Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC (2011) Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol 70:59-69.
Schweizer S, Meisel A, Märschenz S (2013) Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab 33:1335-46.
Sharp FR (1993) Transplants for stroke patients? Ann Neurol 34:322-3.
Sohni A, Verfaillie CM (2011) Multipotent adult progenitor cells. Best Practice & Research Clinical Haematology. 24:3-11.
Sozmen EG, Hinman JD, Carmichael ST (2012) Models that matter: white matter stroke models. Neurotherapeutics. 9:349-58.
Steinberg GK, Kondziolka D, Bates D, SB623 Stroke Phase 1/2A Study Group (2016) Response by Steinberg et al to Letter Regarding Article, “Clinical Outcomes of Transplanted Modified Bone Marrow–Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2A Study”. Stroke. 47(12):e269.
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN, Bates D, Poggio G, Case C, McGrogan M, Yankee EW, Schwartz NE (2018) Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow–derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg 131:1462-72.
Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV (2017) Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 158:94-131.
Sun JM, Kurtzberg J (2015) Cord blood for brain injury. Cytotherapy. 17:775-85.
Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, Hernadez-Ontiveros DG, Shinozuka K, Ishikawa H, Kaneko Y, Yankee E, McGrogan M, Case C, Borlongan CV (2014) Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci 8:116.
Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, Case C, Borlongan CV (2013) Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 8(9):e74857-e.
Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, Xu L, Hess DC, Borlongan CV (2007) Transplantation of bone marrow-derived stem cells: A promising therapy for stroke. Cell Transplant 16:159-69.
Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, Sakata H, Matsuzaka Y, Mushiake H, Tominaga T, Borlongan CV, Dezawa M (2015) Transplantation of unique subpopulation of fibroblasts, muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 34:160-73.
U.S. National Library of Medicine. MultiStem® Administration for Stroke Treatment and Enhanced Recovery Study (MASTERS-2). Available at https://clinicaltrials.gov/ct2/show/NCT03545607. Accessed March 11, 2019.
van Velthoven CTJ, Gonzalez F, Vexler ZS, Ferriero DM (2014) Stem cells for neonatal stroke- the future is here. Front Cell Neurosci 8:207.
Veighey K (2020) Ischemic conditioning in organ transplantation Cond Med 1:212-219.
Venkat P, Chopp M, Chen J (2018) Cell-based and exosome therapy in diabetic stroke. Stem Cells Transl Med 7:451-5.
Yang B, Fung A, Pac-Soo C, Ma D (2016) Vascular surgery-related organ injury and protective strategies: update and future prospects. Br J Anaesth 117:ii32–43.
Yasuhara T, Hara K, Maki M, Mays RW, Deans RJ, Hess DC, Carroll JE, Borlongan CV (2008) Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J Cereb Blood Flow Metab 28:1804-10.
Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM, Yu SJ, Bae E, Yu G, Xu L, McGrogan M, Bankiewicz K, Case C, Borlongan CV (2009) Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells and Development. 18:1501-14.
Yu H, Xu Z, Qu G, Wang H, Lin L, Li X, , Xie X, Lei Y, He X, Chen Y, Li Y (2021) Hypoxic preconditioning enhances the efficacy of mesenchymal stem cells-derived conditioned medium in switching microglia toward anti-inflammatory polarization in ischemia/reperfusion. Cell Mol Neurobiol 41:505–24.
Zhang R, Zhang Z, Chopp M (2016) Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab 36:2034-43.
Zhang Y, Ma L, Su Y, Su L, Lan X, Wu D, Han S, Li J, Kvederis L, Corey S, Borlongan CV, Ji X (2019) Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res 1725:146432.
Zhao Y, Zhang M, Lu G-L, Huang B-X, Wang D-W, Shao Y, Lu MJ (2020) Hypoxic preconditioning enhances cellular viability and pro-angiogenic paracrine activity: The roles of VEGF-A and SDF-1a in rat adipose stem cells. Front Cell Dev Biol 8:580131.
References
Chase Kingsbury1*
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Blaise Cozene2*
2Tulane University, 6823 St. Charles Ave, New Orleans, LA 70118, USA.
Justin Cho1*
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Zhen-Jie Wang1
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Alma R. Lezama3
3Centro de Investigación en Ciencias de la Salud (CICSA); FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
Felipe Esparza3
3Centro de Investigación en Ciencias de la Salud (CICSA); FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
German Rivera Monroy3
3Centro de Investigación en Ciencias de la Salud (CICSA); FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, México.
Madeline Saft4
4University of Michigan, 701 E. University Ave, Ann Arbor, MI 48109, USA.
Alex Shear5
5University of Florida, 205 Fletcher Drive, Gainesville, FL 32611, USA.
Nadia Sadanandan6
6Georgetown University, 3700 O St NW, Washington, DC 20057, USA.
Reed Berlet7
7Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064 USA.
Jea-Young Lee1
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Cesario V. Borlongan1#
1Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
*These authors contributed equally to this paper.
Corresponding author:
Prof. Cesar V. Borlongan
Email: cborlong@usf.edu

In a new window | Download PPT
Figure 1: Conditioning of stem cells. Exposing cultured stem cells to hypoxic stress and oxygen deprivation prior to transplantation enhances ischemic tolerance. These conditioned stem cells may be more tolerant to the harsh environment of the ischemic brain, allowing them to survive and function better than non-conditioned stem cells.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6199 | 25 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA