Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Patching the scarred heart
Time:2021-06-08
Number:6714
Shuo Cong1,2,3, Jasper Chua4, Sauri Hernandez-Resendiz1,2
Author Affiliations


- 1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. Author affiliation.
- 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.
- 3Yong Loo Lin Medical School, National University of Singapore, Singapore.
- 4National Dental Research Institute Singapore, National Dental Centre Singapore, Singapore.
Conditioning Medicine 2021. 4(2): 100-112.
Abstract
Acute myocardial infarction (AMI) and heart failure (HF) that often follows remain the leading causes of morbidity and mortality worldwide. Following MI, lost cardiomyocytes (CM) are replaced by non-contractile scar tissue that increases ventricular wall stress while diminishing myocardial performance. With the negligible regenerative capacity of the heart, the field of heart engineering and regenerative therapy for MI remains a challenge. Cardiac patch often combines the use of cells and synthetic/biomaterials with the ultimate aim of improving myocardial function. Through the years, there have been remarkable breakthroughs in the fields of stem cell and biomaterials research. The advent of human-induced pluripotent stem cells provides a potentially unlimited source of cardiomyocytes for regenerative therapy. By combining this with 3D bioprinting, it was possible to generate a cardiac patch with cell and structural organizations similar to that of the native heart. Even with vast technological advancements, the promise of the cardiac patch to treat MI has not been fulfilled. Subsequent studies revealed that exosomes, rather than the cellular component of the cardiac patch, is one of the main contributors to its cardioprotection. In this review, we present and discuss perspectives of the cardiac patch and its drawbacks and future relevance as a promising intervention for MI patients.
Keywords: Cardiac patch, engineered heart tissue, regenerative medicine, acute myocardial infarction, cardioprotection, stem cell therapy
Abstract
Acute myocardial infarction (AMI) and heart failure (HF) that often follows remain the leading causes of morbidity and mortality worldwide. Following MI, lost cardiomyocytes (CM) are replaced by non-contractile scar tissue that increases ventricular wall stress while diminishing myocardial performance. With the negligible regenerative capacity of the heart, the field of heart engineering and regenerative therapy for MI remains a challenge. Cardiac patch often combines the use of cells and synthetic/biomaterials with the ultimate aim of improving myocardial function. Through the years, there have been remarkable breakthroughs in the fields of stem cell and biomaterials research. The advent of human-induced pluripotent stem cells provides a potentially unlimited source of cardiomyocytes for regenerative therapy. By combining this with 3D bioprinting, it was possible to generate a cardiac patch with cell and structural organizations similar to that of the native heart. Even with vast technological advancements, the promise of the cardiac patch to treat MI has not been fulfilled. Subsequent studies revealed that exosomes, rather than the cellular component of the cardiac patch, is one of the main contributors to its cardioprotection. In this review, we present and discuss perspectives of the cardiac patch and its drawbacks and future relevance as a promising intervention for MI patients.
Keywords: Cardiac patch, engineered heart tissue, regenerative medicine, acute myocardial infarction, cardioprotection, stem cell therapy
Acute myocardial infarction
Acute myocardial infarction (AMI) and the often-ensuing heart failure (HF) are leading causes of morbidity and mortality globally, exerting huge burdens on healthcare and economy (Reed et al., 2017). MI occurs as a result of cardiomyocyte death due to prolonged ischemia and is clinically classified based on the presence or absence of ST-segment elevation on the electrocardiogram (ECG) – ST-elevation MI (STEMI) and non-STEMI (NSTEMI) (Anderson and Morrow, 2017).
Reperfusion, using either thrombolytic therapy or percutaneous coronary intervention (PCI), is mandatory following AMI to salvage ischemic cell death and improve clinical outcome (Saleh and Ambrose, 2018), with the latter being the method of choice (Grines et al., 2003). With reperfusion along with appropriate medical and lifestyle intervention, most patients can be discharged within two to three days and resume normal or near normal lives (Saleh and Ambrose, 2018). While reperfusion reduces myocardial ischemic injury and limits the infarct size, the process of reperfusion independently activates a cascade of cellular injuries and exacerbates myocardial injury (Shin et al., 2017), causing excessive cardiomyocyte cell death and increasing the infarct size. This phenomenon, termed myocardial reperfusion injury, was shown to contribute to up to 50% of the final infarct size (Yellon and Hausenloy, 2007). The lost cardiomyocytes are replaced by non-contractile scar tissue consisting of cardiac fibroblasts and collagen (Jugdutt, 2009). Though scar formation preserves structural integrity, excessive collagen deposition can cause cardiomyocyte atrophy and arrhythmogenicity (Leask, 2015). Therefore, despite effective reperfusion therapy, survivors remain at risk of severe sequelae, including sudden cardiac death (SCD), HF, and left ventricular systolic dysfunction (Docherty et al., 2020), prompting the need for new therapies against AMI.
Given that the heart has very limited capacity for regeneration (van Berlo and Molkentin, 2014), stem cell transplantation has emerged as a promising avenue to improve function following myocardial insult (Bartunek et al., 2013; Suncion et al., 2014; Perin et al., 2015; Bartunek et al., 2017; Emmert et al., 2017; Yau et al., 2019). Some clinical trials have yielded positive results, showing improved left ventricular ejection fraction (LVEF) – a major determinant of long-term prognosis for STEMI – following administration of mesenchymal stem cells (MSC) (Kim et al., 2018) and bone marrow mononuclear stem cells (Stamm et al., 2007; Laguna et al., 2018). The benefit of stem cell transplantation extends beyond myocardial regeneration, and include angiogenesis (Tse et al., 2003; Li et al., 2010), and improvements in tissue perfusion and fibrotic burden (Karantalis et al., 2014). However, several recent clinical trials have yielded neutral results with no significant LVEF improvements (Nasseri et al., 2014; Wollert et al., 2017; Laguna et al., 2018; Nicolau et al., 2018; Traverse et al., 2018) (see Table 1).
Stem cell transplatation
Following MI, up to one billion cardiomyocytes in the infarct zone are lost (Lin and Pu, 2014). Over the past two decades, studies have attempted to replace the lost myocardial cells using cell injection. However, poor inferior cell engraftment and cell survival at the ischemic region coupled with rapid (hours to days following transplantation) cell loss have resulted in the low efficacy of stem cell therapy (Nguyen et al., 2016).
Stem cell transplantation has yielded promising results (Liu et al., 2018). However, it appears current stem cell transplantation has yet to reach its full potential of alleviating cardiac injury. Several limitations have been eluded from previous studies, with the major limitation being poor cell survival and retention at the intended site (Hou et al., 2005; Sekine et al., 2011; Wang et al., 2013; Lepperhof et al., 2014; Roche et al., 2014; Yan et al., 2017) limiting the regenerative application of stem cell therapy. Furthermore, the harsh ischemic microenvironment of the injured myocardium is not favorable for the transplanted cells (Hu et al., 2016). Efforts to circumvent this include the use of intrinsic cellular homing (such as sphingosine-1 phosphate-sphingosine-1 phosphate receptor 2 (S1P-S1PR2) axis in Muse cells) (Yamada et al., 2018), nanoparticles to facilitate homing of cells (Huang et al., 2013; Lee et al., 2020), and cardiac patch (Roche et al., 2014; Bellamy et al., 2015; Zhang, 2015; Sugiura et al., 2016). While the former two entail a less invasive approach to improve cell retention, they do not necessarily improve the microenvironment to encourage cell survival. On the other hand, cardiac patch, which employs the use of biomaterials, may require more invasive procedures, but the use of biomaterials could provide a more favorable microenvironment to improve cell survival (Sun et al., 2020). Herein we review the recent progress on cardiac patches and discuss how it remains a promising endeavour to alleviate post-MI sequelae.
Cardiac patch
In contrast to cell therapy alone, cardiac patch is a three-dimensional heart tissue engineered in vitro and implanted over the infarcted tissue. It has been widely reported to enhance cell retention and survival at the implanted sites, thereby increasing the success of cell therapies (Madonna et al., 2019). Several animal studies have shown the effectiveness of cardiac patch in alleviating cardiac injury in MI (Table 2). A recent study has demonstrated the high degree of retention and vascularization over 14 days of transplanted cardiac patches that were printed with bioinks (composed of cardiac extracellular matrix (cECM), human heart progenitor cells (hCPCs), and gelatin methacrylate (GelMA) in rat models of MI (Bejleri et al., 2018). The effectiveness of cardiac patches was also seen in a study using a porcine model of MI, where a pair of clinically relevant cardiac patches containing trilineage cardiac cells had engraftment rates beyond 10% at four weeks post-implant and was associated with significant improvements of the left ventricular function coupled with reduced infarct size and myocardial wall stress in the peri-scar boarder zone of the myocardium (Gao et al., 2018). To date, there are only a few human studies being done that used cardiac patch to alleviate post-infarct sequelae (Menasche et al., 2015; Menasche et al., 2018; Prat-Vidal et al., 2020).
The earliest cardiac patch that was implanted in a human contained cardiac-committed human embryonic stem cells (hESCs) – Isl-1+ SSEA-1+ (stage-specific embryonic antigen-1) cells – embedded in a fibrin scaffold. The 68-year-old patient presented with HF as a result of a previous MI, and was designated for surgical anterior myocardial revascularization via coronary artery bypass graft (CABG). During the CABG, the cardiac patch was secured between the pericardium and epicardium with the use of sutures. Functional improvements were observed after three months, with the patient having increased LVEF, 6 min walking distance, and decreased LV end-diastolic and end-systolic volumes (Menasche et al., 2015). Though these improvements were only seen in one patient, the authors conducted a similar study subsequently on six patients with severe ischemic LV dysfunction resulting from previous MI. That study yielded similar optimistic results in the aforementioned parameters (Menasche et al., 2018), demonstrating both the efficacy as well as safety of cardiac patches. Moving forward, the recent first-in-human study scaled up a 2 cm2 preclinical construct (Galvez-Monton et al., 2017) to a 16 cm2 decellularized human pericardial matrix colonized with 12.5 million human viable Wharton’s jelly-derived MSCs (WJ-MSCs) (Prat-Vidal et al., 2020). The cardiac patch was applied by surgical glue over non-revascularizable myocardial scar tissue while CABG was being performed for revascularizable regions. The patient showed ~9% reduction in scar mass in the treated area (Prat-Vidal et al., 2020). Cardiac patches offer a promising integrative approach to repair the injured heart following MI, with optimistic results ranging from improvement of cell retention and engraftment to reducing adverse LV remodeling, preventing LV dilation, and thinning, and enhancing LV function (see Table 3). Nonetheless, cardiac patch has its limitation, and is currently not applicable for clinical application (Zhang et al., 2018).
The challenges of patching the heart
The main challenges in engineered heart tissue (EHT), or cardiac patch, includes but are not limited to (1) the optimization of scaffold mechanics and biocompatibility, (2) cell maturation and contractile ability, (3) electromechanical integration, (4) immune rejection, (5) tissue vascularization and oxygen supplementation (Jackson et al., 2020).
Scaffold mechanics and biocompatibility
An ideal scaffold for cardiac tissue engineering should possess excellent mechanical properties and electrical conductivity to perform normal physiological functions of the heart (Qasim et al., 2019b). Thus, an ideal scaffold must possess sufficient porosity for cell ingrowth (Loh and Choong, 2013; Bruzauskaite et al., 2016), architecture that facilitate proper cardiomyocyte alignment (Homma et al., 2020), mechanical properties to withstand surgical implantation, and surface characteristics to support firm cell adhesion and growth (Prasad and Krishnan, 2008; Khalili and Ahmad, 2015). Recent advances in biomaterials have provided a variety of different approaches to create scaffolds for tissue engineering, which include the use of nanofibers (Joshi and Kothapalli, 2015), hydrogels (El-Sherbiny and Yacoub, 2013; Radhakrishnan et al., 2014), injectable gels (Alagarsamy et al., 2019), decellularization (Rana et al., 2017; Daley et al., 2018), and 3D printing (Mosadegh et al., 2015; Qasim et al., 2019a). Among which, generating scaffolds from decellularization have been particularly attractive as it preserves the structural “blueprint” of the actual heart. In a breakthrough study in 2008, researchers successfully created a physiologically functional heart by first decellularizing the rat whole heart via coronary perfusion and later incorporating the decellularized scaffolds with cardiac and endothelial cells (Ott et al., 2008). Subsequently, in 2016, researchers successfully repopulated decellularized human myocardial slices (200 µm thick with hiPSC-CMs) to generate myocardial tissue capable of spontaneous contraction (Guyette et al., 2016). More recently, a pre-clinical study on swine models of MI demonstrated that the implantation of such cardiac patch led to restoration of ventricular function and better recovery following MI. The cardiac patch was made of decellularized myocardial and pericardial tissues that were repopulated with adipose tissue derived MSCs (Perea-Gil et al., 2018).
However, decellularization and repopulation of cells are not without imperfections. The balance between the removal of cellular components and preservation of structural and biomechanical integrity of the three-dimensional extracellular matrix remains to be optimized (Di Meglio et al., 2017; Kc et al., 2019). Other challenges of using decellularized extracellular matrix (dECM) includes the homogenous distribution of repopulated cells within the scaffold compartments, and the diffusion limit of thick cardiac dECM (Kc et al., 2019).
Other noteworthy innovation includes the recent three-dimensional (3D) bioprinting, which uses a layer-by-layer approach to deposit bioink filaments to generate cardiac patches (Gardin et al., 2020). The advent of additive manufacturing 3D bioprinting technology allows heterogenous cell types, biomaterials, and signalling factors to be precisely deposited and arranged in organized geometries similar to those found in the native counterparts (Alonzo et al., 2019). Some of the biomaterials that were utilized for myocardial tissue printing include alginate ((Rastogi and Kandasubramanian, 2019), collagen (Jakab et al., 2008), gelatin (Gaetani et al., 2015), fibrin (Barsotti et al., 2011), and synthetic biomaterials (Ho et al., 2017). Not surprisingly, 3D bioprinting has been explored in the context of dECM (Pati et al., 2014). Three-dimensional printed dECM-based cardiac patch retains the microenvironmental cues that facilitates cardiomyocyte differentiation and maturation (Das et al., 2019). Such results were echoed in another recent study that derived dECM from porcine heart. Human MSCs were then printed onto porcine dECM to generate the cardiac patch, which was implanted on the epicardial infarct region of a rat model of MI. The implanted patch provided a conducive microenvironment and paracrine factors that promote vascular regeneration, resulting in restoration of cardiac function (Park et al., 2019).
Cell maturation and contractile ability
One of the early limitations of using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) is their immature phenotype. Since then, many have attempted to facilitate the maturation of hiPSC-CMs to achieve a more adult-like phenotype – including extended culture, electrical and mechanical stimulation, extracellular matrix modulation, and using microRNAs (Ramachandra et al., 2021). In the context of serum-free culture, several factors were identified to be crucial for the maturation of hiPSC-CMs. These include triiodothyronine (T3), insulin-like growth factor 1(IGF-1), and glucocorticoid dexamethasone. T3 is essential for cardiomyocyte excitability and contractibility was found to enhance the resting membrane potential while IGF-1 and dexamethasone produce synergistic effects on cellular bioenergetics and traction force (Birket et al., 2015). Additionally, further functional and metabolic maturation of hiPSC-CMs were observed when these three factors were coupled with peroxisome proliferator-activated receptor alpha (PPAR-α) activation and hypoxia inducible factor-1 alpha (HIF-1α) inhibition (Gentillon et al., 2019). Direct supplementation of metabolic substrate such as palmitate/oleate (fatty acids) was also found to promote metabolic maturation of hiPSC-CMs, with better mitochondrial oxidative phosphorylation, and ATP production (Ramachandra et al., 2018).
Interestingly, 3D cultures of hiPSC-CMs generate greater mitochondrial mass as compared to 2D hiPSC-CMs cultures, and produce mitochondrial proteomic profiles similar to adult human cardiomyocytes (Ulmer et al., 2018). By combining different cell populations – hiPSC-CMs, cardiac fibroblasts, and cardiac endothelial cells – maturation is further enhanced, where 3D cultures of hiPSC-CMs exhibit improved sarcomeric structures with transverse tubules, greater contractility and mitochondrial respiration, and more mature electrophysiological properties. The study found that the enhanced maturation was associated with the coupling of hiPSC-CMs with cardiac fibroblast via connexin 43 (CX43) gap junctions and increased intracellular cAMP (Giacomelli et al., 2020). Similarly, co-culturing hiPSC-CMs with MSCs showed improved contractile properties, with the hiPSC-CMs exhibiting aligned myofibrils with A-, H-, and I-bands that are able to contract and relax rapidly (Yoshida et al., 2018).
Remarkably, the microenvironment has a huge impact on cardiomyocyte maturation, where myocardial-grafted cardiac patch resulted in nascent tissue-like organization with aligned cardiomyocytes in the scaffold that was accompanied by a greater degree of neovascularization and microvascular maturation as compared to the peripherally grafted cardiac patch (Ja et al., 2018). In addition, hiPSC-CMs alone may not be optimal for cardiac regeneration. In particular, a stem cell study showed that when injected into the peri-infarcted anterior free wall of a murine model of MI, hiPSC-derived cardiac progenitors provided greater protection compared to hiPSC-CMs, resulting in greater functional recovery of the heart (Ja et al., 2016). By incorporating hiPSC-CMs with human dermal fibroblasts into fibrin hydrogels and applying electrical stimulation to induce auxotonic contractions, the cardiac patch exhibit adult-like gene expression profiles, organized ultrastructure, sarcomere length, and mitochondrial density. Functionally, these cardiac patches have a positive force-frequency relationship and functional calcium handling. Cardiomyocyte elongation and alignment were facilitated by the passive tension created by the stretching motion within the cardiac patch (Ronaldson-Bouchard et al., 2018). Also, instead of electrical stimulation, a separate study has stimulated similar cardiac patch using a dynamic (rocking) platform, and also resulted in matured cardiomyocytes – with presence of intercalated disk-like structures (Gao et al., 2018).
Moreover, it seems that the presence of other cell population, such as endothelial cells, smooth muscle cells, and MSCs, may significantly improve the contractility (Burridge et al., 2014) and therapeutic effects (Ye et al., 2014) of cardiac patches.
Electromechanical Integration
Besides the cellular immaturity of cardiac patches, another important hurdle to overcome is the electromechanical integration between the implanted cardiac patch and the host myocardium since contraction-competent cardiac patches may disrupt the cardiac syncytium and de-synchronize cardiac rhythm, and potentially exacerbate cardiac arrhythmia, which could lead to pathological conditions (Puig-Sanvicens et al., 2015). Non-human primate studies have shown that the direct injection of hiPSC-CMs has the potential to cause dangerously abnormal ventricular electrical activities (Chong et al., 2014), while epicardial injection of hESC-CMs resulted in graft-induced arrhythmias (Liu et al., 2018).
To tackle this, conductive polymers – such as polydimethysiloxane (Jackman et al., 2018), polypyrrole (Cui et al., 2018), and poly-3-amino-4-methoxybenzoic acid (Zhang et al., 2020) – were incorporated into the scaffolds (Solazzo et al., 2019). A recent study has demonstrated that an injection of conductive polypyrrole-chitosan hydrogel into the scar zone following MI improved the electrical conduction across the fibrotic scar and resynchronized the cardiac contraction of the rat’s heart (He et al., 2020). Another study conjugated a choline-based bio-ionic liquid onto gelatin methacryloyl to generate a scaffold with better conductive and adhesive property. After which, primary cardiomyocytes and cardiac fibroblasts were incorporated into the scaffold, and the resulting cardiac patch attenuated post-MI remodeling in murine hearts (Walker et al., 2019).
Taken together, conductive scaffolds offer a platform to improve the electromechanical integration of cardiac patches while improving the synchronization of cardiac contraction. Nonetheless, further research is required to optimize the conductivity of cardiac patches to achieve similar properties of the native heart (Baei et al., 2020).
Immune rejection
Immunogenicity is expected to be less of a problem in clinical practice because hiPSC-CMs can be generated from the patient’s own somatic cells. However, the urgency for prompt treatment following MI to minimize cardiac injury (Lesneski, 2010) precludes the use of autologous hiPSC-CMs as a substantial amount of time is needed for hiPSC reprogramming and subsequent differentiation into hiPSC-CMs (Blair and Barker, 2016; Lipsitz et al., 2016). On the other hand, while using pre-made allogenic hiPSC-CMs for cardiac patches is more practical, it runs the risk of immune rejection due to the human leukocyte antigen (HLA) – the human major histocompatibility complex (MHC) (de Rham and Villard, 2014). Thus, the risk versus benefit ratio of the use of hiPSC-CMs remains highly debatable.
By inactivating MHC class I and II genes and overexpressing CD47, researchers were able to generate hypoimmunogenic hiPSCs that retained the ability to differentiate into spontaneously beating hiPSC-CMs (Deuse et al., 2019). This opens the exciting possibility of having a universally compatible source of readily available hiPSC-CMs for regenerative medicine. Alternatively, the use of MHC haplotype homozygous cells could also overcome immunorejection for allogenic hiPSC-CM transplantation (Kawamura et al., 2016), with a recent clinical study demonstrating the usefulness of hiPSC banking for hematologic and nonhematologic malignancies (Morishima et al., 2020). All of the paired 39 donor HLA homozygous donor to patient heterozygous, except one (early death), engrafted neutrophils (the primary outcome of the study) with incidence of acute graft-versus-host disease of 17/38 (grades II to IV) and 3/38 (grades III to IV). The study concluded that HLA-homo hiPSC transplantation led to favorable engraftment (Morishima et al., 2020). More interestingly, a recent study demonstrated that syngeneic MSCs reduces immune rejection after transplantation of allogenic hiPSC-CMs in mice (Yoshida et al., 2020). Such findings add to the aforementioned benefits of incorporating MSCs (i.e., enhanced maturation and cardioprotection) in cardiac patches. Furthermore, syngeneic MSCs can be readily harvested using methods such as AdiPrep® (Dragoo and Chang, 2017).
Vascularization and oxygen supply
Poor perfusion of cardiac patches remains a major issue for cells-containing cardiac patches. Previously, because of the lack of vasculature, the viable cardiac cell sheet-layered tissues were limited to three layers (~80 µm) (Shimizu et al., 2006). Moreover, the suboptimal neovascularization and microvascular maturation could be an intrinsic limitation of the current hiPSC-CMs as a study showed that cardiac patch containing human embryonic stem cell-derived cardiomyocytes (hESC-CMs) generated significantly more robust and mature microvasculature as compared to cardiac patches containing hiPSC-CMs (Ja et al., 2018).
Strategies to rapidly perfuse thick, cell-dense cardiac patches to avoid hypoxia and necrosis are critical to support the long-term survival of the implanted cardiac patches (Chang and Niklason, 2017; Tang et al., 2018). Recent advancements have improved the perfusion of cardiac patches. By incorporating different cell types besides hiPSC-CMs, such as endothelial cells and MSCs, the hybrid cardiac patch was able to generate micro-vessels and integrate rapidly with the host (Huang et al., 2019). Recently, the use of microfluidic hydrodynamics focusing has become an interesting strategy to vascularize cardiac patches as researchers were able to construct biomimetic microvessels. Subsequently, these biomimetic microvessels were incorporated into the cardiac patch resulting in a vascularized cardiac patch that has the natural architecture and function of capillaries. When implanted in a rat model of AMI, the cardiac patch induced cardiomyocyte proliferation at the peri-infarct region four-weeks post-treatment with a significant increase in myocardial capillary density as compared to the conventional cardiac patch (Su et al., 2018).
The mechanism of aciton
Structural Support
Following MI, the injured myocardium is replaced by a thin fibrotic scar that increases wall stress in the surrounding tissues. Thus, the addition of wall thickness and rigidity by a cardiac patch may improve myocardial performance and prevent detrimental LV remodeling (Domenech et al., 2016). Decrease infarct size and reduced wall thinning were also seen (in rat model of MI) from a cardiac patch without cardiomyocytes. This fibrin-based, stretch-conditioned cardiac patch served as a proof-of-concept of the benefits conferred by structural support by the scaffold rather than the replenishment of cardiomyocytes (Wendel et al., 2014). In another study, acellular cardiac patches, generated using type I collagen, attenuated LV remodeling with reduced fibrosis and formation of an interconnected blood vessels at the infarct site, resulting in significant protection against cardiac injury at both the anatomical and functional levels following MI (Serpooshan et al., 2013).
Direct remuscularization
The original goal of the cardiac patch is to replace fibrotic scar tissue with electromechanically functional and vascularized tissue (Lakshmanan et al., 2012; Ye et al., 2013). While a study has shown that cardiac patches containing hiPSC-CMs, fibroblasts, and endothelial cells were able to reduce the infarct size and increase the vessel numbers following MI (Yeung et al., 2019), another study (employing a cryo-injury guinea pig model) demonstrated that cardiac patches only resulted in partial remuscularization of the injured heart (Querdel et al., 2021). Given the poor retention and survival of transplanted cells, multiple stem cell transplantation studies concluded that the functional improvements seen were not due to direct remuscularization but rather the paracrine effects by the transplanted cells (Gnecchi et al., 2006; Takahashi et al., 2006; Uemura et al., 2006; van der Spoel et al., 2012; Zuo et al., 2012; Bao et al., 2017; Wu et al., 2017; Dougherty et al., 2018; Zhu et al., 2018). As more data has emerged, it appears that remuscularization by the cardiac patch is controversial, especially with the suboptimal biophysical integration of the cardiac patch and the host myocardium (Huang et al., 2019). Current research attributes the main contributor of cardioprotective effects by cardiac patches to be the paracrine factors (Hodgkinson et al., 2016). Such a turn of events could be seen as a blessing since clinically relevant cell-based therapy would require billions of cardiomyocytes (Chong et al., 2014; Liu et al., 2018), making it highly laborious and time-consuming.
Paracrine Effect
Current evidence suggests the majority of the benefits associated with cardiac patches likely revolves from the paracrine activity of implanted cells (Qasim et al., 2019b), with a recent study showing the secretome from hiPSCs and MSCs is able to produce significant improvement of cardiac function and remodeling following MI (Alrefai et al., 2019), thereby circumventing the need for exogenous cell implantation. Furthermore, a cardiac patch containing hiPSC-CMs, fibroblasts, and endothelial cells, produced improvement of heart function that is correlated with patch production of extracellular vesicles (i.e. exosomes). In addition, the cardiac patch led to regeneration of cardiac tissues, angiogenesis in the infarcted area, and reduced scar tissue formation (Yeung et al., 2019).
Adult stem cells secrete a variety of growth factors and chemokines - including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and IGF-1 – which alters the microenvironment of the myocardium and regulate remodeling following MI (Hodgkinson et al., 2016; Broughton et al., 2018). To facilitate the direct delivery of the secreted regenerative factors into the injured myocardium, microneedles were engineered on a cardiac patch incorporated with cardiac stromal cells. The resulting cardiac patch effectively augmented the myocardial function and enhanced angiomyogenesis (Tang et al., 2018).
Future directions
With many optimistic results thus far, the cardiac patch remains a promising avenue to pursue to attenuate cardiac injury associated with MI. However, more evidence suggests that the beneficial effects of the cardiac patch are largely due to the non-cellular part of the cardiac patch (Domenech et al., 2016; Qasim et al., 2019b). Therefore, we envision that the next generation of cardiac patch would be an acellular one that is focused on providing structural support while serving as a delivery vehicle for the continuous release of cardioprotective secretomes. Being cell-free not only reduces the risk of immune rejection but overcomes the need for perfusion, electromechanical integration, and cell maturation – all of which are the major roadblocks of today’s cardiac patch.
Conclusion
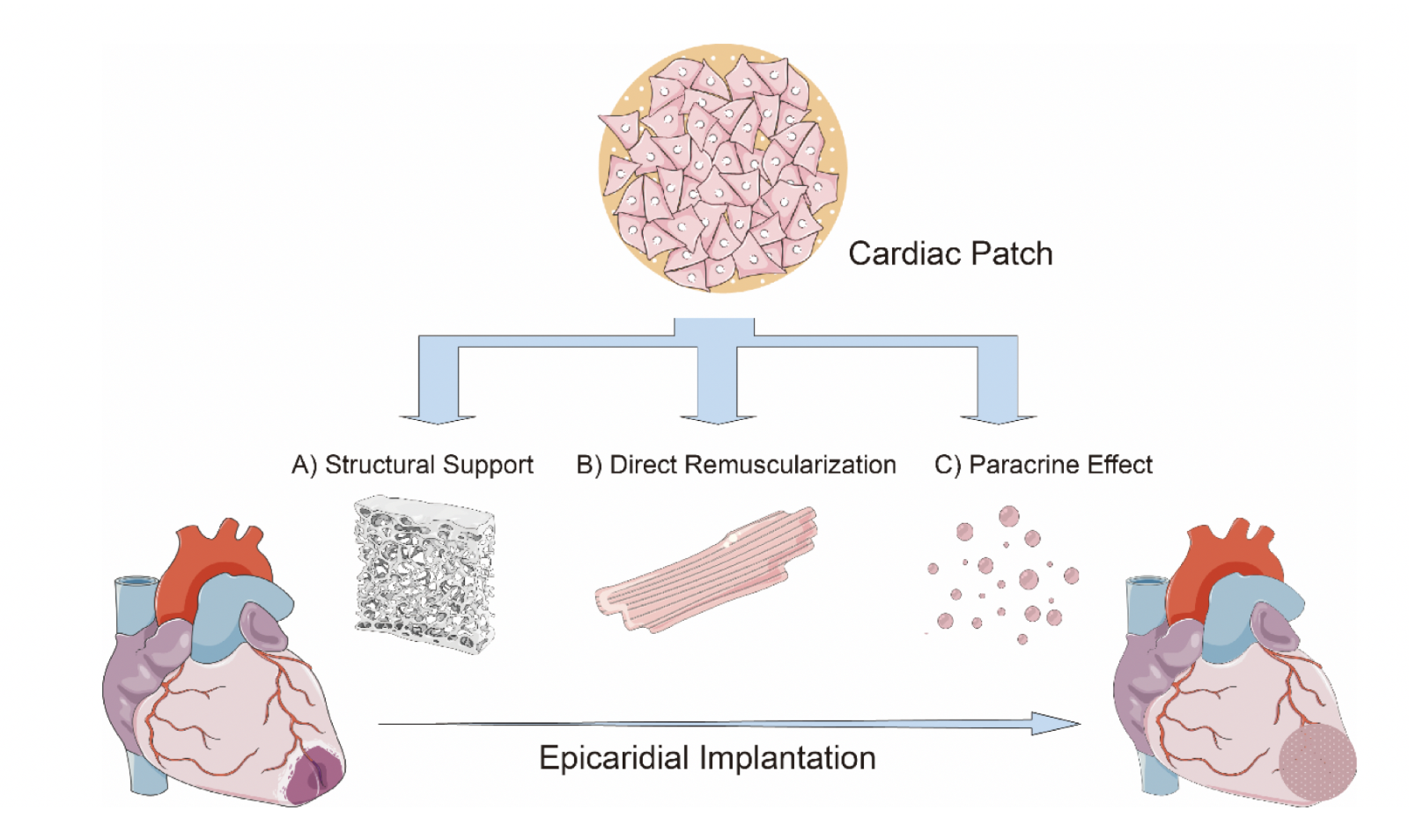
AMI remains as one of the leading causes of death despite tremendous leaps in medical advancements. Over the years, research has led to mechanistic insights on the cardioprotective benefits conferred by cardiac patches. These include (1) providing structural support, (2) promoting direct remuscularization, and (3) secretion of cardioprotective paracrine factors (see Figure 1). Earlier studies have focused on “direct remuscularization”, optimizing the delivery and incorporation of cardiomyocytes and other cell types to the infarct zone in an attempt to regenerate and repair myocardium. Soon, it became clear that cardiomyocytes were not the only important cell type. Other important cell types include MSCs, cardiac fibroblasts, and endothelial cells. However, increasing evidence have suggested that the key contributors to the protective effects of cardiac patches are the secretomes or exosomes produced by the implanted cells rather than the cells themselves. Therefore, we envision that the next generation of cardiac patches would be an acellular one that deliver protective secretomes. Being acellular would help overcome most of the major obstacles faced by the current cardiac patches. With optimistic data of cardiac patches presented by numerous studies, we believe that the cardiac patch remains a promising avenue to attenuate post-MI sequelae.
In a new window | Download PPT
Figure 1: Schematic description of the mechanistic action of cardiac patch. The major mechanisms include (A) providing structural support, (B) promoting direct myocardial remuscularization, and (C) producing cardioprotective paracrine factors. (A) Since a thin fibrotic scar replaces the myocardium at the infarct zone, the rigid scaffold of the cardiac patch provides additional wall thickness and rigidity, which could enhance myocardial function while preventing detrimental left ventricular remodeling. (B) With the tremendous loss of cardiomyocytes following AMI, early studies focus on regenerating the injured myocardium by introducing cells with therapeutic properties. While there were optimistic results that implanted cells were able to reduce the infarct size and stimulate vascularization, there were also contrasting results that showed partial remuscularization with suboptimal biophysical integration of the cardiac patch. Currently, more evidence is attributing the cardioprotective effects of cardiac patch to the paracrine factors produced by the implanted cells. (C) The implanted cells such as adult stem cells which secrete a variety of paracrine factors which is capable of altering the myocardial microenvironment and influence myocardial remodeling.
Acknowledgements
Sauri Hernandez-Resendiz is supported by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Young Individual Research Grant (OF-YIRG)-[NMRC/OFYIRG/0078/2018].
References
Alagarsamy KN, Yan W, Srivastava A, Desiderio V, Dhingra S (2019) Application of injectable hydrogels for cardiac stem cell therapy and tissue engineering. Rev Cardiovasc Med 20:221-230.
Alonzo M, AnilKumar S, Roman B, Tasnim N, Joddar B (2019) 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl Res 211:64-83.
Alrefai MT, Tarola CL, Raagas R, Ridwan K, Shalal M, Lomis N, Paul A, Alrefai MD, Prakash S, Schwertani A, Shum-Tim D (2019) Functional Assessment of Pluripotent and Mesenchymal Stem Cell Derived Secretome in Heart Disease. Ann Stem Cell Res 2:29-36.
Anderson JL, Morrow DA (2017) Acute Myocardial Infarction. N Engl J Med 376:2053-2064.
Baei P, Hosseini M, Baharvand H, Pahlavan S (2020) Electrically conductive materials for in vitro cardiac microtissue engineering. J Biomed Mater Res A 108:1203-1213.
Bao L, Meng Q, Li Y, Deng S, Yu Z, Liu Z, Zhang L, Fan H (2017) C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J Card Fail 23:403-415.
Barsotti MC, Felice F, Balbarini A, Di Stefano R (2011) Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem 58:301-310.
Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A (2013) Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 61:2329-2338.
Bartunek J et al. (2020) Cardiopoietic stem cell therapy in ischaemic heart failure: long-term clinical outcomes. ESC Heart Fail.
Bartunek J et al. (2017) Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 38:648-660.
Bejleri D, Streeter BW, Nachlas ALY, Brown ME, Gaetani R, Christman KL, Davis ME (2018) A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv Healthc Mater 7:e1800672.
Bellamy V, Vanneaux V, Bel A, Nemetalla H, Emmanuelle Boitard S, Farouz Y, Joanne P, Perier MC, Robidel E, Mandet C, Hagege A, Bruneval P, Larghero J, Agbulut O, Menasche P (2015) Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Heart Lung Transplant 34:1198-1207.
Birket MJ, Ribeiro MC, Kosmidis G, Ward D, Leitoguinho AR, van de Pol V, Dambrot C, Devalla HD, Davis RP, Mastroberardino PG, Atsma DE, Passier R, Mummery CL (2015) Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep 13:733-745.
Blair NF, Barker RA (2016) Making it personal: the prospects for autologous pluripotent stem cell-derived therapies. Regen Med 11:423-425.
Broughton KM, Wang BJ, Firouzi F, Khalafalla F, Dimmeler S, Fernandez-Aviles F, Sussman MA (2018) Mechanisms of Cardiac Repair and Regeneration. Circ Res 122:1151-1163.
Bruzauskaite I, Bironaite D, Bagdonas E, Bernotiene E (2016) Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology 68:355-369.
Burridge PW, Metzler SA, Nakayama KH, Abilez OJ, Simmons CS, Bruce MA, Matsuura Y, Kim P, Wu JC, Butte M, Huang NF, Yang PC (2014) Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am J Transl Res 6:724-735.
Chang WG, Niklason LE (2017) A short discourse on vascular tissue engineering. NPJ Regen Med 2.
Chong JJ et al. (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510:273-277.
Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, Pellerin D, Locca D, Westwood M, Rakhit R, Crake T, Kastrup J, Parmar M, Agrawal S, Jones D, Martin J, Mathur A (2016) A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trialdagger. Eur Heart J 37:256-263.
Cui H, Liu C, Esworthy T, Huang Y, Yu ZX, Zhou X, San H, Lee SJ, Hann SY, Boehm M, Mohiuddin M, Fisher JP, Zhang LG (2020) 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction. Sci Adv 6:eabb5067.
Cui Z, Ni NC, Wu J, Du GQ, He S, Yau TM, Weisel RD, Sung HW, Li RK (2018) Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 8:2752-2764.
Daley MC, Fenn SL, Black LD, 3rd (2018) Applications of Cardiac Extracellular Matrix in Tissue Engineering and Regenerative Medicine. Adv Exp Med Biol 1098:59-83.
Das S, Kim SW, Choi YJ, Lee S, Lee SH, Kong JS, Park HJ, Cho DW, Jang J (2019) Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater 95:188-200.
de Rham C, Villard J (2014) Potential and limitation of HLA-based banking of human pluripotent stem cells for cell therapy. J Immunol Res 2014:518135.
Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S (2019) Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37:252-258.
Di Meglio F, Nurzynska D, Romano V, Miraglia R, Belviso I, Sacco AM, Barbato V, Di Gennaro M, Granato G, Maiello C, Montagnani S, Castaldo C (2017) Optimization of Human Myocardium Decellularization Method for the Construction of Implantable Patches. Tissue Eng Part C Methods 23:525-539.
Docherty KF, Ferreira JP, Sharma A, Girerd N, Gregson J, Duarte K, Petrie MC, Jhund PS, Dickstein K, Pfeffer MA, Pitt B, Rossignol P, Zannad F, McMurray JJV (2020) Predictors of sudden cardiac death in high-risk patients following a myocardial infarction. Eur J Heart Fail 22:848-855.
Domenech M, Polo-Corrales L, Ramirez-Vick JE, Freytes DO (2016) Tissue Engineering Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng Part B Rev 22:438-458.
Domenge O, Ragot H, Deloux R, Crepet A, Revet G, Boitard SE, Simon A, Mougenot N, David L, Delair T, Montembault A, Agbulut O (2021) Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: impact of the acetylation degree of chitosan. Acta Biomater 119:125-139.
Dougherty JA, Kumar N, Noor M, Angelos MG, Khan M, Chen CA, Khan M (2018) Extracellular Vesicles Released by Human Induced-Pluripotent Stem Cell-Derived Cardiomyocytes Promote Angiogenesis. Front Physiol 9:1794.
Dragoo JL, Chang W (2017) Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells From the Infrapatellar Fat Pad. Am J Sports Med 45:3119-3127.
El-Sherbiny IM, Yacoub MH (2013) Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract 2013:316-342.
Emmert MY, Wolint P, Jakab A, Sheehy SP, Pasqualini FS, Nguyen TDL, Hilbe M, Seifert B, Weber B, Brokopp CE, Macejovska D, Caliskan E, von Eckardstein A, Schwartlander R, Vogel V, Falk V, Parker KK, Gyongyosi M, Hoerstrup SP (2017) Safety and efficacy of cardiopoietic stem cells in the treatment of post-infarction left-ventricular dysfunction - From cardioprotection to functional repair in a translational pig infarction model. Biomaterials 122:48-62.
Fernandez-Aviles F et al. (2018) Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ Res 123:579-589.
Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP (2015) Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 61:339-348.
Galvez-Monton C, Bragos R, Soler-Botija C, Diaz-Guemes I, Prat-Vidal C, Crisostomo V, Sanchez-Margallo FM, Llucia-Valldeperas A, Bogonez-Franco P, Perea-Gil I, Roura S, Bayes-Genis A (2017) Noninvasive Assessment of an Engineered Bioactive Graft in Myocardial Infarction: Impact on Cardiac Function and Scar Healing. Stem Cells Transl Med 6:647-655.
Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J (2018) Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 137:1712-1730.
Gardin C, Ferroni L, Latremouille C, Chachques JC, Mitrecic D, Zavan B (2020) Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 9.
Gentillon C, Li D, Duan M, Yu WM, Preininger MK, Jha R, Rampoldi A, Saraf A, Gibson GC, Qu CK, Brown LA, Xu C (2019) Targeting HIF-1alpha in combination with PPARalpha activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol 132:120-135.
Giacomelli E et al. (2020) Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 26:862-879 e811.
Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ (2006) Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20:661-669.
Grines CL, Serruys P, O'Neill WW (2003) Fibrinolytic therapy: is it a treatment of the past? Circulation 107:2538-2542.
Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, Gershlak JR, Okamoto T, Gonzalez G, Milan DJ, Gaudette GR, Ott HC (2016) Bioengineering Human Myocardium on Native Extracellular Matrix. Circ Res 118:56-72.
He S, Wu J, Li SH, Wang L, Sun Y, Xie J, Ramnath D, Weisel RD, Yau TM, Sung HW, Li RK (2020) The conductive function of biopolymer corrects myocardial scar conduction blockage and resynchronizes contraction to prevent heart failure. Biomaterials 258:120285.
Ho CM, Mishra A, Lin PT, Ng SH, Yeong WY, Kim YJ, Yoon YJ (2017) 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol Biosci 17.
Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ (2016) Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res 118:95-107.
Homma J, Shimizu S, Sekine H, Matsuura K, Shimizu T (2020) A novel method to align cells in a cardiac tissue-like construct fabricated by cell sheet-based tissue engineering. J Tissue Eng Regen Med.
Hosoyama K, Ahumada M, McTiernan CD, Davis DR, Variola F, Ruel M, Liang W, Suuronen EJ, Alarcon EI (2018) Nanoengineered Electroconductive Collagen-Based Cardiac Patch for Infarcted Myocardium Repair. ACS Appl Mater Interfaces 10:44668-44677.
Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112:I150-156.
Hu X et al. (2016) A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ Res 118:970-983.
Huang K, Hu S, Cheng K (2019) A New Era of Cardiac Cell Therapy: Opportunities and Challenges. Adv Healthc Mater 8:e1801011.
Huang K, Ozpinar EW, Su T, Tang J, Shen D, Qiao L, Hu S, Li Z, Liang H, Mathews K, Scharf V, Freytes DO, Cheng K (2020) An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med 12.
Huang Z, Shen Y, Sun A, Huang G, Zhu H, Huang B, Xu J, Song Y, Pei N, Ma J, Yang X, Zou Y, Qian J, Ge J (2013) Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res Ther 4:149.
Ja KP, Lim KP, Chen A, Ting S, Li SQ, Tee N, Ramachandra C, Mehta A, Wong P, Oh S, Shim W (2018) Construction of a vascularized hydrogel for cardiac tissue formation in a porcine model. J Tissue Eng Regen Med 12:e2029-e2038.
Ja KP, Miao Q, Zhen Tee NG, Lim SY, Nandihalli M, Ramachandra CJA, Mehta A, Shim W (2016) iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J Cell Mol Med 20:323-332.
Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, Bursac N (2018) Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159:48-58.
Jackson AO, Rahman GA, Yin K, Long S (2020) Enhancing Matured Stem-Cardiac Cell Generation and Transplantation: A Novel Strategy for Heart Failure Therapy. J Cardiovasc Transl Res.
Jakab K, Norotte C, Damon B, Marga F, Neagu A, Besch-Williford CL, Kachurin A, Church KH, Park H, Mironov V, Markwald R, Vunjak-Novakovic G, Forgacs G (2008) Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A 14:413-421.
Jiang Y, Sun SJ, Zhen Z, Wei R, Zhang N, Liao SY, Tse HF (2021) Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction. Stem Cell Res Ther 12:13.
Joshi J, Kothapalli CR (2015) Nanofibers based tissue engineering and drug delivery approaches for myocardial regeneration. Curr Pharm Des 21:2006-2020.
Jugdutt BI (2009) Limiting fibrosis after myocardial infarction. N Engl J Med 360:1567-1569.
Karantalis V et al. (2014) Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 114:1302-1310.
Kastrup J, Haack-Sorensen M, Juhl M, Harary Sondergaard R, Follin B, Drozd Lund L, Monsted Johansen E, Ali Qayyum A, Bruun Mathiasen A, Jorgensen E, Helqvist S, Jorgen Elberg J, Bruunsgaard H, Ekblond A (2017) Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl Med 6:1963-1971.
Kawamura T, Miyagawa S, Fukushima S, Maeda A, Kashiyama N, Kawamura A, Miki K, Okita K, Yoshida Y, Shiina T, Ogasawara K, Miyagawa S, Toda K, Okuyama H, Sawa Y (2016) Cardiomyocytes Derived from MHC-Homozygous Induced Pluripotent Stem Cells Exhibit Reduced Allogeneic Immunogenicity in MHC-Matched Non-human Primates. Stem Cell Reports 6:312-320.
Kc P, Hong Y, Zhang G (2019) Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: advantages and challenges. Regen Biomater 6:185-199.
Khalili AA, Ahmad MR (2015) A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int J Mol Sci 16:18149-18184.
Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, Lee MG, Kang WY, Lee KS, Ahn YK, Jeong MH, Kim HS (2018) Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther 32:329-338.
Laguna G, S DIS, Maroto L, Fulquet E, Echevarria JR, Revilla A, Uruena N, Sevilla T, Arnold R, Ramos B, Gutierrez H, Serrador A, San Roman JA (2018) Effect of direct intramyocardial autologous stem cell grafting in the sub-acute phase after myocardial infarction. J Cardiovasc Surg (Torino) 59:259-267.
Lakshmanan R, Krishnan UM, Sethuraman S (2012) Living cardiac patch: the elixir for cardiac regeneration. Expert Opin Biol Ther 12:1623-1640.
Leask A (2015) Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 116:1269-1276.
Lee JR, Park BW, Kim J, Choo YW, Kim HY, Yoon JK, Kim H, Hwang JW, Kang M, Kwon SP, Song SY, Ko IO, Park JA, Ban K, Hyeon T, Park HJ, Kim BS (2020) Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv 6:eaaz0952.
Lepperhof V, Polchynski O, Kruttwig K, Bruggemann C, Neef K, Drey F, Zheng Y, Ackermann JP, Choi YH, Wunderlich TF, Hoehn M, Hescheler J, Saric T (2014) Bioluminescent imaging of genetically selected induced pluripotent stem cell-derived cardiomyocytes after transplantation into infarcted heart of syngeneic recipients. PLoS One 9:e107363.
Lesneski L (2010) Factors influencing treatment delay for patients with acute myocardial infarction. Appl Nurs Res 23:185-190.
Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M (2010) Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol 299:H1772-1781.
Liang S, Zhang Y, Wang H, Xu Z, Chen J, Bao R, Tan B, Cui Y, Fan G, Wang W, Wang W, Liu W (2018) Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv Mater 30:e1704235.
Lin Z, Pu WT (2014) Strategies for cardiac regeneration and repair. Sci Transl Med 6:239rv231.
Lipsitz YY, Timmins NE, Zandstra PW (2016) Quality cell therapy manufacturing by design. Nat Biotechnol 34:393-400.
Liu YW et al. (2018) Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36:597-605.
Loh QL, Choong C (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 19:485-502.
Madonna R et al. (2019) ESC Working Group on Cellular Biology of the Heart: position paper for Cardiovascular Research: tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc Res 115:488-500.
Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J (2020) Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail 22:884-892.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J (2015) Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 36:2011-2017.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J (2018) Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 71:429-438.
Miyagawa S, Domae K, Yoshikawa Y, Fukushima S, Nakamura T, Saito A, Sakata Y, Hamada S, Toda K, Pak K, Takeuchi M, Sawa Y (2017) Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J Am Heart Assoc 6.
Morishima Y, Morishima S, Murata M, Arima N, Uchida N, Sugio Y, Takahashi S, Matsuhashi Y, Onizuka M, Eto T, Nagafuji K, Onishi Y, Inoue M, Atsuta Y, Fukuda T, Ichinohe T, Kato S, Kanda J (2020) Impact of Homozygous Conserved Extended HLA Haplotype on Single Cord Blood Transplantation: Lessons for Induced Pluripotent Stem Cell Banking and Transplantation in Allogeneic Settings. Biol Blood Marrow Transplant 26:132-138.
Mosadegh B, Xiong G, Dunham S, Min JK (2015) Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater 10:034002.
Munarin F, Kant RJ, Rupert CE, Khoo A, Coulombe KLK (2020) Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 251:120033.
Naseri MH et al. (2018) COMPARE CPM-RMI Trial: Intramyocardial Transplantation of Autologous Bone Marrow-Derived CD133+ Cells and MNCs during CABG in Patients with Recent MI: A Phase II/III, Multicenter, Placebo-Controlled, Randomized, Double-Blind Clinical Trial. Cell J 20:267-277.
Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R, Stamm C (2014) Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 35:1263-1274.
Nguyen PK, Neofytou E, Rhee JW, Wu JC (2016) Potential Strategies to Address the Major Clinical Barriers Facing Stem Cell Regenerative Therapy for Cardiovascular Disease: A Review. JAMA Cardiol 1:953-962.
Nicolau JC, Furtado RHM, Silva SA, Rochitte CE, Rassi A, Jr., Moraes J, Jr., Quintella E, Costantini CR, Korman APM, Mattos MA, Castello HJ, Jr., Caixeta A, Dohmann HFR, de Carvalho ACC, MiHeart AMII (2018) Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double-blind randomized trial. Clin Cardiol 41:392-399.
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA (2008) Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14:213-221.
Paitazoglou C, Bergmann MW, Vrtovec B, Chamuleau SAJ, van Klarenbosch B, Wojakowski W, Michalewska-Wludarczyk A, Gyongyosi M, Ekblond A, Haack-Sorensen M, Jaquet K, Vrangbaek K, Kastrup J, Investigators S (2019) Rationale and design of the European multicentre study on Stem Cell therapy in IschEmic Non-treatable Cardiac diseasE (SCIENCE). Eur J Heart Fail 21:1032-1041.
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ (2019) Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun 10:3123.
Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5:3935.
Perea-Gil I, Galvez-Monton C, Prat-Vidal C, Jorba I, Segu-Verges C, Roura S, Soler-Botija C, Iborra-Egea O, Revuelta-Lopez E, Fernandez MA, Farre R, Navajas D, Bayes-Genis A (2018) Head-to-head comparison of two engineered cardiac grafts for myocardial repair: From scaffold characterization to pre-clinical testing. Sci Rep 8:6708.
Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD (2015) A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res 117:576-584.
Prasad CK, Krishnan LK (2008) Regulation of endothelial cell phenotype by biomimetic matrix coated on biomaterials for cardiovascular tissue engineering. Acta Biomater 4:182-191.
Prat-Vidal C et al. (2020) First-in-human PeriCord cardiac bioimplant: Scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine 54:102729.
Puig-Sanvicens VA, Semino CE, Zur Nieden NI (2015) Cardiac differentiation potential of human induced pluripotent stem cells in a 3D self-assembling peptide scaffold. Differentiation 90:101-110.
Qasim M, Haq F, Kang MH, Kim JH (2019a) 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int J Nanomedicine 14:1311-1333.
Qasim M, Arunkumar P, Powell HM, Khan M (2019b) Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci 229:233-250.
Qayyum AA, Mathiasen AB, Helqvist S, Jorgensen E, Haack-Sorensen M, Ekblond A, Kastrup J (2019) Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J Transl Med 17:360.
Querdel E et al. (2021) Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation.
Radhakrishnan J, Krishnan UM, Sethuraman S (2014) Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv 32:449-461.
Ramachandra CJA, Chua J, Cong S, Kp MMJ, Shim W, Wu JC, Hausenloy DJ (2021) Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies. Cardiovasc Res 117:694-711.
Ramachandra CJA, Mehta A, Wong P, Ja K, Fritsche-Danielson R, Bhat RV, Hausenloy DJ, Kovalik JP, Shim W (2018) Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes. Int J Cardiol 272:288-297.
Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S, Ramalingam M (2017) Development of decellularized scaffolds for stem cell-driven tissue engineering. J Tissue Eng Regen Med 11:942-965.
Rastogi P, Kandasubramanian B (2019) Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 11:042001.
Raval AN, Johnston PV, Duckers HJ, Cook TD, Traverse JH, Altman PA, Dhingra R, Hematti P, Borrello I, Anderson RD, Pepine CJ (2021) Point of care, bone marrow mononuclear cell therapy in ischemic heart failure patients personalized for cell potency: 12-month feasibility results from CardiAMP heart failure roll-in cohort. Int J Cardiol 326:131-138.
Reed GW, Rossi JE, Cannon CP (2017) Acute myocardial infarction. Lancet 389:197-210.
Roche ET, Hastings CL, Lewin SA, Shvartsman D, Brudno Y, Vasilyev NV, O'Brien FJ, Walsh CJ, Duffy GP, Mooney DJ (2014) Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials 35:6850-6858.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G (2018) Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556:239-243.
Saleh M, Ambrose JA (2018) Understanding myocardial infarction. F1000Res 7.
Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, Kobayashi E, Yamato M, Okano T (2011) Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A 17:2973-2980.
Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, Mahmoudi M, Malkovskiy AV, Rajadas J, Butte MJ, Bernstein D, Ruiz-Lozano P (2013) The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 34:9048-9055.
Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T (2006) Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J 20:708-710.
Shin B, Cowan DB, Emani SM, Del Nido PJ, McCully JD (2017) Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury. Adv Exp Med Biol 982:595-619.
Soetisna TW, Sukmawan R, Setianto B, Mansyur M, Murni TW, Listiyaningsih E, Santoso A (2020) Combined transepicardial and transseptal implantation of autologous CD 133+ bone marrow cells during bypass grafting improves cardiac function in patients with low ejection fraction. J Card Surg 35:740-746.
Solazzo M, O'Brien FJ, Nicolosi V, Monaghan MG (2019) The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering. APL Bioeng 3:041501.
Song C, Zhang X, Wang L, Wen F, Xu K, Xiong W, Li C, Li B, Wang Q, Xing MMQ, Qiu X (2019) An Injectable Conductive Three-Dimensional Elastic Network by Tangled Surgical-Suture Spring for Heart Repair. ACS Nano 13:14122-14137.
Song X, Wang X, Zhang J, Shen S, Yin W, Ye G, Wang L, Hou H, Qiu X (2021) A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials 273:120811.
Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G (2007) Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg 133:717-725.
Steinhoff G et al. (2017) Cardiac Function Improvement and Bone Marrow Response -: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133(+) Application After Myocardial Infarction. EBioMedicine 22:208-224.
Su T, Huang K, Daniele MA, Hensley MT, Young AT, Tang J, Allen TA, Vandergriff AC, Erb PD, Ligler FS, Cheng K (2018) Cardiac Stem Cell Patch Integrated with Microengineered Blood Vessels Promotes Cardiomyocyte Proliferation and Neovascularization after Acute Myocardial Infarction. ACS Appl Mater Interfaces 10:33088-33096.
Sugiura T, Hibino N, Breuer CK, Shinoka T (2016) Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardiomyocytes promoted the regeneration of host cardiomyocytes in a rat model. J Cardiothorac Surg 11:163.
Sun X, Wu J, Qiang B, Romagnuolo R, Gagliardi M, Keller G, Laflamme MA, Li RK, Nunes SS (2020) Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci Transl Med 12.
Suncion VY et al. (2014) Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res 114:1292-1301.
Surder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, Soncin S, Turchetto L, Radrizzani M, Zuber M, Windecker S, Moschovitis A, Buhler I, Kozerke S, Erne P, Luscher TF, Corti R (2016) Effect of Bone Marrow-Derived Mononuclear Cell Treatment, Early or Late After Acute Myocardial Infarction: Twelve Months CMR and Long-Term Clinical Results. Circ Res 119:481-490.
Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K (2006) Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 291:H886-893.
Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, Hensley MT, Caranasos TG, Zhang J, Gu Z, Cheng K (2018) Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv 4:eaat9365.
Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, Zhao DXM, Ellis SG, Forder JR, Perin EC, Penn MS, Hatzopoulos AK, Chambers JC, Baran KW, Raveendran G, Gee AP, Taylor DA, Moye L, Ebert RF, Simari RD (2018) TIME Trial: Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ Res 122:479-488.
Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP (2003) Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 361:47-49.
Uemura R, Xu M, Ahmad N, Ashraf M (2006) Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98:1414-1421.
Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, Funcke S, Murphy E, Eschenhagen T, Hansen A (2018) Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Reports 10:834-847.
van Berlo JH, Molkentin JD (2014) An emerging consensus on cardiac regeneration. Nat Med 20:1386-1393.
van der Spoel TI, Vrijsen KR, Koudstaal S, Sluijter JP, Nijsen JF, de Jong HW, Hoefer IE, Cramer MJ, Doevendans PA, van Belle E, Chamuleau SA (2012) Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: a study on delivery efficiency. J Cell Mol Med 16:2768-2776.
Walker BW, Lara RP, Yu CH, Sani ES, Kimball W, Joyce S, Annabi N (2019) Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials 207:89-101.
Wang WE, Yang D, Li L, Wang W, Peng Y, Chen C, Chen P, Xia X, Wang H, Jiang J, Liao Q, Li Y, Xie G, Huang H, Guo Y, Ye L, Duan DD, Chen X, Houser SR, Zeng C (2013) Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ Res 113:288-300.
Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang JJ (2014) Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A 20:1325-1335.
Wollert KC et al. (2017) Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J 38:2936-2943.
Wu SZ, Li YL, Huang W, Cai WF, Liang J, Paul C, Jiang L, Wu ZC, Xu M, Zhu P, Wang Y (2017) Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem Funct 35:113-123.
Xu JY et al. (2019) Transplantation efficacy of autologous bone marrow mesenchymal stem cells combined with atorvastatin for acute myocardial infarction (TEAM-AMI): rationale and design of a randomized, double-blind, placebo-controlled, multi-center, Phase II TEAM-AMI trial. Regen Med 14:1077-1087.
Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, Baba S, Shigemoto T, Kuroda Y, Kanamori H, Amin M, Kawasaki M, Nishigaki K, Taoka M, Isobe T, Muramatsu C, Dezawa M, Minatoguchi S (2018) S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ Res 122:1069-1083.
Yan W, Guo Y, Tao L, Lau WB, Gan L, Yan Z, Guo R, Gao E, Wong GW, Koch WL, Wang Y, Ma XL (2017) C1q/Tumor Necrosis Factor-Related Protein-9 Regulates the Fate of Implanted Mesenchymal Stem Cells and Mobilizes Their Protective Effects Against Ischemic Heart Injury via Multiple Novel Signaling Pathways. Circulation 136:2162-2177.
Yau TM et al. (2019) Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial. JAMA 321:1176-1186.
Ye L, Zimmermann WH, Garry DJ, Zhang J (2013) Patching the heart: cardiac repair from within and outside. Circ Res 113:922-932.
Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J (2014) Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15:750-761.
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121-1135.
Yeung E, Fukunishi T, Bai Y, Bedja D, Pitaktong I, Mattson G, Jeyaram A, Lui C, Ong CS, Inoue T, Matsushita H, Abdollahi S, Jay SM, Hibino N (2019) Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J Tissue Eng Regen Med 13:2031-2039.
Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K, Sawa Y (2018) Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol Ther 26:2681-2695.
Yoshida S, Miyagawa S, Toyofuku T, Fukushima S, Kawamura T, Kawamura A, Kashiyama N, Nakamura Y, Toda K, Sawa Y (2020) Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation. Sci Rep 10:4593.
Zhang C, Hsieh MH, Wu SY, Li SH, Wu J, Liu SM, Wei HJ, Weisel RD, Sung HW, Li RK (2020) A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials 231:119672.
Zhang J (2015) Engineered Tissue Patch for Cardiac Cell Therapy. Curr Treat Options Cardiovasc Med 17:399.
Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G (2018) Can We Engineer a Human Cardiac Patch for Therapy? Circ Res 123:244-265.
Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K (2021) Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun 12:1412.
Zhu K et al. (2018) Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ Res 122:958-969.
Zuo S, Jones WK, Li H, He Z, Pasha Z, Yang Y, Wang Y, Fan GC, Ashraf M, Xu M (2012) Paracrine effect of Wnt11-overexpressing mesenchymal stem cells on ischemic injury. Stem Cells Dev 21:598-608.
References
Shuo Cong1-3*
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. Author affiliation. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.3Yong Loo Lin Medical School, National University of Singapore, Singapore.
Jasper Chua4*
4National Dental Research Institute Singapore, National Dental Centre Singapore, Singapore。
Sauri Hernandez-Resendiz1,2#
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. Author affiliation. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.
*Both authors contributed equally to this article.
Corresponding author:
Dr. Sauri Hernandez-Resendiz
Email: sauri.hdz@duke-nus.edu.sgl

In a new window | Download PPT
Figure 1: Schematic description of the mechanistic action of cardiac patch. The major mechanisms include (A) providing structural support, (B) promoting direct myocardial remuscularization, and (C) producing cardioprotective paracrine factors. (A) Since a thin fibrotic scar replaces the myocardium at the infarct zone, the rigid scaffold of the cardiac patch provides additional wall thickness and rigidity, which could enhance myocardial function while preventing detrimental left ventricular remodeling. (B) With the tremendous loss of cardiomyocytes following AMI, early studies focus on regenerating the injured myocardium by introducing cells with therapeutic properties. While there were optimistic results that implanted cells were able to reduce the infarct size and stimulate vascularization, there were also contrasting results that showed partial remuscularization with suboptimal biophysical integration of the cardiac patch. Currently, more evidence is attributing the cardioprotective effects of cardiac patch to the paracrine factors produced by the implanted cells. (C) The implanted cells such as adult stem cells which secrete a variety of paracrine factors which is capable of altering the myocardial microenvironment and influence myocardial remodeling.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6714 | 12 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA