Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Stem cell metabolism: from embryonic development to stem cell differentiation
Time:2021-06-08
Number:7187
Jarmon G. Lees, Shiang Y. Lim, David K. Gardner, Alexandra J. Harvey
Author Affiliations


- 1O’Brien Institute Department, St. Vincent’s Institute of Medical Research, VIC, Australia.
- 2Department of Medicine, Melbourne Medical School, University of Melbourne, VIC, Australia.
- 3Department of Surgery, Melbourne Medical School, University of Melbourne, VIC, Australia.
- 4School of BioSciences, University of Melbourne, VIC, Australia.
Conditioning Medicine 2021. 4(2): 88-99.
Abstract
Transitions through pluripotent stem cell states, differentiation, and reprogramming, involve substantial metabolic remodeling. These metabolic transitions are only just beginning to be understood, often relying on transcriptional data to infer metabolism, rather than actual metabolic outputs. Here, we consider the metabolism of the early embryo through development, and look at the nutrient milieu within the developing stem cell niche. We discuss what is known about the distinct metabolic states captured in vitro by the 2-cell-like, naïve, blastocyst-like, formative, and primed states of pluripotency. We explore the recently described metabolic surge event that occurs as pluripotency is lost and stem cells commit to differentiate. Finally, we explore the most recent understanding of germ layer-specific metabolic remodeling. To establish protocols for the safe and efficient differentiation of healthy cells for therapies, we must develop a better understanding of the dynamic continuum of metabolic states that span pluripotency and differentiation, and how to influence them.
Keywords: Stem cells, Pluripotency, Metabolism, Germ layers, Embryo, Differentiation
Abstract
Transitions through pluripotent stem cell states, differentiation, and reprogramming, involve substantial metabolic remodeling. These metabolic transitions are only just beginning to be understood, often relying on transcriptional data to infer metabolism, rather than actual metabolic outputs. Here, we consider the metabolism of the early embryo through development, and look at the nutrient milieu within the developing stem cell niche. We discuss what is known about the distinct metabolic states captured in vitro by the 2-cell-like, naïve, blastocyst-like, formative, and primed states of pluripotency. We explore the recently described metabolic surge event that occurs as pluripotency is lost and stem cells commit to differentiate. Finally, we explore the most recent understanding of germ layer-specific metabolic remodeling. To establish protocols for the safe and efficient differentiation of healthy cells for therapies, we must develop a better understanding of the dynamic continuum of metabolic states that span pluripotency and differentiation, and how to influence them.
Keywords: Stem cells, Pluripotency, Metabolism, Germ layers, Embryo, Differentiation
Introduction
Metabolism is central to pluripotency and differentiation, with lasting impacts on derived cell identity through regulation of the epigenetic landscape (Donohoe and Bultman, 2012; Harvey et al., 2016; Harvey et al., 2019; Wu et al., 2019). Akin to the dynamic nutrient requirements of the developing embryo, discrete in vitro cell states have distinct metabolic profiles (Zhou et al., 2012; Cliff and Dalton, 2017), with nutrients capable of inducing and maintaining alternate cell states (Shyh-Chang et al., 2013; Shiraki et al., 2014; Moussaieff et al., 2015; Lees et al., 2020). This places metabolism at the forefront of development and cell state decisions. The developing embryo, from which pluripotent stem cells originate, undergoes a series of dynamic metabolic transitions synchronized to its molecular development. It is only through understanding embryonic metabolism and development that we can derive and maintain different in vitro stem cell states for disease modeling and therapies.
Early embryo development
The fertilized oocyte is totipotent, with resultant blastomeres capable of generating all three primary germ layers, ectoderm, mesoderm, and endoderm, in addition to the extra embryonic tissues. Up to the 2-cell embryo, blastomeres remain totipotent (Garner and McLaren, 1974). In the mouse, a transporting epithelium is established around the 8-16-cell stage through a process known as compaction where cell definition is lost and the outer cells of the embryo form tight junctions, giving rise to the blastocyst. The blastocyst comprises the inner cell mass (ICM), which gives rise to the three primary germ layers and consequently the fetus, and the trophectoderm (TE), which gives rise to the extraembryonic and placental tissue. It is from the ICM cells that embryonic stem cells (ESCs) can be isolated, expanded in culture, and differentiated for downstream applications including disease modeling and cell therapies.
The molecular signature of mouse and human stem cells
Isolated mouse ESCs retain the in vivo pluripotency of their parent ICM cells indicated by a lack of lineage priming genes, and are able to contribute to chimeric animals and form teratocarcinomas composed of representatives of all three germ layers (Gardner, 1968; Evans and Kaufman, 1981). Mouse ESCs are proposed to be equivalent to the E3.5 – 4.5 ICM (Nichols and Smith, 2012), supported in culture by leukemia inhibitory factory (LIF) activation of the Janus kinase/signal transducers and activators of transcriptions (JAK-STAT3) signaling pathway (Boeuf et al., 1997). Stimulation of extracellular signal-regulated kinase (Erk) and glycogen synthase kinase 3-beta (GSK3β) pathways in mouse ESCs, transitions them towards the primed mouse ESC state. The primed state is a stable pluripotent state in culture, where cells are more permissive to differentiation signals, equivalent to the E6.5 epiblast, termed mouse epiblast stem cells (EpiSCs) (Tesar et al., 2007). Comprehensive reviews have covered the transcriptional and epigenetic differences between mouse EpiSCs derived from the post-implantation epiblast and mouse ESCs (Brons et al., 2007; Tesar et al., 2007). Mouse EpiSCs derived from pre-gastrula to late gastrula stages and from different tissues of origin display similar global transcriptional profiles (Kojima et al., 2014). This suggests that current culture conditions are woefully inadequate to sustain the complex continuum of pluripotent stem cell states. The human ESC state is analogous to the mouse EpiSC state, representing a primed stage of development. The primed state is replicated by human induced pluripotent stem cells (iPSCs; somatic cells that have been reprogrammed to a pluripotent state) and resemble primed human ESCs in their capacity for self-renewal, in vitro differentiation potential, and surprisingly, can contribute to viable chimeras in mice, where mouse EpiSCs rarely can (Okita et al., 2007).
The in vitro naïve state in the mouse corresponds to the Nanog positive cells within the ICM at E3.5 during blastocyst formation (Silva et al., 2009). Naïve human ESCs have been isolated in culture from primed human ESCs using combinations of GSK and MEK/ERK inhibitors (2i), LIF and Activin (Hanna et al., 2010; Theunissen et al., 2014) among others (Gafni et al., 2013; Takashima et al., 2014; Ware et al., 2014; O'Reilly et al., 2019), with no consistent protocol identified for their generation. However, all these conditions fail to establish a human naïve state equivalent to the mouse as they do not incorporate efficiently into chimeras, although a modest increase in efficiency can be achieved by depleting cells of p53 tumour suppressor protein (Bayerl et al., 2021). Indeed, the human naïve state requires a more complex suite of pathways to be simultaneously inhibited than other species, which has given rise to sophisticated reporter screening strategies to uncover its molecular basis (Theunissen et al., 2014; Bayerl et al., 2021). Measuring our success in obtaining embryonic-like stem cell states against valuable human embryos will continue to be a challenging but necessary process. This raises the question of what indicators are most appropriate, acknowledging that core transcription factors such as Oct4, Nanog, and Sox2 are too robustly expressed across stem cell states to properly discriminate along the continuum (Davidson et al., 2015). Instead, changes to metabolism and the epigenome could potentially be employed as reliable outcome measures for bona fide embryonic-like stem cell states. Metabolism is central to pluripotency and responds rapidly to changes in culture, with lasting impacts on cell state through regulation of the epigenetic landscape (Donohoe and Bultman, 2012; Harvey et al., 2016; Harvey et al., 2019; Wu et al., 2019). Indeed, human naïve cells isolated directly from the ICM into culture establish a hypomethylated state relative to their parent ICM cells (Guo et al., 2016), indicating that current naïve culture conditions are insufficient for establishing a bona fide human naïve state in vitro.
Relatively recently, several other embryonic stages have been modeled with in vitro cultures including 2-cell-like cells, formative or intermediate stem cells, and blastocyst-like structures. Two-cell-like cells occur spontaneously in mouse ESCs cultured in 2i/LIF conditions (Macfarlan et al., 2012; Rodriguez-Terrones et al., 2020) and display similar molecular and chromatin features as the totipotent 2-cell blastomeres of the mouse embryo. However, 2-cell-like cells are not truly totipotent nor do they contain the maternal RNAs and proteins of their in vivo counterparts (Genet and Torres-Padilla, 2020). While a promising tool to study this rare developmental stage, 2-cell-like cells have not yet been isolated in the human and are not capable of self-renewal. Formative stem cells describe an intermediate state of pluripotency between the naïve and primed states (Smith, 2017). Significantly, and unlike mouse ESCs or EpiSCs, formative stem cells can give rise to primordial germ cells, and transcriptional and epigenetic analyses match them to the pre-gastrulation formative epiblast (Kinoshita et al., 2021; Wang et al., 2021).
The earliest attempts to generate blastocyst-like structures were disorganized embryoid bodies made from the spontaneous differentiation of mouse ESCs (Doetschman et al., 1985). Bioengineering strategies were then employed attempting to replicate the structure of a blastocyst using 3D matrices (Poh et al., 2014) and micropatterned surfaces (Warmflash et al., 2014). The most recent advances have come through aggregating mouse ESCs, trophoblast stem cells, and/or extra-embryonic endoderm stem cells, harnessing the cross-talk between these stem cell types to drive embryo-like morphogenesis (Harrison et al., 2017; Sozen et al., 2018; Li et al., 2019; Zhang et al., 2019). Blastocyst-like structures have since been derived from human naïve stem cells (Yu et al., 2021), and from both mouse and human reprogrammed somatic cells by either transitioning through a 2-cell-like state (Li et al., 2019) or exploiting the inherent heterogeneity of the reprogramming process (Liu et al., 2021b). These blastocyst-like structures, or blastoids, model the general architecture of the blastocyst including a blastocoel-like cavity and TE-like and ICM-like cells, and can undergo implantation in utero (Harrison et al., 2017; Sozen et al., 2018; Li et al., 2019; Zhang et al., 2019; Liu et al., 2021b; Yu et al., 2021). Single cell RNA sequencing of blastocyst-like structures against human blastocysts has matched them to both pre-implantation (Yu et al., 2021) and post-implantation (Liu et al., 2021b) human embryonic states. However, while an amazing step towards being able to model mammalian embryo development in vitro, blastocyst-like structures are still in their infancy with reasonable questions surrounding their ability to make TE lineages (Zhao et al., 2021), their ability to model post-implantation events including primitive streak formation and gastrulation, the haphazard arrangement of tissues following implantation, a complete absence of any metabolic data, and the ethical question of whether the 14-day termination rule of embryonic development will need to apply to these structures as they approach embryonic-like developmental competency. Physiological oxygen (5%) greatly enhances the formation efficiency of the blastocoel-like cavity in blastocyst-like structures (Sozen et al., 2018), consistent with the extensively documented impact of physiological oxygen on embryo development (Wale and Gardner, 2010), stem cell differentiation (Burridge et al., 2011), and reprogramming (Spyrou et al., 2019b). The implementation of physiological levels of other nutrients, amino acids, and carbohydrates, which have been shown to be beneficial in embryo development and in vitro stem cell differentiation studies (Zhu et al., 2012; Wu et al., 2013; Bardy et al., 2015; Ermisch et al., 2020), is likely to support other challenging aspects of blastocyst-like development such as primitive streak formation and gastrulation. The utility of blastocyst-like structures as models of early human development will depend on our ability to pattern discrete developmental cell populations such as the TE at the earliest stages to subsequently achieve timely and organized development.
The epigenetic landscape of pluripotency
Epigenetic mechanisms are required during development to silence pluripotent genes and activate lineage-specific genes in a dynamic and temporal manner (Chen and Dent, 2014; Sirard, 2019). Human ESCs exhibit a euchromatic (accessible) and highly dynamic chromatin landscape (Boland et al., 2014) corresponding to elevated global transcriptional activity (Efroni et al., 2008). The combination of repressive H3K27me3 and active H3K3me3 at specific developmental genes, termed bivalent methylation, establishes a primed epigenetic state poised for differentiation (Bernstein et al., 2006; Voigt et al., 2013). Differentiation requires global changes in the epigenetic landscape, characterized by restricted gene expression, extensive regions of heterochromatin, H3K9me3 marks, and other repressive DNA methylation patterns (Hawkins et al., 2010; Harvey et al., 2019).
To establish and maintain this epigenetic landscape, epigenetic modifiers that regulate DNA methylation, histone modification, and chromatin organization are required. DNA methylation and demethylation are regulated by DNA methyltransferases and Ten-Eleven Translocation dioxygenases respectively (Wu and Zhang, 2014). Histone acetylation by histone acetyltransferases induces a euchromatic state and facilitates transcription while histone deacetylation, via histone deacetylases, is associated with transcriptional repression. Epigenetic modifiers also require specific metabolites as co-factors, which has been comprehensively reviewed by Donohoe and Bultman (2012) and Harvey et al., (2016). Specifically, S-adenosylmethionine, generated through one carbon metabolism and essential for mouse (Shyh-Chang et al., 2013) and human (Shiraki et al., 2014) ESC pluripotency, acts as the methyl donor for DNA and histone methylation. Tricarboxylic acid (TCA) cycle products α-ketoglutarate and succinate regulate Ten-Eleven Translocation activity (Xiao et al., 2012), and class III histone deacetylases activity is regulated by NAD+-dependent mechanisms (Cantó et al., 2015). Therefore, metabolic activity has the capacity to modulate the epigenome through the availability of these epigenetic modifiers (Harvey et al., 2016; Tsogtbaatar et al., 2020).
Metabolism as a regulator of embryo development and stem cell pluripotency
The nutrient environment and metabolism of the embryo
Lessons learned from embryonic development highlight the significance of establishing an appropriate metabolic environment, as sub-optimal nutrient compositions can significantly impair embryo development (Gardner and Lane, 1993; Gardner, 1998b; de Lima et al., 2020) and human birth rates (Meintjes et al., 2009; Waldenström et al., 2009). Prior to implantation, embryos develop within the reproductive tract as free-floating entities, reliant on nutrients and other signaling molecules contained within reproductive tract fluid for survival. The uterine and oviductal fluid in which the embryo and ICM cells develop, comprises a rich and complex mixture of proteins and metabolites (Leese, 1988) that changes throughout embryonic development to support the needs of the developing embryo (Gardner et al., 1996). One such dynamic nutrient is oxygen, which ranges from its highest point of ~9% in the oviduct (Fischer and Bavister, 1993) to ~5.3% in the uterus at the time of implantation, and falls to 2.5% as the blastocyst implants into the uterine wall. In the oxygen rich oviduct, the cleavage stage embryo undergoes aerobic oxidation of pyruvate and lactate (Figure 1) (Leese and Barton, 1985; Gardner et al., 1996). It is in the oviduct that activation of the embryonic genome occurs, at the 2-cell stage in the mouse, and at the 4- and 8-cell stage in the human. The 8-cell embryo and compacting morula encounter a higher glucose environment in the uterus (Gardner et al., 1996), which begins the transition to glucose oxidation for rapid ATP production. From the morula to the blastocyst stage, aerobic glycolysis nearly doubles (Obeidat et al., 2019) reflecting the biosynthetic and energetic requirements at this stage of development (Gardner, 1998a; Gardner and Harvey, 2015). The mouse blastocyst ICM (E3.5 – 4.5) is almost exclusively glycolytic as it begins proliferating and priming for epiblast and primitive endoderm lineages (Hewitson and Leese, 1993b; Houghton, 2006), while the TE cells convert approximately 50% of consumed glucose to lactate even in the presence of oxygen. In the uterine environment of ~5.3% oxygen, oxygen consumption by the TE cells increases 3-fold at the time of blastocyst formation, to provide the energy required as they expand to form and maintain the blastocoel cavity, as well as supplying the carbon moieties required for nucleic acid biosynthesis, and the reducing capacity for lipid synthesis (Gardner and Harvey, 2015). TE specification is uniquely controlled by glucose metabolism at this stage, not through characteristic ATP generation, but through the pentose phosphate pathway and hexosamine biosynthetic pathway control of transcription (Chi et al., 2020). Significantly, establishment of the TE during development is important as it allows a level of control over the internal environment of the blastocyst in which the ICM resides (Wicklow et al., 2014; Harvey, 2019).
Blastocyst implantation takes place at E5.0 and E7.0 in the mouse and human respectively, at which point the embryo resides in a low oxygen environment (~2.5%) until the syncytiotrophoblasts can invade the uterine wall to reach the spiral arterioles and promote maternal vascularisation (Genbacev et al., 1997; Caniggia et al., 2000). Oxygen consumption by the implanted embryo drops to pre-blastocyst levels (Houghton et al., 1996), and glycolysis becomes the primary metabolic pathway, together with extensive pentose phosphate pathway use to support proliferation (Clough and Whittingham, 1983). From E6.0 – 9.5, when the neural tube, neural crest, and brain vesicles are forming, mouse embryos are highly glycolytic, converting over 90% of consumed glucose to lactate (Clough and Whittingham, 1983). Glycolysis is important during the formation of the multi-layered gastrula from the ICM, as glucose phosphate isomerase-knockout mice develop abnormalities at E7.5 (Kelly and West, 1996). This exclusive reliance on anaerobic metabolism post-implantation indicates that in vivo development takes place in an anoxic environment (Rogers et al., 1982). The distinct and transient metabolic states exhibited by the developing embryo, and the consequences of perturbation, highlight how complex nutrient environments are required to maintain bona fide pluripotent states in vitro and support efficient differentiation.
Mitochondria in development
Mitochondria are the site of oxidative phosphorylation (OxPhos). Mitochondria are dynamic organelles that undergo fission and fusion events to produce fragmented and elongated mitochondrial phenotypes, respectively. These processes are tightly regulated by a group of highly conserved dynamin superfamily of large guanosine triphosphate hydrolases. These include mitofusin-1, mitofusin-2, and optical atrophy-1 for mitochondrial fusion; and dynamin related protein-1, mitochondrial fission-1, mitochondrial fission factor, mitochondrial dynamic protein of 49 kDa and 51 kDa for mitochondrial fission (Chen et al., 2012; Mishra and Chan, 2014). Throughout embryo development, mitochondria provide ATP for growth and maintain a signaling axis with the nucleus (Lees et al., 2017; Harvey, 2019). Mitochondrial morphology and localization is dynamic throughout development, reflecting the changing metabolic requirements of the embryo (Collins et al., 2002). Morphologies can range from round or spherical organelles, with electron sparse matrices and few peripheral arched cristae that have a relatively small inner mitochondrial membrane surface for the assembly of electron transport chain complexes, to long filamentous organelles with dense matrices, and many transverse cristae that maximize the surface area for OxPhos. Mitochondria form complexes with other organelles including smooth endoplasmic reticulum and vesicles in the post-ovulation oocyte to generate the necessary cellular components for fertilization (Motta et al., 2000). In response to increased bioenergetic demands by the cell, mitochondria undergo cristae and electron transport chain complex reorganization to maximize metabolic efficiency (Patten et al., 2014; Picard et al., 2015; Jarosz et al., 2017; Ghosh et al., 2018; Liu et al., 2021a). Mitochondria are perinuclear in ICM cells (Motta et al., 2000), likely a response to their high proliferative demands (Lees et al., 2017), and are dispersed throughout the cytoplasm in somatic cells (St John et al., 2005; Folmes et al., 2011). Mitochondrial dispersal occurs within 3–7 days of stem cell differentiation (St John et al., 2005; Facucho-Oliveira et al., 2007; Mandal et al., 2011). However, reprogramming somatic cells to a pluripotent state causes the mitochondria to reassume a perinuclear localization (Folmes et al., 2011) indicating a link between perinuclear localization and pluripotency (Lees et al., 2017).
Mitochondria in pluripotent stem cells
In vitro pluripotent stem cell mitochondria poorly recapitulate in vivo mitochondrial morphologies, which has likely contributed to the difficulty in achieving complete pluripotency or totipotency in vitro given the critical role of mitochondrial dynamics in pluripotency and differentiation (Zhong et al., 2019). While the 2-cell embryo has spherical electron dense mitochondria, 2-cell-like cell mitochondria more closely resemble blastocyst stage mitochondria (Sathananthan and Trounson, 2000; Lees et al., 2017) with elongated electron-poor matrices (Rodriguez-Terrones et al., 2020). Incomplete reprogramming of the mitochondria may contribute to the reduced developmental potential of 2-cell-like cells, which fail to contribute to chimeras. Naïve ESC mitochondrial morphology is round and vacuolated with few cristae compared to primed ESCs (Ware et al., 2014; Bahat et al., 2018). However, naïve human ESCs do not attain a mitochondrial morphology equivalent to that of in vivo human or mouse ICM cells, typified by a round/elongated mitochondrial complement with some developed cristae (Sathananthan and Trounson, 2000; Lees et al., 2017). Mitochondria of formative stem cells are small and round with sparse cristae, but with a high rate of oxygen consumption (Cornacchia et al., 2019) similar to the naïve state (Ware et al., 2014). On the other hand, primed state mitochondria have a sperical morphology with clear matrices and few arching cristae, which is in contrast to the elongated morphology found in the post-implantation blastocyst (Cho et al., 2006; Varum et al., 2011; Lees et al., 2019a). This chaotic picture, in which none of the in vitro mitochondrial morphologies completely recapitulate in vivo mitochondrial morphologies indicates that metabolic reprogramming (Harvey et al., 2018; Spyrou et al., 2019a), like molecular reprogramming, has not been faithfully accomplished.
Pluripotent stem cell metabolism
Just as the molecular signatures of 2-cell-like naïve, formative, and primed stem cell states differ, so too do their metabolic states and nutrient requirements (Figure 1). Similar to the 2-cell embryo, 2-cell-like cells rely on the oxidation of pyruvate and lactate for energy (Kaneko, 2016; Leese and Barton, 1984; Rodriguez-Terrones et al., 2020), and generally exhibit a quiescent metabolism with low levels of both glycolytic and mitochondrial OxPhos (Hu et al., 2020; Rodriguez-Terrones et al., 2020).
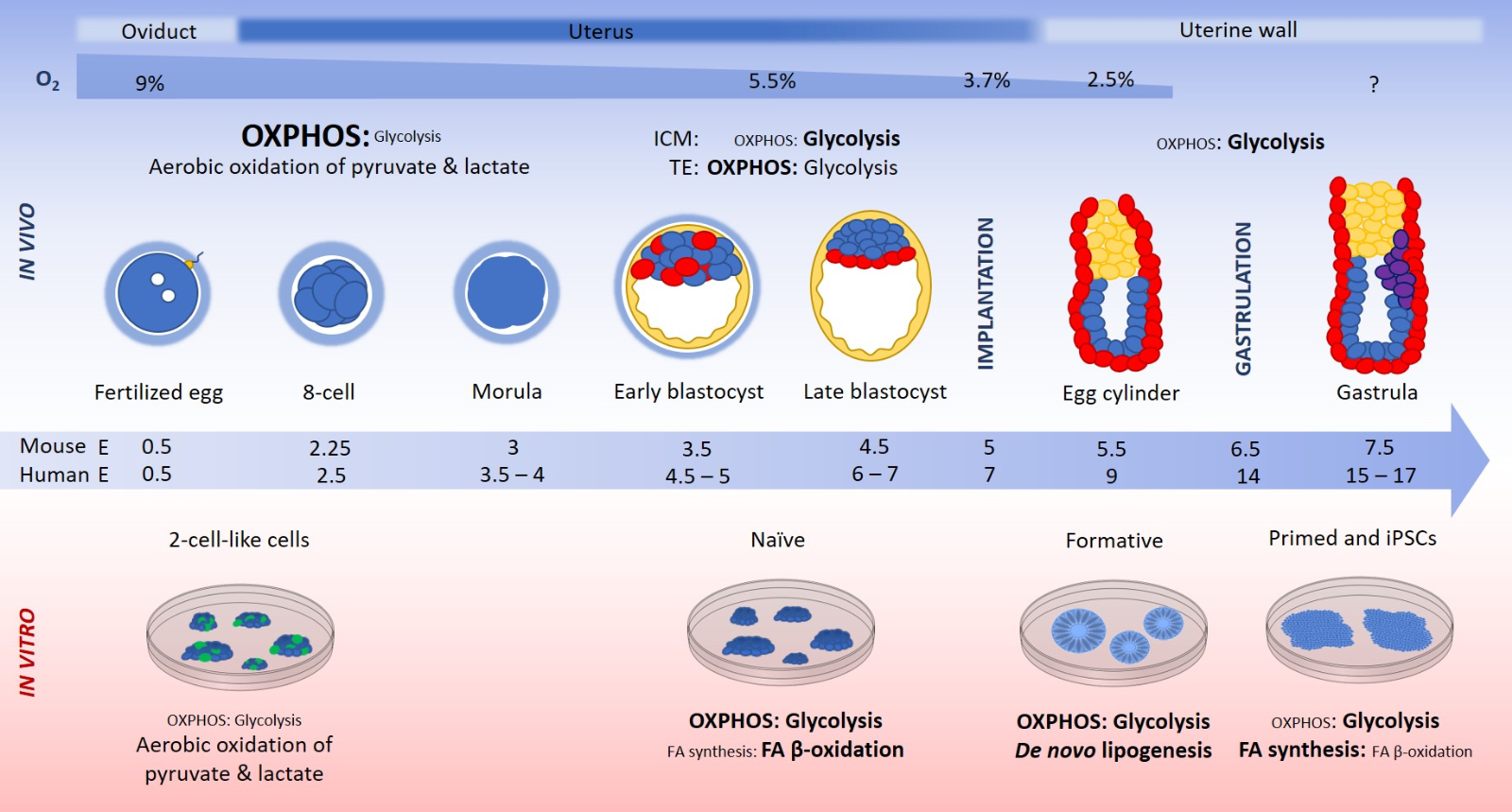
In a new window | Download PPT
Figure 1: Fertilization of the mammalian egg occurs at embryonic day (E) 0.5 in the oviduct, which resides at ~9% oxygen (Fischer and Bavister, 1993). The cleavage stage embryo is surrounded by the zona pellucida (blue cloud), a glycoprotein coat that protects against polyspermy and premature hatching, and the cumulus cells, which generate pyruvate and lactate from glucose, creating a pyruvate- and lactate-rich environment for the cleavage stage embryo (Leese and Barton, 1985; Gardner et al., 1996). The metabolism of the cleavage stage embryo consists primarily of the aerobic oxidation of pyruvate and lactate (Gardner and Harvey, 2015). Zygotic/embryonic genome activation occurs in the mouse 2-cell embryo, and at the 4- and 8-cell stage in the human. Cleavage division to the 8-cell stage and the compaction of the morula occur in the uterus, which has a relatively higher glucose concentration (3.15 mM) than that of the oviduct (0.5 mM) (Gardner et al., 1996). The higher glucose concentration in the uterus, and the declining concentrations of pyruvate and lactate, coincide with the increased demand for ATP, which alleviates the block in glycolysis present in the cleavage stages, and signals the switch to a primarily glucose driven metabolism at the blastocyst stage. Blastocyst formation takes place in the uterus under ~5.3% oxygen (Maas et al., 1976) at E3.5 in the mouse and E5 in the human, when the trophectoderm (TE; yellow) cells expand to encompass the blastocoel cavity, and the inner cell mass (ICM; blue) cells begin random priming for epiblast (blue) and primitive endoderm linages (red) (Chazaud et al., 2006). Oxygen consumption increases 3-fold at the blastocyst stage, its highest during embryonic development (Houghton et al., 1996), in order to create the ATP required for blastocoel formation. The blastocyst comprises two discrete cell types, the ICM and the TE, each with distinct nutritional requirements and metabolism. In the mouse, the TE converts ~50% of consumed glucose to lactate, while the ICM is almost exclusively glycolytic, converting most consumed glucose to lactate (Hewitson and Leese, 1993a). Oxygen consumption by the TE cells increases after compaction, and mitochondria develop from spherical organelles into elongated morphologies with transverse cristae (Lees et al., 2017). Implantation occurs at E5 and E7 in the mouse and human respectively, at which time the oxygen concentration drops within the uterus to 3.7% (Maas et al., 1976). During implantation, synctiotrophoblasts invade the uterine wall, recorded at ~2.5% oxygen (Genbacev et al., 1997; Caniggia et al., 2000). Following implantation, the polar TE cells proliferate to form the extraembryonic ectoderm (yellow), which will contribute to the embryonic part of the placenta. Oxygen consumption by the egg cylinder stage embryo drops to pre-blastocyst levels (Houghton et al., 1996), and glycolysis becomes the primary metabolic pathway together with extensive pentose phosphate pathway use for proliferation (Clough and Whittingham, 1983). Gastrulation occurs at E6.5 and E14 in the mouse and human respectively giving rise to the gastrula and the formation of the primitive streak and primordial germ cells (purple). In the mouse, in vitro derived ESCs from the E3.5 ICM are termed mouse ESCs, representing the naïve state of pluripotency and are maintained in culture by LIF/STAT3 signalling (Nichols and Smith, 2012). 2-cell-like cells (green) arise spontaneously in mouse ESC culture and represent the 2-cell blastomere stage of embryonic development. Mouse ESCs derived from the E6.5 – 7.5 post-implantation epiblast are termed epiblast stem cells (EpiSCs). In the human, ESCs derived from the E3.5 – 4.5 ICM and maintained in vitro in 2i/LIF/Activin are termed naïve human ESCs, equivalent to mouse ESCs (Hanna et al., 2010; Theunissen et al., 2014). If human ESCs derived from the pre-implantation blastocyst are not maintained in naïve culture conditions, and instead are maintained in the standard hESC system (bFGF/TGFβ/Activin), they transition to the primed state, termed human ESCs, equivalent to mouse EpiSCs. Mouse epiblast-like cells, and human formative or intermediate stem cells are representative of the E5.5 mouse epiblast and can give rise to primordial germ cells in culture. Mouse 2-cell-like cells oxidise lactate and pyruvate like the 2-cell embryo. Mouse and human naïve ESCs have a bivalent metabolism, making use of glycolytic and mitochondrial glucose metabolism and glutamine for anaplerosis (Zhou et al., 2012; Carey et al., 2014; Vardhana et al., 2019). Formative stem cells exhibit a high level of de novo lipogenesis and a bivalent metabolism, while primed state human ESCs and mouse EpiSCs are reliant on glycolysis, the absence of which severely reduces cell viability and growth (Zhou et al., 2012). Mouse and human primed state ESCs display increased fatty acid (FA) synthesis compared to the naïve state, and a reduced capacity for fatty acid β-oxidation (Sperber et al., 2015b). The thickness and size of the text represents the relative contribution of the pathway to metabolism.
Metabolism in mouse and human naïve ESCs is characterized by high rates of mitochondrial OxPhos concurrent with glycolysis, a flexible metabolic state termed bivalent metabolism (Zhou et al., 2012; Takashima et al., 2014; Sperber et al., 2015a; Gu et al., 2016; O'Reilly et al., 2019). Naïve mouse ESCs consume large amounts of glutamine, required to synthesize α-ketoglutarate for H3K27me3 demethylation (Carey et al., 2014; Zhang et al., 2016; Tischler et al., 2019) and maintain the general hypomethylated state characteristic of the pre-implantation embryonic ICM (Okae et al., 2014).
Formative or intermediate stem cell metabolism has only recently come under examination. However, it is clear that discrete metabolic states can be induced between the naïve and primed states. In particular, de novo lipogenesis has emerged as a shared transcriptional signature of both the intermediate pluripotent state (Du et al., 2018; Wang et al., 2021) and the pre-implantation human epiblast in vivo (Cornacchia et al., 2019). Interestingly, the addition of proline to mouse ESC culture is sufficient to promote the transition from mouse ESCs to an intermediate EpiSC state (Washington et al., 2010; Casalino et al., 2011). Conversely, a formative stem cell state can be induced from primed human ESCs through the addition of the metabolite NAD+, which induces a bivalent metabolism and increases pluripotency markers and self-renewal (Lees et al., 2020). Relative to the primed state, the NAD+-induced formative state doubles the uptake of glutamine and results in a global reduction in H3K27me3 (Lees et al., 2020), consistent with the shift towards the hypomethylated state observed in the late blastocyst ICM (Okae et al., 2014) and mouse and human naïve stem cells (Gafni et al., 2013; Takashima et al., 2014; Sperber et al., 2015a).
Human ESC and iPSC primed state metabolism is characterized by a heavy dependency on glycolysis, with ~50-70% of glucose being converted to lactate (Zhang et al., 2011; Zhou et al., 2012; Harvey et al., 2015; Spyrou et al., 2019a). This glycolytic dependency is seen in the mammalian blastocyst and other highly proliferative cells, including tumour cells (Warburg, 1956; Gott et al., 1990; Wu et al., 2007). The reliance of human ESCs on glycolysis is likely necessary to maintain cellular NAD+ levels for continuous glycolysis, and allow for rapid biomass generation to support proliferation (Lunt and Vander Heiden, 2011; Gardner and Harvey, 2015). Proliferation is also supported in the primed state through the uptake of large amounts of the amino acid tryptophan, which increases the production of N-formylkynurenine, an upstream metabolite in the kynurenine pathway, although this proliferative effect is independent of de novo NAD+ synthesis and glycolysis (Someya et al., 2021). Mitochondrial OxPhos occurs at relatively low levels in primed human ESCs compared to the naïve state and differentiated cells (Varum et al., 2011; Zhou et al., 2012), and human ESCs contain comparatively lower mitochondrial DNA copy numbers (Facucho-Oliveira et al., 2007; Armstrong et al., 2010) and mitochondrial mass (Cho et al., 2006; Varum et al., 2011). Despite their limited oxidative capacity, mouse and human primed state stem cells constantly undergo the maximum rate of mitochondrial activity available to them (Varum et al., 2011; Zhou et al., 2012). The reduced mitochondrial OxPhos and highly glycolytic metabolism of the primed state is in keeping with post-implantation embryo metabolism, which takes place in a low oxygen environment and is heavily reliant on aerobic glycolysis.
Metabolism during pluripotent stem cell differentiation and reprogramming
A metabolic surge event accompanies the exit from pluripotency in vitro
Changes to metabolism and mitochondria precede lineage marker upregulation during pluripotent stem cell differentiation (Mandal et al., 2011; Zhou et al., 2012), suggesting that metabolism is a key initiator of the exit from pluripotency. Recent studies have described a key metabolic surge event that occurs within the first 24 hours of differentiation to ectoderm (Lees et al., 2018) and mesoderm (Richard et al., 2019), highlighting the driving role of metabolism in the initial stages of differentiation. Concurrent with the loss of pluripotency, there is a transient surge in glycolytic and mitochondrial metabolism, which dissipates by 48 hours of differentiation. It is as yet unclear whether the timing of this metabolic event is precisely orchestrated to facilitate differentiation, or if current culture conditions are simply unable to meet the dynamic metabolic requirements of differentiation. This metabolic surge includes increases in glucose uptake, glycolytic rate, mitochondrial oxygen consumption, mitochondrial superoxide production, and mitochondrial mass (Lees et al., 2018; Richard et al., 2019). It is understandable that this transitional metabolic event has been missed until now, given that metabolic analyses of stem cell differentiation are rare, and usually occur at 48-hour, 72-hour, or weekly intervals (Cho et al., 2006; Prigione and Adjaye, 2010; Varum et al., 2011; Mondragon-Teran et al., 2013). Just as distinct waves of transcriptional and proteomic remodeling take place during differentiation and reprogramming (Polo et al., 2012), this transient metabolic surge may be required to shift pluripotent stem cells out of the stable primed state (Enver et al., 2009) and begin metabolic remodeling for the new cell state.
Metabolic remodeling during germ layer specification
The switch from aerobic glycolysis in pluripotent stem cells to mitochondrial OxPhos during differentiation was long believed to be a universal event (Varum et al., 2011; Zhang et al., 2011; Gu et al., 2016; Cliff and Dalton, 2017). In vivo, the gastrula-stage definitive endoderm, which gives rise to the fetal gut tube and all subsequent endodermal organs, develops elongated mitochondria with dense matrices and many transverse cristae likely to support a high rate of OxPhos (Solter et al., 1974; Tam et al., 1993). While mesoderm, which gives rise to muscle and connective tissues, develops spherical mitochondria during gastrulation (Tam et al., 1993). As pluripotent stem cells differentiate into mesoderm and endoderm in vitro over 1 – 3 weeks, there is a metabolic remodeling from primed state aerobic glycolysis to mitochondrial OxPhos, which supports the differentiated cell state (Cliff et al., 2017; Benlamara et al., 2019; Lu et al., 2019; Richard et al., 2019; Song et al., 2019). Differentiating mesodermal and endodermal mitochondria develop into a reticulated mature network (St John et al., 2005; Chung et al., 2007; Hoque et al., 2018) similar to that observed in cardiomyocytes, fibroblasts, or liver cells, which are capable of more efficient oxygen consumption compared to pluripotent stem cells (Varum et al., 2011; Lees et al., 2019b). Similarly, in vitro mesoderm and endoderm differentiation increases mitochondrial mass (St John et al., 2005) and mitochondrial DNA copy number (Facucho-Oliveira et al., 2007).
However, this understanding of remodeling from glycolytic to oxidative has been based on metabolic studies of mesoderm and endoderm differentiations. Initial studies into ectoderm specification noted a surprising decrease in mitochondrial oxygen consumption, mitochondrial DNA copy number, and mitochondrial mass as pluripotent stem cells committed to a neural fate (Armstrong et al., 2010; Birket et al., 2011), which was at odds with the dogma around mesoderm and endoderm metabolic remodeling. In vivo, mitochondria of the post-gastrulation mouse ectoderm are spherical, vacuolated, and have electron-poor matrices suggesting a limited capacity for mitochondrial OxPhos (Poelmann, 1981). Indeed, it was only relatively recently determined that pluripotent stem cell differentiation to ectoderm required the maintenance of a high level of glycolytic flux until the specification of the neural floor plate at day 10, and that this is regulated by MYC and MYCN (Cliff et al., 2017). Subsequent studies have shown increased glycolytic rates as human pluripotent stem cells differentiate into neural stem cells (Lees et al., 2018; Li et al., 2020). Specification of ectoderm over mesoderm and endoderm has also been associated with the suppression of cholesterol synthesis (Xu et al., 2021), reduced glutamine incorporation into TCA cycle metabolites (Lu et al., 2019), and the accumulation of the TCA cycle metabolite and epigenetic cofactor, α-ketoglutarate (Benlamara et al., 2019). Taken together these studies suggest a pivotal role for TCA cycle enzymes in determining ectoderm lineage specification through metabolic regulation and the regulation of epigenetic cofactor availability (Martínez-Reyes et al., 2016).
Metabolic remodeling during iPSC reprogramming
IPSCs represent the primed ESC state and consequently reprogramming of somatic cells is accompanied by the remodeling of metabolism from OxPhos to glycolysis (Prigione et al., 2010; Folmes et al., 2011; Panopoulos et al., 2011; Hansson et al., 2012; Son et al., 2013; Mathieu et al., 2014; O’Reilly et al., 2019). However, this remodeling is generally incomplete, including only a partial reversion of somatic mitochondria to an ESC-like morphology (Prigione et al., 2010; Folmes et al., 2011), the partial retention of somatic cell metabolism, and an insensitivity to nutrient availability (Harvey et al., 2018; Spyrou et al., 2019a), which is atypical of the primed ESC state (Lees et al., 2015). How and when metabolism is restructured has largely been extrapolated from transcriptional studies (Polo et al., 2010; Prigione et al., 2010; Tonge et al., 2014) or by comparing somatic and reprogrammed pluripotent stem cell types (Varum et al., 2011). Metabolic reprogramming occurs within 24 hours (Hansson et al., 2012), preceding the key events in transcriptional remodeling (Folmes et al., 2011; Polo et al., 2012), and before the first global transcriptional event at approximately 72 hours post-reprogramming (Polo et al., 2012). During iPSC formation, a stoichiometric change in electron transport chain complexes occurs within the first 72 hours, while glycolytic proteins undergo a more gradual increase over two weeks (Hansson et al., 2012). Complex I and IV protein levels rapidly decrease within 72 hours of reprogramming while complexes II, III, and V gradually increase suggesting an orchestrated alteration in the efficiency of mitochondrial OxPhos. Interestingly, at the very early stages of metabolic reprogramming when metabolism is becoming more glycolytic, there is a transient surge in mitochondrial activity (Kida et al., 2015; Hawkins et al., 2016; Mathieu and Ruohola-Baker, 2017), which is strikingly similar to the transient metabolic surge observed as pluripotency is lost and stem cells commence differentiation (Lees et al., 2018; Richard et al., 2019). Consequently, the optimization of reprogramming and differentiation will require a series of defined medium systems designed to support not only the developing transcriptional network, but also the developing metabolism.
Conclusions and future directions
Metabolic pathways do not exist in isolation. No cell resides in a binary state of either glycolysis or mitochondrial OxPhos. While the naïve state has a clear bivalent metabolism (Zhou et al., 2012), primed state pluripotent stem cells are an excellent example of a dominant glycolytic metabolism concomitant with an absolute requirement for mitochondrial OxPhos (Mandal et al., 2011; Lees et al., 2020). Indeed, it is not surprising that profound molecular transitions such as the onset of differentiation and reprogramming would require the combined efforts of multiple metabolic pathways, not simply to meet energy requirements, but to install the new epigenetic landscape through the synthesis of metabolic cofactors (Harvey et al., 2016).
To date, non-human embryo development has been employed as a suboptimal benchmark for human in vitro differentiation. The advent of human blastocyst-like structures obtainable from patient-specific cells (Liu et al., 2021b) will greatly assist the way we study development and disease. Nevertheless, these human models still require significant optimization and detailed metabolic profiling. Comprehensive human embryo data, which can be obtained non-invasively through metabolic analysis of conditioned medium and morphokinetic analysis of time-lapse imaging (Ferrick et al., 2020), can be employed to benchmark this next step of in vitro stem cell modeling.
For the safe and efficient differentiation of healthy cells for therapies, we must develop a detailed understanding of the metabolic states that span pluripotency and differentiation, and how to influence them. Only a handful of studies have examined the metabolic changes that occur during in vitro lineage specification in detail, and there are even fewer in vivo studies as a benchmark. We are only now starting to unravel the different metabolic trajectories of mesoderm, endoderm, and ectoderm specification, for which detailed metabolite flux analyses will be necessary. With the advent of single cell metabolic profiling techniques (Argüello et al., 2020; Hartmann et al., 2021), the stem cell field is poised to uncover the fundamental metabolic processes that maintain stem cell pluripotency and drive differentiation. These insights will not only facilitate directed differentiation, but through reverse engineering, facilitate the development of more effective reprogramming conditions. Single cell metabolomics will also help to establish in silico models for the prediction of metabolic perturbations for drug development and toxicity testing. Ongoing research into the metabolic determinants of pluripotency and differentiation will therefore help to ensure the full therapeutic potential of stem cells is realized.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The O’Brien Institute Department of St Vincent’s Institute of Medical Research receives Operational Infrastructure Support from the Victorian State Government’s Department of Innovation, Industry and Regional Development.
References
Argüello RJ, Combes AJ, Char R, Gigan J-P, Baaziz AI, Bousiquot E, Camosseto V, Samad B, Tsui J, Yan P, Boissonneau S, Figarella-Branger D, Gatti E, Tabouret E, Krummel MF, Pierre P (2020) SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab 32:1063-1075.e1067.
Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28:661-673.
Bahat A, Goldman A, Zaltsman Y, Khan DH, Halperin C, Amzallag E, Krupalnik V, Mullokandov M, Silberman A, Erez A, Schimmer AD, Hanna JH, Gross A (2018) MTCH2-mediated mitochondrial fusion drives exit from naïve pluripotency in embryonic stem cells. Nature Communications 9:5132.
Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Böhnke L, Boyer L, Simon S, Gage FH (2015) Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proceedings of the National Academy of Sciences 112:E2725-E2734.
Bayerl J et al. (2021) Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell Stem Cell.
Benlamara S, Aubry L, Fabregue J, Bénit P, Rustin P, Rak M (2019) Distinctive Krebs cycle remodeling in iPSC-derived neural and mesenchymal stem cells. Biochem Biophys Res Commun 511:658-664.
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315-326.
Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X (2011) A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci 124:348-358.
Boeuf H, Hauss C, De Graeve F, Baran N, Kedinger C (1997) Leukemia Inhibitory Factor–dependent Transcriptional Activation in Embryonic Stem Cells. The Journal of cell biology 138:1207-1217.
Boland MJ, Nazor KL, Loring JF (2014) Epigenetic regulation of pluripotency and differentiation. Circ Res 115:311-324.
Brons IGM, Smithers LE, Trotter MW, Rugg-Gunn P, Bowen S, de Sousa Lopes SMC, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191.
Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET (2011) A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PloS one 6:e18293.
Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ 3. The Journal of clinical investigation 105:577-587.
Cantó C, Menzies KJ, Auwerx J (2015) NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab 22:31-53.
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB (2014) Intracellular [agr]-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518:413-416.
Casalino L, Comes S, Lambazzi G, De Stefano B, Filosa S, De Falco S, De Cesare D, Minchiotti G, Patriarca EJ (2011) Control of embryonic stem cell metastability by L-proline catabolism. Journal of molecular cell biology 3:108-122.
Chazaud C, Yamanaka Y, Pawson T, Rossant J (2006) Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 10:615-624.
Chen CT, Hsu SH, Wei YH (2012) Mitochondrial bioenergetic function and metabolic plasticity in stem cell differentiation and cellular reprogramming. Biochim Biophys Acta 1820:571-576.
Chen T, Dent SY (2014) Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet 15:93-106.
Chi F, Sharpley MS, Nagaraj R, Roy SS, Banerjee U (2020) Glycolysis-Independent Glucose Metabolism Distinguishes TE from ICM Fate during Mammalian Embryogenesis. Dev Cell 53:9-26.e24.
Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park do J, Park KS, Lee HK (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun 348:1472-1478.
Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med 4(S1):S60-67.
Cliff TS, Dalton S (2017) Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Current opinion in genetics & development 46:44-49.
Cliff TS, Wu T, Boward BR, Yin A, Yin H, Glushka JN, Prestegaard JH, Dalton S (2017) MYC Controls Human Pluripotent Stem Cell Fate Decisions through Regulation of Metabolic Flux. Cell Stem Cell 21:502-516.
Clough J, Whittingham D (1983) Metabolism of [14C] glucose by postimplantation mouse embryos in vitro. Development 74:133-142.
Collins TJ, Berridge MJ, Lipp P, Bootman MD (2002) Mitochondria are morphologically and functionally heterogeneous within cells. The EMBO journal 21:1616-1627.
Cornacchia D, Zhang C, Zimmer B, Chung SY, Fan Y, Soliman MA, Tchieu J, Chambers SM, Shah H, Paull D, Konrad C, Vincendeau M, Noggle SA, Manfredi G, Finley LWS, Cross JR, Betel D, Studer L (2019) Lipid Deprivation Induces a Stable, Naive-to-Primed Intermediate State of Pluripotency in Human PSCs. Cell Stem Cell 25:120-136.e110.
Davidson KC, Mason EA, Pera MF (2015) The pluripotent state in mouse and human. Development 142:3090-3099.
de Lima CB, dos Santos ÉC, Ispada J, Fontes PK, Nogueira MFG, dos Santos CMD, Milazzotto MP (2020) The dynamics between in vitro culture and metabolism: embryonic adaptation to environmental changes. Scientific Reports 10:15672.
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27-45.
Donohoe DR, Bultman SJ (2012) Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. J Cell Physiol 227:3169-3177.
Du P, Pirouz M, Choi J, Huebner AJ, Clement K, Meissner A, Hochedlinger K, Gregory RI (2018) An Intermediate Pluripotent State Controlled by MicroRNAs Is Required for the Naive-to-Primed Stem Cell Transition. Cell Stem Cell 22:851-864.e855.
Efroni S, Duttagupta R, Cheng J, Dehghani H, Hoeppner DJ, Dash C, Bazett-Jones DP, Le Grice S, McKay RD, Buetow KH (2008) Global transcription in pluripotent embryonic stem cells. Cell Stem Cell 2:437-447.
Enver T, Pera M, Peterson C, Andrews PW (2009) Stem cell states, fates, and the rules of attraction. Cell Stem Cell 4:387-397.
Ermisch AF, Herrick JR, Pasquariello R, Dyer MC, Lyons SM, Broeckling CD, Rajput SK, Schoolcraft WB, Krisher RL (2020) A novel culture medium with reduced nutrient concentrations supports the development and viability of mouse embryos. Scientific Reports 10:9263.
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154-156.
Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120:4025-4034.
Ferrick L, Lee YSL, Gardner DK (2020) Metabolic activity of human blastocysts correlates with their morphokinetics, morphological grade, KIDScore and artificial intelligence ranking. Hum Reprod 35:2004-2016.
Fischer B, Bavister BD (1993) Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 99:673-679.
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14:264-271.
Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A (2013) Derivation of novel human ground state naive pluripotent stem cells. Nature 504:282-286.
Gardner DK (1998a) Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 49:83-102.
Gardner DK (1998b) Development of serum-free media for the culture and transfer of human blastocysts. Hum Reprod 13:218-225.
Gardner DK, Lane M (1993) Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod 48:377-385.
Gardner DK, Harvey AJ (2015) Blastocyst metabolism. Reproduction, Fertility and Development 27:638-654.
Gardner DK, Lane M, Calderon I, Leeton J (1996) Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril 65:349-353.
Gardner RL (1968) Mouse chimaeras obtained by the injection of cells into the blastocyst. Nature 220:596-597.
Garner W, McLaren A (1974) Cell distribution in chimaeric mouse embryos before implantation. Development 32:495-503.
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ (1997) Regulation of human placental development by oxygen tension. Science 277:1669-1672.
Genet M, Torres-Padilla M-E (2020) The molecular and cellular features of 2-cell-like cells: a reference guide. Development 147:dev189688.
Ghosh S, Tran K, Delbridge LMD, Hickey AJR, Hanssen E, Crampin EJ, Rajagopal V (2018) Insights on the impact of mitochondrial organisation on bioenergetics in high-resolution computational models of cardiac cell architecture. PLOS Computational Biology 14:e1006640.
Gott A, Hardy K, Winston R, Leese H (1990) Non-invasive measurement of pyruvate and glucose uptake and lactate production by sigle human preimplantation embryos. Hum Reprod 5:104-108.
Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR (2016) Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell 19:476-490.
Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J (2016) Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem cell reports 6:437-446.
Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proceedings of the National Academy of Sciences 107:9222-9227.
Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J (2012) Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell reports 2:1579-1592.
Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M (2017) Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356.
Hartmann FJ, Mrdjen D, McCaffrey E, Glass DR, Greenwald NF, Bharadwaj A, Khair Z, Verberk SGS, Baranski A, Baskar R, Graf W, Van Valen D, Van den Bossche J, Angelo M, Bendall SC (2021) Single-cell metabolic profiling of human cytotoxic T cells. Nat Biotechnol 39:186-197.
Harvey A, Caretti G, Moresi V, Renzini A, Adamo S (2019) Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance. Stem Cell Reports 13:573-589.
Harvey AJ (2019) Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction 157:R159-r179.
Harvey AJ, Rathjen J, Gardner DK (2016) Metaboloepigenetic Regulation of Pluripotent Stem Cells. Stem cells international 2016:1-15.
Harvey AJ, Rathjen J, Yu LJ, Gardner DK (2015) Oxygen modulates human embryonic stem cell metabolism in the absence of changes in self-renewal. Reproduction, Fertility and Development 28:446-458.
Harvey AJ, O’Brien C, Lambshead J, Sheedy JR, Rathjen J, Laslett AL, Gardner DK (2018) Physiological oxygen culture reveals retention of metabolic memory in human induced pluripotent stem cells. PLOS ONE 13:e0193949.
Hawkins KE, Joy S, Delhove JM, Kotiadis VN, Fernandez E, Fitzpatrick LM, Whiteford JR, King PJ, Bolanos JP, Duchen MR (2016) NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming. Cell reports 14:1883-1891.
Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S (2010) Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell 6:479-491.
Hewitson LC, Leese HJ (1993a) Energy metabolism of the trophectoderm and inner cell mass of the mouse blastocyst. The Journal of experimental zoology 267:337-343.
Hewitson LC, Leese HJ (1993b) Energy metabolism of the trophectoderm and inner cell mass of the mouse blastocyst. The Journal of experimental zoology 267:337-343.
Hoque A, Sivakumaran P, Bond ST, Ling NXY, Kong AM, Scott JW, Bandara N, Hernández D, Liu G-S, Wong RCB, Ryan MT, Hausenloy DJ, Kemp BE, Oakhill JS, Drew BG, Pébay A, Lim SY (2018) Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell Death Discovery 4:39.
Houghton FD (2006) Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation 74:11-18.
Houghton FD, Thompson JG, Kennedy CJ, Leese HJ (1996) Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 44:476-485.
Jarosz J, Ghosh S, Delbridge LMD, Petzer A, Hickey AJR, Crampin EJ, Hanssen E, Rajagopal V (2017) Changes in mitochondrial morphology and organization can enhance energy supply from mitochondrial oxidative phosphorylation in diabetic cardiomyopathy. American Journal of Physiology-Cell Physiology 312:C190-C197.
Kelly A, West JD (1996) Genetic evidence that glycolysis is necessary for gastrulation in the mouse. Dev Dyn 207:300-308.
Kida YS, Kawamura T, Wei Z, Sogo T, Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR (2015) ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell 16:547-555.
Kinoshita M, Barber M, Mansfield W, Cui Y, Spindlow D, Stirparo GG, Dietmann S, Nichols J, Smith A (2021) Capture of Mouse and Human Stem Cells with Features of Formative Pluripotency. Cell Stem Cell 28:453-471.e458.
Kojima Y, Kaufman-Francis K, Studdert JB, Steiner KA, Power MD, Loebel DA, Jones V, Hor A, de Alencastro G, Logan GJ (2014) The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell 14:107-120.
Lees JG, Cliff TS, Gammilonghi A, Ryall JG, Dalton S, Gardner DK, Harvey AJ (2019a) Oxygen regulates human pluripotent stem cell metabolic flux. Stem Cells International 2019.
Lees JG, Gardner DK, Harvey AJ (2017) Pluripotent Stem Cell Metabolism and Mitochondria: Beyond ATP. Stem Cells International 2017:2874283.
Lees JG, Gardner DK, Harvey AJ (2018) Mitochondrial and glycolytic remodeling during nascent neural differentiation of human pluripotent stem cells. Development 145:dev168997.
Lees JG, Gardner DK, Harvey AJ (2020) Nicotinamide adenine dinucleotide induces a bivalent metabolism and maintains pluripotency in human embryonic stem cells. Stem Cells 38:624-638.
Lees JG, Rathjen J, Sheedy JR, Gardner DK, Harvey AJ (2015) Distinct profiles of human embryonic stem cell metabolism and mitochondria identified by oxygen. Reproduction 150:367.
Lees JG, Kong AM, Chen YC, Sivakumaran P, Hernández D, Pébay A, Harvey AJ, Gardner DK, Lim SY (2019b) Mitochondrial Fusion by M1 Promotes Embryoid Body Cardiac Differentiation of Human Pluripotent Stem Cells. Stem Cells International 2019:6380135.
Leese H (1988) The formation and function of oviduct fluid. J Reprod Fertil 82:843-856.
Leese HJ, Barton AM (1985) Production of pyruvate by isolated mouse cumulus cells. The Journal of experimental zoology 234:231-236.
Li D, Ding Z, Gui M, Hou Y, Xie K (2020) Metabolic Enhancement of Glycolysis and Mitochondrial Respiration Are Essential for Neuronal Differentiation. Cellular Reprogramming 22:291-299.
Li R, Zhong C, Yu Y, Liu H, Sakurai M, Yu L, Min Z, Shi L, Wei Y, Takahashi Y, Liao H-K, Qiao J, Deng H, Nuñez-Delicado E, Rodriguez Esteban C, Wu J, Izpisua Belmonte JC (2019) Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 179:687-702.e618.
Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng Y, Shi L, Meloni BP, Zhang C, Zheng M, Gao J (2021a) Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduction and Targeted Therapy 6:65.
Liu X, Tan JP, Schröder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G, Zhou Y, Poppe D, Lister R, Clark AT, Rackham OJL, Zenker J, Polo JM (2021b) Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature.
Lu V, Dahan P, Ahsan FM, Patananan AN, Roy IJ, Torres A, Nguyen RMT, Huang D, Braas D, Teitell MA (2019) Mitochondrial metabolism and glutamine are essential for mesoderm differentiation of human pluripotent stem cells. Cell Research 29:596-598.
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441-464.
Maas DH, Storey BT, Mastroianni L (1976) Oxygen Tension in the Oviduct of the Rhesus Monkey (Macaca Mulatta). Fertil Steril 27:1312-1317.
Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A, Singer O, Trono D, Pfaff SL (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487:57-63.
Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U (2011) Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 29:486-495.
Martínez-Reyes I, Diebold Lauren P, Kong H, Schieber M, Huang H, Hensley Christopher T, Mehta Manan M, Wang T, Santos Janine H, Woychik R, Dufour E, Spelbrink Johannes N, Weinberg Samuel E, Zhao Y, DeBerardinis Ralph J, Chandel Navdeep S (2016) TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol Cell 61:199-209.
Mathieu J, Ruohola-Baker H (2017) Metabolic remodeling during the loss and acquisition of pluripotency. Development 144:541-551.
Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H (2014) Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14:592-605.
Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, Barnett BD, Madden JD (2009) A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program†. Hum Reprod 24:300-307.
Mishra P, Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15:634-646.
Mondragon-Teran P, Tostoes R, Mason C, Lye GJ, Veraitch FS (2013) Oxygen-controlled automated neural differentiation of mouse embryonic stem cells. Regenerative medicine 8:171-182.
Motta PM, Nottola SA, Makabe S, Heyn R (2000) Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod 15:129-147.
Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21:392-402.
Nichols J, Smith A (2012) Pluripotency in the embryo and in culture. Cold Spring Harbor perspectives in biology 4:a008128.
O'Reilly C, Qi Q, Peters JL, Cheng Y, Yoon S-O, Han M-J (2019) The primitive growth factor NME7(AB) induces mitochondrially active naïve-like pluripotent stem cells. Biochem Biophys Rep 20:100656-100656.
O’Reilly C, Cho J-H, Qi Q, Peters JL, Fukuda Y, Frase S, Peng J, Schuetz JD, Cheng Y, Yoon S-O, Han M-J (2019) Metabolic switching in pluripotent stem cells reorganizes energy metabolism and subcellular organelles. Exp Cell Res 379:55-64.
Obeidat Y, Catandi G, Carnevale E, Chicco AJ, DeMann A, Field S, Chen T (2019) A multi-sensor system for measuring bovine embryo metabolism. Biosens Bioelectron 126:615-623.
Okae H, Chiba H, Hiura H, Hamada H, Sato A, Utsunomiya T, Kikuchi H, Yoshida H, Tanaka A, Suyama M (2014) Genome-wide analysis of DNA methylation dynamics during early human development. PLoS genetics 10:e1004868.
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313-317.
Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerías A, Batchelder EM, Plongthongkum N, Lutz M (2011) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell research 22:168-177.
Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, Harper M-E, Germain M, Slack RS (2014) OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. The EMBO Journal 33:2676-2691.
Picard M, McManus MJ, Csordás G, Várnai P, Dorn Ii GW, Williams D, Hajnóczky G, Wallace DC (2015) Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nature Communications 6:6259.
Poelmann RE (1981) The formation of the embryonic mesoderm in the early post-implantation mouse embryo. Anat Embryol 162:29-40.
Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS, Wang N (2014) Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun 5:4000.
Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28:848-855.
Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, Borkent M, Apostolou E, Alaei S, Cloutier J (2012) A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151:1617-1632.
Prigione A, Adjaye J (2010) Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol 54:1729-1741.
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28:721-733.
Richard A, Vallin E, Romestaing C, Roussel D, Gandrillon O, Gonin-Giraud S (2019) Erythroid differentiation displays a peak of energy consumption concomitant with glycolytic metabolism rearrangements. PloS one 14:e0221472.
Rodriguez-Terrones D, Hartleben G, Gaume X, Eid A, Guthmann M, Iturbide A, Torres-Padilla ME (2020) A distinct metabolic state arises during the emergence of 2-cell-like cells. EMBO reports 21:e48354.
Rogers P, Murphy C, Gannon B (1982) Absence of capillaries in the endometrium surrounding the implanting rat blastocyst. Micron (1969) 13:373-374.
Sathananthan AH, Trounson A (2000) Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod 15:148-159.
Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K, Endo F, Kume S (2014) Methionine Metabolism Regulates Maintenance and Differentiation of Human Pluripotent Stem Cells. Cell Metab 19:780-794.
Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339:222-226.
Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A (2009) Nanog is the gateway to the pluripotent ground state. Cell 138:722-737.
Sirard M-A (2019) Distribution and dynamics of mitochondrial DNA methylation in oocytes, embryos and granulosa cells. Scientific reports 9:11937-11937.
Smith A (2017) Formative pluripotency: the executive phase in a developmental continuum. Development 144:365-373.
Solter D, Biczysko W, Pienkowski M, Koprowski H (1974) Ultrastructure of mouse egg cylinders developed in vitro. The Anatomical Record 180:263-279.
Someya S, Tohyama S, Kameda K, Tanosaki S, Morita Y, Sasaki K, Kang M-I, Kishino Y, Okada M, Tani H, Soma Y, Nakajima K, Umei T, Sekine O, Moriwaki T, Kanazawa H, Kobayashi E, Fujita J, Fukuda K (2021) Tryptophan metabolism regulates proliferative capacity of human pluripotent stem cells. iScience 24:102090.
Son MJ, Jeong BR, Kwon Y, Cho YS (2013) Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition. The international journal of biochemistry & cell biology 45:2512-2518.
Song C, Xu F, Ren Z, Zhang Y, Meng Y, Yang Y, Lingadahalli S, Cheung E, Li G, Liu W, Wan J, Zhao Y, Chen G (2019) Elevated Exogenous Pyruvate Potentiates Mesodermal Differentiation through Metabolic Modulation and AMPK/mTOR Pathway in Human Embryonic Stem Cells. Stem Cell Reports 13:338-351.
Sozen B, Amadei G, Cox A, Wang R, Na E, Czukiewska S, Chappell L, Voet T, Michel G, Jing N, Glover DM, Zernicka-Goetz M (2018) Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat Cell Biol 20:979-989.
Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H (2015a) The metabolome regulates the epigenetic landscape during naive to primed human embryonic stem cell transition. Nat Cell Biol 17:1523.
Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H (2015b) The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol 17:1523-1535.
Spyrou J, Gardner DK, Harvey AJ (2019a) Metabolomic and transcriptional analyses reveal atmospheric oxygen during human induced pluripotent stem cell generation impairs metabolic reprogramming. Stem Cells.
Spyrou J, Gardner DK, Harvey AJ (2019b) Metabolism Is a Key Regulator of Induced Pluripotent Stem Cell Reprogramming. Stem Cells International 2019.
St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP (2005) The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning and Stem Cells 7:141-153.
Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158:1254-1269.
Tam PP, Williams EA, Chan W (1993) Gastrulation in the mouse embryo: ultrastructural and molecular aspects of germ layer morphogenesis. Microsc Res Tech 26:301-328.
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196-199.
Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:471-487.
Tischler J, Gruhn WH, Reid J, Allgeyer E, Buettner F, Marr C, Theis F, Simons BD, Wernisch L, Surani MA (2019) Metabolic regulation of pluripotency and germ cell fate through α-ketoglutarate. The EMBO journal 38:e99518.
Tonge PD, Corso AJ, Monetti C, Hussein SM, Puri MC, Michael IP, Li M, Lee D-S, Mar JC, Cloonan N (2014) Divergent reprogramming routes lead to alternative stem-cell states. Nature 516:192-197.
Tsogtbaatar E, Landin C, Minter-Dykhouse K, Folmes CD (2020) Energy metabolism regulates stem cell pluripotency. Frontiers in cell and developmental biology 8.
Vardhana SA, Arnold PK, Rosen BP, Chen Y, Carey BW, Huangfu D, Carmona-Fontaine C, Thompson CB, Finley LWS (2019) Glutamine independence is a selectable feature of pluripotent stem cells. Nature Metabolism 1:676-687.
Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAt, Ramalho-Santos J, Van Houten B, Schatten G (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6:e20914.
Voigt P, Tee W-W, Reinberg D (2013) A double take on bivalent promoters. Genes & development 27:1318-1338.
Waldenström U, Engström A-B, Hellberg D, Nilsson S (2009) Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril 91:2461-2465.
Wale PL, Gardner DK (2010) Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online 21:402-410.
Wang X, Xiang Y, Yu Y, Wang R, Zhang Y, Xu Q, Sun H, Zhao Z-A, Jiang X, Wang X, Lu X, Qin D, Quan Y, Zhang J, Shyh-Chang N, Wang H, Jing N, Xie W, Li L (2021) Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Research.
Warburg O (1956) On the origin of cancer cells. Science 123:309-314.
Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S (2014) Derivation of naive human embryonic stem cells. Proceedings of the National Academy of Sciences 111:4484-4489.
Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH (2014) A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11:847-854.
Washington JM, Rathjen J, Felquer F, Lonic A, Bettess MD, Hamra N, Semendric L, Tan BS, Lake JA, Keough RA, Morris MB, Rathjen PD (2010) L-Proline induces differentiation of ES cells: a novel role for an amino acid in the regulation of pluripotent cells in culture. American Journal of Physiology-Cell Physiology 298:982-992.
Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, Ralston A (2014) HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst. PLOS Genetics 10:e1004618.
Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z (2013) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241-256.
Wu H, Zhang Y (2014) Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156:45-68.
Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. American Journal of Physiology-Cell Physiology 292:C125-C136.
Wu S, Zhang J, Li F, Du W, Zhou X, Wan M, Fan Y, Xu X, Zhou X, Zheng L (2019) One-carbon metabolism links nutrition intake to embryonic development via epigenetic mechanisms. Stem cells international 2019.
Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y (2012) Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes & development 26:1326-1338.
Xu X, Du Y, Ma L, Zhang S, Shi L, Chen Z, Zhou Z, Hui Y, Liu Y, Fang Y, Fan B, Liu Z, Li N, Zhou S, Jiang C, Liu L, Zhang X (2021) Mapping germ-layer specification preventing genes in hPSCs via genome-scale CRISPR screening. iScience 24:101926.
Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, Wu J (2021) Blastocyst-like structures generated from human pluripotent stem cells. Nature.
Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M (2016) LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell stem cell 19:66-80.
Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, Jung HJ, McCaffery JM, Kurland IJ, Reue K, Lee WN, Koehler CM, Teitell MA (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. The EMBO journal 30:4860-4873.
Zhang S et al. (2019) Implantation initiation of self-assembled embryo-like structures generated using three types of mouse blastocyst-derived stem cells. Nat Commun 10:496.
Zhao C, Reyes AP, Schell JP, Weltner J, Ortega N, Zheng Y, Björklund ÅK, Rossant J, Fu J, Petropoulos S, Lanner F (2021) Reprogrammed iBlastoids contain amnion-like cells but not trophectoderm. bioRxiv:2021.2005.2007.442980.
Zhong X, Cui P, Cai Y, Wang L, He X, Long P, Lu K, Yan R, Zhang Y, Pan X, Zhao X, Li W, Zhang H, Zhou Q, Gao P (2019) Mitochondrial Dynamics Is Critical for the Full Pluripotency and Embryonic Developmental Potential of Pluripotent Stem Cells. Cell Metab 29:979-992.e974.
Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H (2012) HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31:2103-2116.
Zhu J, Aja S, Kim E-K, Park MJ, Ramamurthy S, Jia J, Hu X, Geng P, Ronnett GV (2012) Physiological oxygen level is critical for modeling neuronal metabolism in vitro. J Neurosci Res 90:422-434.
References
Jarmon G. Lees1,2 *
1O’Brien Institute Department, St. Vincent’s Institute of Medical Research, VIC, Australia. 2Department of Medicine, Melbourne Medical School, University of Melbourne, VIC, Australia.
Shiang Y. Lim1,3
1O’Brien Institute Department, St. Vincent’s Institute of Medical Research, VIC, Australia. 3Department of Surgery, Melbourne Medical School, University of Melbourne, VIC, Australia.
David K. Gardner4
4School of BioSciences, University of Melbourne, VIC, Australia.
Alexandra J. Harvey4
4School of BioSciences, University of Melbourne, VIC, Australia.
Corresponding author:
Jarmon G. Lees
Email: jlees@svi.edu.au

In a new window | Download PPT
Figure 1: Fertilization of the mammalian egg occurs at embryonic day (E) 0.5 in the oviduct, which resides at ~9% oxygen (Fischer and Bavister, 1993). The cleavage stage embryo is surrounded by the zona pellucida (blue cloud), a glycoprotein coat that protects against polyspermy and premature hatching, and the cumulus cells, which generate pyruvate and lactate from glucose, creating a pyruvate- and lactate-rich environment for the cleavage stage embryo (Leese and Barton, 1985; Gardner et al., 1996). The metabolism of the cleavage stage embryo consists primarily of the aerobic oxidation of pyruvate and lactate (Gardner and Harvey, 2015). Zygotic/embryonic genome activation occurs in the mouse 2-cell embryo, and at the 4- and 8-cell stage in the human. Cleavage division to the 8-cell stage and the compaction of the morula occur in the uterus, which has a relatively higher glucose concentration (3.15 mM) than that of the oviduct (0.5 mM) (Gardner et al., 1996). The higher glucose concentration in the uterus, and the declining concentrations of pyruvate and lactate, coincide with the increased demand for ATP, which alleviates the block in glycolysis present in the cleavage stages, and signals the switch to a primarily glucose driven metabolism at the blastocyst stage. Blastocyst formation takes place in the uterus under ~5.3% oxygen (Maas et al., 1976) at E3.5 in the mouse and E5 in the human, when the trophectoderm (TE; yellow) cells expand to encompass the blastocoel cavity, and the inner cell mass (ICM; blue) cells begin random priming for epiblast (blue) and primitive endoderm linages (red) (Chazaud et al., 2006). Oxygen consumption increases 3-fold at the blastocyst stage, its highest during embryonic development (Houghton et al., 1996), in order to create the ATP required for blastocoel formation. The blastocyst comprises two discrete cell types, the ICM and the TE, each with distinct nutritional requirements and metabolism. In the mouse, the TE converts ~50% of consumed glucose to lactate, while the ICM is almost exclusively glycolytic, converting most consumed glucose to lactate (Hewitson and Leese, 1993a). Oxygen consumption by the TE cells increases after compaction, and mitochondria develop from spherical organelles into elongated morphologies with transverse cristae (Lees et al., 2017). Implantation occurs at E5 and E7 in the mouse and human respectively, at which time the oxygen concentration drops within the uterus to 3.7% (Maas et al., 1976). During implantation, synctiotrophoblasts invade the uterine wall, recorded at ~2.5% oxygen (Genbacev et al., 1997; Caniggia et al., 2000). Following implantation, the polar TE cells proliferate to form the extraembryonic ectoderm (yellow), which will contribute to the embryonic part of the placenta. Oxygen consumption by the egg cylinder stage embryo drops to pre-blastocyst levels (Houghton et al., 1996), and glycolysis becomes the primary metabolic pathway together with extensive pentose phosphate pathway use for proliferation (Clough and Whittingham, 1983). Gastrulation occurs at E6.5 and E14 in the mouse and human respectively giving rise to the gastrula and the formation of the primitive streak and primordial germ cells (purple). In the mouse, in vitro derived ESCs from the E3.5 ICM are termed mouse ESCs, representing the naïve state of pluripotency and are maintained in culture by LIF/STAT3 signalling (Nichols and Smith, 2012). 2-cell-like cells (green) arise spontaneously in mouse ESC culture and represent the 2-cell blastomere stage of embryonic development. Mouse ESCs derived from the E6.5 – 7.5 post-implantation epiblast are termed epiblast stem cells (EpiSCs). In the human, ESCs derived from the E3.5 – 4.5 ICM and maintained in vitro in 2i/LIF/Activin are termed naïve human ESCs, equivalent to mouse ESCs (Hanna et al., 2010; Theunissen et al., 2014). If human ESCs derived from the pre-implantation blastocyst are not maintained in naïve culture conditions, and instead are maintained in the standard hESC system (bFGF/TGFβ/Activin), they transition to the primed state, termed human ESCs, equivalent to mouse EpiSCs. Mouse epiblast-like cells, and human formative or intermediate stem cells are representative of the E5.5 mouse epiblast and can give rise to primordial germ cells in culture. Mouse 2-cell-like cells oxidise lactate and pyruvate like the 2-cell embryo. Mouse and human naïve ESCs have a bivalent metabolism, making use of glycolytic and mitochondrial glucose metabolism and glutamine for anaplerosis (Zhou et al., 2012; Carey et al., 2014; Vardhana et al., 2019). Formative stem cells exhibit a high level of de novo lipogenesis and a bivalent metabolism, while primed state human ESCs and mouse EpiSCs are reliant on glycolysis, the absence of which severely reduces cell viability and growth (Zhou et al., 2012). Mouse and human primed state ESCs display increased fatty acid (FA) synthesis compared to the naïve state, and a reduced capacity for fatty acid β-oxidation (Sperber et al., 2015b). The thickness and size of the text represents the relative contribution of the pathway to metabolism.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7187 | 76 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA