Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Mending a broken heart: can gene modulation bolster therapeutic performance of adult stem cells?
Time:2021-06-08
Number:6245
En Ping Yap1,2,3, Xavier Chan1,2,4, Fan Yu1,2,3, Shuo Cong1,2,3, Chrishan J. A. Ramachandra1,2
Author Affiliations


- 1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.
- 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 4Faculty of Science, National University of Singapore, Singapore.
Conditioning Medicine 2021. 4(2): 71-87.
Abstract
Myocardial infarction and the congestive heart failure (HF) that follows are poised to be the leading cause of death in the modern world. Although advances in technology and patient management have helped reduce the mortality rate, the ability to sustain long-term recovery of the heart post-infarction is nearly impossible. This is mainly due to the inability of the adult heart to repair the infarcted region as it has limited regenerative capacity. The implementation of adult stem cells (ASCs) for cardiac therapy has been associated with improvements in cardiac function in several preclinical HF models, however, their effectiveness in the clinical setting has been inconsistent with several large clinical trials reporting disappointing outcomes. To enhance the effectiveness of ASCs, the incorporation of transgenes that bolster their homing and engraftment, anti-apoptotic, anti-inflammation, and pro-angiogenic properties has been proposed and in support, recent findings show transgenic ASCs to be more effective than their naïve counterpart by attenuating disease progression and improving cardiac recovery. Moreover, in addition to improving the therapeutic performance of ASCs, gene modulation provides insight on the fundamental mechanisms underlying the effectiveness of the respective therapy. Here, we review the current status on ASC therapy for cardiac repair and discuss the implementation of transgenic ASCs for restoring the failing heart and improving clinical outcomes.
Keywords: Heart failure, Adult stem cells, Regeneration, Paracrine effects, Induced pluripotent stem cells (iPSCs), Bioengineering
Abstract
Myocardial infarction and the congestive heart failure (HF) that follows are poised to be the leading cause of death in the modern world. Although advances in technology and patient management have helped reduce the mortality rate, the ability to sustain long-term recovery of the heart post-infarction is nearly impossible. This is mainly due to the inability of the adult heart to repair the infarcted region as it has limited regenerative capacity. The implementation of adult stem cells (ASCs) for cardiac therapy has been associated with improvements in cardiac function in several preclinical HF models, however, their effectiveness in the clinical setting has been inconsistent with several large clinical trials reporting disappointing outcomes. To enhance the effectiveness of ASCs, the incorporation of transgenes that bolster their homing and engraftment, anti-apoptotic, anti-inflammation, and pro-angiogenic properties has been proposed and in support, recent findings show transgenic ASCs to be more effective than their naïve counterpart by attenuating disease progression and improving cardiac recovery. Moreover, in addition to improving the therapeutic performance of ASCs, gene modulation provides insight on the fundamental mechanisms underlying the effectiveness of the respective therapy. Here, we review the current status on ASC therapy for cardiac repair and discuss the implementation of transgenic ASCs for restoring the failing heart and improving clinical outcomes.
Keywords: Heart failure, Adult stem cells, Regeneration, Paracrine effects, Induced pluripotent stem cells (iPSCs), Bioengineering
Introduction
Congestive heart failure (HF) due to myocardial infarction (MI) is the leading cause of mortality and morbidity worldwide. Currently, an estimated 64.3 million people have been diagnosed with HF worldwide, and the incidence of HF ranges between 1 to 9 cases per 1000 person-years among Europe and the United States (Groenewegen et al., 2020). Coronary vessel occlusion (and MI) triggers a hypoxic environment that induces cardiomyocyte death via necrosis or apoptosis, resulting in irreversible damage to the myocardium. On the cellular level, ~26% of the 2-4 billion cardiomyocytes that are present in the human left ventricle (LV) could undergo apoptosis in just a few days following MI (Abbate et al., 2002; Ramachandra et al., 2020). The inflammation that ensues leads to the elimination of non-viable cardiomyocytes, which in turn causes fibrosis that replaces the damaged myocardium with a fibrous scar, comprising several extracellular matrix (ECM) components. This scarring process, though intended to support the weakened LV wall post-MI, renders the heart stiff, resulting in adverse LV remodeling with decreased contractility and increased workload on the surviving tissue, leading to HF.
The extremely limited proliferation ability of adult cardiomyocytes makes the heart one of the least regenerative organs in the human body. Though cardiac stem cells have been proposed to reside within the heart, their numbers are not sufficient to replace the damaged myocardium and restore normal heart function (Cruz-Samperio et al., 2021). A coronary artery bypass graft (CABG) could assist in restoring normal blood flow to the heart and prolong life-expectancy; however, it does not address the underlying condition, i.e., restoring the damaged myocardium (Konijnenberg et al., 2020). Heart transplantation is the most viable option for serious cases; however, lack of organ donors together with complications associated with immune suppression hinders this approach from being universally adopted. The lack of pharmacological and surgical intervention to restore the damaged myocardium has hence led researchers to find alternate ways to prevent HF and improve cardiac function.
Over the past few decades, adult stem cells (ASCs) have garnered much recognition as they have been implemented in several experimental and clinical studies for HF (Broughton and Sussman, 2018; Desgres and Menasche, 2019). The transplantation of skeletal myoblasts, bone marrow cells, mesenchymal stem cells, hematopoietic stem cells, and cardiac stem cells has shown much promise in several preclinical animal models of HF, where they improve cardiac function by enhancing hemodynamics and attenuate cardiac remodeling by either differentiation, neovascularization, cell fusion, or secretion of paracrine factors (Lancaster et al., 2018; Yang, 2018; Vazir et al., 2019). However, when it comes to myocardium repair and heart function restoration in humans (Banerjee et al., 2018; Ghiroldi et al., 2018; Sterner et al., 2018), ASC therapy has fallen short in providing substantial clinical benefits, despite being proven to be safe for transplantation. To overcome these disappointing findings, studies have focused on (i) the type and number of cells to be delivered, (ii) the timing of delivery, (iii) the route and frequency of administration, and (iv) their beneficial effects (Braunwald, 2018), and though there is a surplus of literature addressing these points, there is not much focus on the use of genetically modified ASCs, which are reported to be more effective than their naïve counterparts (Lionetti and Recchia, 2010). While the major concern over the use of transgenic ASCs is the incorporation of foreign genetic material, the implementation of ex vivo gene therapy strategies in clinical trials for other disorders (Evans et al., 2013; Evans and Huard, 2015; Holzinger et al., 2016), together with the disappointing outcomes from naïve ASCs, would suggest that it may be appropriate to consider transgenic ASCs for cardiac therapy. In this review, we highlight the current status of ASC-based cardiac therapy and discuss how transgenic ASCs have been instrumental in improving heart function in various models and why they could be considered for future clinical trials.
The current state of cardiac therapy
During first-generation stem cell clinical trials, skeletal myoblasts, mesenchymal stem cells, mesenchymal precursor cells, bone marrow cells, and hematopoietic stem cells were identified as potential candidates for improving clinical outcomes post-MI (Figure 1).
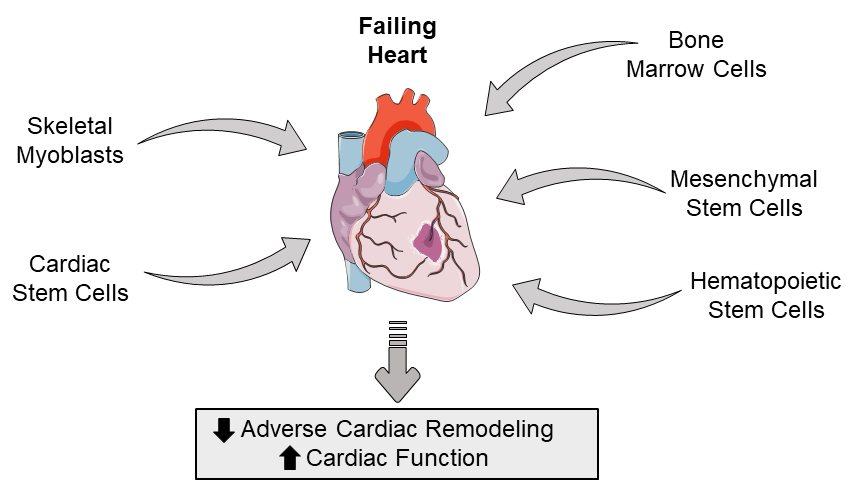
In a new window | Download PPT
Figure 1: Schematic highlighting the types of adult stem cells that can be used to potentially restore the failing heart by attenuatiing adverse cardiac remodeling and improving cardiac function.
Skeletal myoblasts
Skeletal myoblasts (SMs) were the first ASC type considered for cardiac therapy as they could be easily obtained from patient muscle biopsies, be generated in large quantities in vitro, and demonstrated tolerance to hypoxic conditions. The success of SM therapy in experimental models led to their implementation in the first clinical trial in 2003, where intra-myocardial delivery of SMs into the scarred myocardium of patients with severe ischemic cardiomyopathy during CABG was reported to improve heart function (Menasche et al., 2003). Unexpectedly, SM transplantation was found to increase incidences of arrhythmia, which was attributed to the disruption of electrical coupling of engrafted SMs with resident cardiomyocytes (Fukushima et al., 2008). Spontaneous ventricular tachycardia was also observed in animal models due to the accumulation of SM clusters and immune cells (Fukushima et al., 2008). Mechanistically, the global down-regulation of connexin 43 (Cx-43) post-transplantation has been proposed as the reason for arrhythmogenesis (Fukushima et al., 2008; Giraud et al., 2010), which has led to a clinical trial implementing SM overexpressing Cx-43 for treating advanced chronic HF (Gwizdala et al., 2017). These transgenic SMs were found to confer improvement in exercise capacity and myocardial viability of injected segments with no indication of significant ventricular arrhythmia, which could be attributed to better mechanical cooperation of injected cells and injured myocardium with subsequent improvements in electrical coupling. A limitation of this approach however, is that though a large number of cells were cultured from the harvested muscle biopsies, gene transfer of Cx-43 to myoblasts through electroporation led to a vast depletion of cells. To overcome such limitations, less strenuous gene delivery techniques need to be considered, if not, patients would need to undergo repetitive therapies with the provision of several biopsies. In summary, though this clinical trial did show the beneficial effects of transgenic SMs, it was underpowered (n = 13) and lacked a control group for comparison, necessitating the need for further studies to be conducted before the effectiveness of transgenic SM for cardiac therapy can be concluded (Gwizdala et al., 2017).
Bone marrow cells
Bone marrow cells (BMCs) include multiple stem cell populations, including mesenchymal stem cells, hematopoietic stem cells, and endothelial progenitor cells. BMCs can be easily obtained under GMP conditions by Ficoll density gradient centrifugation of bone marrow extracts. In animal models of HF, reports regarding the beneficial effects of BMCs are conflicting (Tomita et al., 1999; Perin et al., 2003) and while BMC transplantation has been shown to increase neovascularization, its effect on improving cardiac function remains controversial. In the first human trial, transendocardial BMC delivery seemed to improve ventricular function in patients with ischemic heart disease by improving blood circulation during a 2- and 4-month follow-up (Perin et al., 2003). However, a 6- and 12-month follow-up revealed no significant improvement in ventricular function, despite the patients exhibiting improvements in myocardial perfusion and exercise capacity (Perin et al., 2004). In other clinical studies, BMC transplantation was reported to significantly improve hemodynamics and ventricular function in patients with ischemic and non-ischemic HF (Hendrikx et al., 2006; Schachinger et al., 2006; Seth et al., 2006). Contrary to these findings, improvements in contractility and reduction in scar size were not observed in patients with chronic ischemic heart disease (Ang et al., 2008; Diederichsen et al., 2010; Perin et al., 2012). Similarly, the First Bone Marrow Mononuclear Cell United States Study in Heart Failure (FOCUS-HF) study (a randomized, double-blind study) reported that BMC transplantation had no significant effect on patients with ischemic cardiomyopathy (Perin et al., 2012). Though there are several advantages for using BMCs (e.g., relatively straight-forward isolation procedures, safe for delivery and negligible arrhythmia incidences), their implementation in cardiac therapy has been met with inconsistent and underwhelming results. To be reconsidered as a viable therapeutic option, improvements in BMC engraftment and differentiation potential are warranted.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent cells of non-hematopoietic origin, that can be isolated from diverse sources (e.g., bone marrow, adipose tissue, placenta, umbilical cord) (Bagno et al., 2018) and can be differentiated into various cell types, including adipocytes, chondrocytes, and osteocytes (Caplan and Dennis, 2006). In the infarcted myocardium they have been found to differentiate into smooth muscle cells and endothelial cells, though their cardiomyogenic potential remains debatable (Nagaya et al., 2005; Silva et al., 2005). The likely mode of action of MSCs in improving cardiac function has been attributed in large to paracrine effects as transplantation of these cells is reported to improve ventricular function via increased vascularization and reduced fibrosis with subsequent attenuation of cardiac remodeling (Mazo et al., 2008; Li et al., 2009; Schuleri et al., 2009; Mazo et al., 2010). Moreover, monolayered MSC sheets have been transplanted onto the scarred myocardium, but unlike fibrous cell sheets that weaken the ventricular wall, MSC sheets were found to improve hemodynamics and reduce mortality rates in rats (Miyahara et al., 2006). These encouraging results in animal models formed the basis for using bone marrow-derived MSCs in the POSEIDON clinical trial. In this study, though improvements in quality of life was observed, HF patients that received different doses of autologous or allogeneic MSCs failed to show drastic improvements in ventricular function, which was in stark contrast to what was previously observed in experimental models (Hare et al., 2012). Similarly, in the PRECISE trial that implemented adipose-derived MSCs, despite increases in patients' exercise capacity, an increase in ventricular function was not observed (Perin et al., 2014). It is important to note that MSCs derived from different sources are not identical and comprise distinct differentiating potential. Moreover, adipose tissue-derived MSCs (AT-MSCs) produce collagen (I, II, and III), whereas bone marrow-derived MSCs (BM-MSCs) possess greater immunosuppressive effects and exhibit heightened pro-angiogenic capacity (Bagno et al., 2018). Such discrepancies arising from the MSC source could be one of the reasons for the unexpected outcomes observed in the clinical trials.
Mesenchymal precursor cells (MPCs) are a restricted subset of MSCs, that when immunoselected for STRO-1 or STRO-3 demonstrate increased proliferative and developmental capabilities (Abdalmula et al., 2017). MPCs also have a unique multimodal mechanism of action that is proposed to result in the polarization of pro-inflammatory type 1 macrophages to an anti-inflammatory type 2 macrophage state in the heart, resulting in the reversal of cardiac and peripheral endothelial dysfunction, inhibition of maladaptive adverse ventricular remodeling, and recovery of deranged vasculature (Borow et al., 2019). In one study assessing the dose-response of allogeneic MPCs in patients with ischemic or non-ischemic HF, a significant reduction of HF-related major adverse cardiac events, as well as improved remodeling was observed in the group that received 150 million MPCs (Perin et al., 2015). In another randomized Phase II clinical trial, intramyocardial injection of MPCs during left ventricular assist device (LVAD) implantation did not seem to improve successful temporary weaning from LVAD support at 6 months, but it did reduce the overall major bleeding rate (Yau et al., 2019). Lastly, in the highly awaited DREAM-HF study (a randomized Phase III study) that assessed the efficacy and safety of MPCs (Rexlemestrocel-L) in patients with advanced chronic HF, a 60% improvement in major adverse cardiac events such as stroke and cardiac arrest was reported regardless of patients' New York Heart Association (NYHA) class, albeit no impact on recurrent hospitalization (www.mesoblast.com). Mechanistically, MPCs are pericyte precursors, which produce high levels of angiopoietin-1, which in turn promotes vessel stability, and hence, these positive results could be attributed to anti-inflammatory effects and improved angiogenesis (Yau et al., 2019). Based on these findings, the implementation of MPCs for cardiac therapy does seem promising, however, their success relies heavily on invasive intra-myocardial delivery methods as intracoronary administration of MSCs/MPCs could lead to heightened risk of embolism with occlusion of coronary microvasculature (Freyman et al., 2006).
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) are rare multipotent, self-renewing cells capable of generating an entire hematopoietic system. HSCs can be isolated from peripheral blood, bone marrow, or cord blood by selecting for CD34+ and CD133+ cells (Ng and Alexander, 2017). In a randomized study, intramyocardial delivery of autologous CD34+ cells during CABG were associated with improved ventricular function in patients with congestive HF (Patel et al., 2005). Similarly, intracoronary infusion of CD133+ and CD133-/CD34+ cells to an infarcted and non-viable region was found to improve ventricular function with favorable remodeling and increased myocardial perfusion in patients with chronic ischemic cardiomyopathy (Manginas et al., 2007). Bone marrow-derived progenitor cells have also been administered in patients with non-ischemic cardiomyopathy (Fischer-Rasokat et al., 2009; Vrtovec et al., 2011), which resulted in improved hemodynamics and cardiac contractility. A similar, but larger study has also yielded promising results where patients with better myocardial homing of the CD34+ cells were reported to have a better response to the therapy as indicated by improvements in ventricular function (Vrtovec et al., 2013). In the PROGENITOR trial, delivery of CD133+ cells in patients with refractory angina was reported to reduce the number of angina episodes per month (Jimenez-Quevedo et al., 2014), while in the ixCELL-DCM randomized phase II trial, transendocardial delivery of ixmyelocel-T in the setting of ischemic dilated cardiomyopathy was associated with significant reductions in adverse cardiac events (Patel et al., 2016). Contrasting findings were documented in other clinical trials that implement the same cells albeit, different delivery methods. For instance, intra-epicardial injection of CD133+ was reported to have no effect on global LV function and clinical symptoms, although there was some improvement in scar size (Nasseri et al., 2014). Moreover, in the underpowered REGENT-VSEL study, transendocardial delivery of CD133+ cells failed to improve myocardial perfusion, LV function, and angina symptoms in patients with refractory angina (Wojakowski et al., 2017), which was further confounded by the inability to recruit sufficient number of patients, thereby severely hampering the ability to conclude on the effectiveness of CD133+ cell therapy. Larger controlled clinical trials are required to further investigate the effectiveness of HSC therapy. Considering that HSCs are not immune-privileged, the main bottleneck lies in finding ways to overcome low cell engraftment and immunological barriers, while minimizing the risk of graft-versus-host disease to yield improvements in cardiac function (Ng and Alexander, 2017).
In summary, due to several conflicting clinical trials, meta-analyses reviewing the effectiveness of first-generation ASCs have been unable to conclusively associate these cells with beneficial effects. Attention has therefore been given to second-generation ASCs, such as cardiac stem/progenitor cells where experimental studies have shown these cardiac-committed cells to possess heightened therapeutic effects as indicated by improvements in engraftment, cardiac function, angiogenesis, and scar size after delivery (Rossini et al., 2011; Li et al., 2012b; Oskouei et al., 2012; Zheng et al., 2013) (Table 1).
.png)
.png)
Cardiac stem cells
Cardiac stem cells (CSCs) are multipotent cells that express markers such as c-kit+ (Beltrami et al., 2003; Messina et al., 2004) and Sca1+ (Oh et al., 2003; Messina et al., 2004). When transplanted near the infarct zone, c-kit+ CSCs were found to invade the scarred myocardium and differentiate into cardiomyocytes and coronary vessels, which in turn improved cardiac function (Rota et al., 2008). Alternatively, cardiosphere-derived cells (CDCs; a cardiac progenitor cell type) can be obtained from ex vivo heart biopsies and differentiate into 3 major cell types; cardiomyocytes, endothelial cells, and smooth muscle cells (Smith et al., 2007). The PERSESUS phase II clinical trial reported that intracoronary infusion of CDCs in patients with congenital HF, led to improvements in cardiac function and HF status. Moreover, these patients were found to exhibit reductions in cardiac fibrosis as a result of reverse remodeling. Despite these promising results, the PERSEUSUS study was an open-labeled study and the effectiveness of CDC therapy should be viewed with caution due to the small sample size (Ishigami et al., 2017). In the CADUCEUS study that assessed the intracoronary delivery of c-kit+ CDCs in MI patients, improvements in heart function were not observed, though reduction in scarring and improvements in regional contractility was reported (Makkar et al., 2012). It can be speculated that the inability to generate target doses through biopsies and the delay in timing between CDC harvest and delivery are major limiting factors for this form of therapy.
Alternatively, consideration could be given for combination therapy as the synergistic action of CDCs and MSCs were found to improve cardiac function in a swine model of ischemia/reperfusion injury (Williams et al., 2013; Karantalis et al., 2015). Currently, the feasibility, safety, and efficacy of transendocardial delivery of MSCs and c-kit+ CSCs is being assessed in the CONCERT-HF study, a 4-arm phase II, randomized and placebo-controlled clinical trial, which would allow for the direct comparison between MSCs and CSCs, as well as their combination in improving cardiac function in patients with chronic ischemic HF (Bolli et al., 2018). While we wait with anticipation for the outcome of this trial, it can be speculated that improvements in cardiac function (if any) may likely be attributed to the therapeutic properties of CSCs rather than MSCs, as the latter is associated with poor engraftment and reduced viability following administration (von Bahr et al., 2012; Wang et al., 2014).
In summary, evidence from experimental studies suggest that both first-generation and second-generation ASCs do have the potential for cardiac therapy, but are confounded by limitations reported in several clinical trials, which hampers their universal adoption as an intervention strategy for improving cardiac function and limiting adverse clinical outcomes in patients with HF. To overcome such obstacles, one strategy could be to bolster the properties of ASCs by introducing transgenes that render them more potent by enhancing their effectiveness during cardiac therapy (Figure 2).

In a new window | Download PPT
Figure 2: Schematic overview of transgenes that can bolster the therapeutic properties of adult stem cells (Up and down arrows indicate overexpression and suppression respectively).
Implementation of transgenic ASCs for cardiac therapy
Anti-apoptotic modifications
A major limitation in cardiac therapy is the poor survival and engraftment of transplanted cells and hence, attempts have been made to enhance such properties by preventing apoptosis mediated by oxidative stress and hypoxia. The enhanced benefits associated with the overexpression of pro-survival genes or through the modulation of microRNAs (miRNAs) that suppress apoptosis-related pathways are discussed below.
In a pioneering study, when MSCs overexpressing Akt1 (a pro-survival gene) were transplanted into the ischemic rat myocardium, profound effects on cardiac remodeling were noted in a dose-dependent manner together with restoration of myocardial volume by four-folds (Mangi et al., 2003). Similar observations were made in large animal models where Akt-overexpressing MSCs were found to be more resistant towards apoptosis and the resultant increase in viable cells post-administration was associated with enhanced repair of the injured myocardium and improved cardiac function (Lim et al., 2006; Yu et al., 2010). In other studies, preconditioning with heat shock proteins (Hsp) was shown to promote cell survival against oxidative stress. For instance, Hsp20 overexpressing rat MSCs were reported to withstand oxidative stress in vitro and exhibited a 2-fold increase in survival when transplanted into infarcted hearts by intracardial injection (Wang et al., 2009). Mechanistically, overexpression of Hsp20 was found to be associated with increased Akt activation and increased growth factor secretion (vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), insulin growth factor-1 (IGF-1)), which reduced fibrosis and enhanced neovascularization with subsequent improvements in cardiac function. Recently, aged MSCs that overexpress part of the shelterin complex, tripeptidyl peptidase 1 (TPP1) were reported to have heightened levels of Akt phosphorylation and meiotic recombination 11 (MRE11) (a double-strand break repair protein) that reduces oxidative stress-mediated apoptosis and improves DNA repair respectively (Yu et al., 2020). Moreover, transplantation of TPP1-MSCs led to augmented cell survival, improved cardiac function, and decreased fibrosis when transplanted into the peri-infarct area in mice. Considering that oxidative stress is a critical mediator of apoptosis, enhancing antioxidant capacity could also be a viable option. In support, overexpression of sirtuin 3 (SIRT3) in aged MSCs led to improvements in antioxidant capacity attributed to up-regulation of Forkhead box O3 (FoxO3a), manganese superoxide dismutase (MnSOD), and catalase, which in turn promoted improvements in cardiac function and reduction in infarct size (Zhang et al., 2018).
H9C2 rat cardiomyoblasts that overexpress human B-cell lymphoma 2 (Bcl-2) were found to be associated with increased graft survival upon transplantation in MI rat models (Kutschka et al., 2006). Moreover, a study evaluating cell sheet technology reported that transgenic sheets comprising Bcl-2-expressing L6 rat skeletal myoblasts survived longer in the infarcted myocardium, increased production of pro-angiogenic factors, and improved cardiac function by reducing fibrosis and increasing angiogenesis in the infarct and border-zone regions in rats (Kitabayashi et al., 2010). In the same study, an increase in the number of proliferating c-kit+ cells were also observed. In the setting of hypoxia, MSCs overexpressing Bcl-2 were found to secrete 60% more VEGF in vitro and when injected into myocardium of MI rats, Bcl-2 expression conferred protection against apoptosis (Li et al., 2007). Interestingly, the capillary density in the infarct border zone was found to be 15% higher and the infarct size was 17% smaller in animals that received Bcl-2-MSC transplantation, thereby exhibiting remarkable functional recovery. In another study, overexpression of FGF-21 in bone marrow-derived MSCs was shown to effectively suppress caspase activation, which in turn protected MSCs from oxidative stress and inflammation mediated apoptosis (Linares et al., 2020), although it is yet to be determined if this approach can improve cardiac function in vivo.
Rat MSCs, which secrete human tissue kallikrein (TK), were reported to be more resistant to hypoxia-induced apoptosis as a result of reduced caspase-3, and transplantation of these cells into the myocardia of MI rats was associated with heightened heart function and improved neovascularization, together with a decrease in the accumulation of inflammatory cells and infarct size, as well as attenuated cardiac remodeling (Gao et al., 2013). In the same study, co-culture of cardiomyocytes with TK-MSC conditioned media was shown to suppress hypoxia-induced apoptosis and increased levels of phosphorylated Akt. The conditioned media was also found to contain elevated levels of VEGF that could stimulate the proliferation, migration, and tube formation of endothelial cells. Similarly, human endothelial progenitor cells (EPCs) that overexpress TK were shown to possess increased tolerance for oxidative stress that was attributed to reduced activation of caspase-3 and caspase-9 with subsequent activation of Akt (Yao et al., 2013). Moreover, as TK overexpression was found to stimulate secretion of VEGF, transplantation of TK-EPCs resulted in increased cardiac function, decreased cardiomyocyte apoptosis, and increased neovascularization and retention in the myocardium. Interestingly, transplanted TK-EPCs were also found to be incorporated into CD31+ capillaries.
In the setting of hypoxia, MSCs that overexpress human heme oyxgenase 1 (HO-1) are reported to secrete several cytokines, including hepatocyte growth factor (HGF), basic FGF, transforming growth factor-beta (TGF-β), VEGF, and interleukin-1beta (IL-1β) (Zeng et al., 2008). Moreover, supernatant enriched with these cytokines was shown to reduce infarct size and cardiomyocyte apoptosis as well as increase angiogenesis in MI rats. Similar beneficial effects have been observed in larger animals, where transplantation of HO-1-overexpressing bone marrow stromal cells was found to improve cardiac function in a porcine ischemia/reperfusion injury model (Jiang et al., 2010). Mechanistically, HO-1-MSCs were found to modulate matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of matrix metalloproteinases (TIMP)) as an increase in TIMP-2 and TIMP-3 with corresponding decrease in MMP-2 and MMP-9 was observed post-transplantation, suggesting that these transgenic MSCs could reverse cardiac remodeling (Shu et al., 2010). Consistent with these findings, exosomes derived from TIMP2-modified human umbilical cord MSCs (huc-exoTIMP2) when injected at the peri-infarct zone of MI rats were shown to increase the expression of anti-apoptotic Bcl-2 and decrease expression of pro-apoptotic Bcl-2 associated X protein (Bax) and pro-caspase-9, which led to reduction of collagen deposition and improvements in cardiac function (Ni et al., 2019).
The overexpression of netrin-1 (a laminin-like protein) in bone marrow-derived Sca-1+ cells has also been investigated. Rat cardiomyocytes and endothelial cells have been reported to express the netrin-1 receptor uncoordinated-5b and hence, when exposed to conditioned media from netrin-1 expressing Sca-1+ cells, a decrease in oxidative stress-mediated apoptosis was observed (Durrani et al., 2012). In the same study, when transplanted to the center and border zone of the infarct, these transgenic cells were shown to reduce ischemia and preserve global heart function in MI rats that could be attributed to elevated activity of nitric oxide synthase (NOS) and increased angiogenesis. The role of apurinic/apyrimidinic endonuclease/redox factor (APE1), a multifunctional enzyme that can activate the DNA repair pathway and induce activation of redox-sensitive transcription factors has also been investigated in cardiac progenitor cells (CPCs) (Aonuma et al., 2016). In this study, APE1-CPCs exposed to H2O2 were found to inhibit reactive oxygen species production and suppress apoptosis via activation of transforming growth factor β-activated kinase 1 (TAK1) and nuclear factor kappa β (NF-κβ) pathway. Moreover, transplantation of APE1-CPCs into ischemic border zone of LV in MI mice was reported to improve LVEF by ~7% and decrease fibrosis by ~6%.
MicroRNAs, a type of non-coding RNA that play a key role in regulating gene expression by directly binding to the 3' untranslated region of their targeted mRNAs, have also been shown to modulate cardiac regeneration and repair (Tao et al., 2015), and much attention has been given to the link between miRNAs and apoptosis regulatory pathways as recently, pro- and anti-apoptotic miRNAs have progressively been identified (Sun et al., 2017). For instance, elevation of miR-133 is reported to be an effective strategy for treatment of MI by reducing hypoxia- and oxidative stress-mediated apoptosis in vitro (Zhang et al., 2012; Xu et al., 2014). Similarly, modified MSCs that overexpress miR-133 were found to drastically reduce their apoptosis when injected at the border zone of infarct in post-MI rats, with a subsequent increase in LVEF (~20%) (Chen et al., 2017). In other studies, MSCs that overexpress miR-25-3p, were shown to alleviate MI by reducing the expression of pro-apoptotic genes, FAS ligand (FASL) and phosphatase and tensin homolog (PTEN) (Peng et al., 2020), while overexpression of miR-301a in human adipose-derived stem cells (hASCs) was shown to reduce apoptosis when transplanted into MI rats by down-regulating apoptosis signal-regulating kinase 1 (ASK1) (Lee et al., 2016). Mechanistically, miR-301a-hASCs were shown to inhibit ASK1 phosphorylation in the setting of hypoxia, which in turn suppressed the downstream p38/ c-Jun N-terminal kinase/NFκB axis.
Though most studies have focused on overexpressing transgenes, studies assessing the effect of gene silencing have also been conducted. For instance, caspase-8 shRNA-modified human MSCs were found to inhibit apoptosis and up-regulate HGF, IGF-1, and Bcl-2 in vitro (Liang et al., 2014). Moreover, when injected into the border zone of the infarcted region of the rat heart, these transgenic MSCs demonstrated enhanced survival and improved heart function with concomitant reduction in fibrosis and cardiomyocyte death.
In summary, these studies demonstrate that both overexpression and silencing of genes that regulate apoptosis could improve the survival and engraftment of transplanted ASCs, which could potentially lead to sustained improvements in cardiac function.
Pro-angiogenic modifications
Since most clinical studies have reported neovascularization to be the main contributor for restoring heart function, it seems appropriate to enhance these features in ASCs. In one study, when rat SMs overexpressing VEGF were seeded on scaffolds and implanted on rat myocardium, no functional recovery was observed, but an increase in vascularization was reported (von Wattenwyl et al., 2012). In a similar study, when MSCs overexpressing VEGF under hypoxic conditions were delivered into rat myocardium (Kim et al., 2011), an ischemia-responsive VEGF secretion was observed that led to increased neovascularization. In the same study, VEGF-MSCs were retained in greater numbers in the infarcted area and were associated with reduced cardiac remodeling. Simultaneous overexpression of FGF-4 and VEGF-A in human primary myoblasts was also shown to induce successful capillary formation, but more importantly did not alter their basic biological functions, including down-regulation of myogenic genes (Zimna et al., 2014).
It is important to note that when generating transgenic cell lines, the type of promoter determines the outcome as much as the transgene itself. For instance, the transplantation of bone marrow-derived EPCs overexpressing VEGF under the control of a hypoxia promoter, was found to express more VEGF and induce more angiogenesis in rats as compared to cells overexpressing VEGF under the control of a constitute cytomegalovirus promoter (She et al., 2012). Moreover, careful consideration must be taken into account that regional higher levels of VEGF could potentially induce abnormal vessel growth, which may counteract its benefits. Therefore, efforts have been made to control VEGF secretion as evidenced by the generation of inducible VEGF-secreting human umbilical cord blood-derived MSCs (hUCB-MSCs) (Cho et al., 2017). These transgenic MSCs, when seeding onto a cardiac patch and grafted onto the injured epicardial anterolateral region in MI rats, were capable of secreting VEGF at physiological concentrations in a controlled setting and were associated with decreased infarct size as well as improved LVEF and fractional shortening.
Human MSCs have been modified to overexpress angiopoietin-1, which improved their survival in the infarcted rat myocardium, increased angiogenesis and arteriogenesis by 11-35%, reduced the infarcted area by 30%, and remarkably thickened the LV wall by 46% (Sun et al., 2007). Similarly, angiogenin modified MSCs were found to exhibit enhanced tolerance against hypoxia in vitro and improved viability in infarcted hearts, assisting in the preservation of contractile function and reducing cardiac remodeling through vasculogenesis as evidenced by the presence of factor VIII and α-smooth muscle actin (αSMA) (Liu et al., 2008). In another study, overexpression of SIRT1 was found to ameliorate the senescent phenotype of aged MSCs and improve their biological functions (Liu et al., 2014), likely mediated by increased expression of pro-angiogenic factors, including angiopoietin-1 and basic FGF.
The endothelial NOS (eNOS) signaling pathway is instrumental in regulating vasodilation and endothelial cell proliferation. In support, transplantation of eNOS overexpressing EPCs into the infarcted heart was shown to decrease tumor necrosis factor- alpha (TNF-α) and IL-1β that led to reductions in infarct size and increased angiogenesis in the peri-infarct region that culminated in improved cardiac function (Chen et al., 2013). Similarly, when human primary myoblasts and C2C12 mouse myoblasts were transfected with eNOS, both cell types were found to secrete large quantities of eNOS, which in turn induced capillary sprouting in human umbilical vein endothelial cells (HUVEC) cells (Janeczek et al., 2013). Unexpectedly, eNOS overexpression in human cells resulted in the down-regulation of myogenin, a transcriptional activator that is required for skeletal muscle development. Whether this down-regulation is deleterious remains to be determined.
Recently, miRNAs have been found to be master regulators of angiogenesis (Ghodrat et al., 2021) and consistently, MSCs overexpressing miR-126 have been found to increase secretion of pro-angiogenic factors and also exhibit greater resistance to hypoxia in vitro (Huang et al., 2013). In the same study, miR-126-MSCs injected into the infarcted region demonstrated increased survival while promoting angiogenesis and improving heart function. Interestingly, these transgenic MSCs expressed elevated levels of delta-like-4 (Dll-4), which was shown to mediate tubulogenesis following transplantation. Importantly, the elevation of all miRNAs is not beneficial and this is shown from the observation that increased miR-377 in HF, is in fact detrimental to stem cell function. However, suppression of miR-377 in transplanted CD34+ cells was able to promote their angiogenic ability and reduce interstitial fibrosis, which resulted in improved LV function in a mouse model of ischemia/reperfusion injury (Joladarashi et al., 2015).
Finally, bone marrow derived-EPCs overexpressing IGF-1 have been found to increase cardiomyocyte proliferation and increase vascularization in the peri-infarct region in MI rats (Sen et al., 2010). Moreover, human CD34+ cells modified to enhance secretion of sonic hedgehog (Shh) were found to protect against ventricular dilation, reduce infarct size, and increase angiogenesis when transplanted into the border zone (Mackie et al., 2012). Interestingly, CD34+ cells were found to store Shh in exosomes and upon secretion; these exosomes could transfer Shh to other cell types, eliciting the induction of canonical Shh signaling. In summary, transgenic modifications that conferred ASCs with more angiogenic ability reveal remarkable improvements to cardiac function and could potentially serve as a therapy for ischemic HF.
Homing enhancing modifications
While intramyocardial injections do guarantee delivery of ASCs to the heart, it is an invasive technique and hence, less-invasive approaches are sought (e.g., intravenous/intracoronary delivery). A major limitation of these approaches is that they are heavily dependent on ASCs to successfully home in on the damaged myocardial region. Hence, understanding the signaling cascades that regulate homing properties could help circumvent this issue. The stromal cell-derived factor 1/C-X-C- motif chemokine receptor 4 (SDF-1)/CXCR4) axis has been reported to be important for homing of progenitor cells to ischemic tissue (Cheng and Qin, 2012; Cheng et al., 2015), and in support, intravenous delivery of CXCR4 overexpressing MSCs were found to possess increased homing ability towards the infarct region and were able to reduce cardiac remodeling and improve ventricular function in rats (Cheng et al., 2008). These findings suggested that intravenous delivery of CXCR4-MSCs is a safe and non-invasive method for attainting functional recovery post-MI. On the contrary, intracoronary delivery of SDF-1α overexpressing EPCs have been found to only increase angiogenesis, unlike myocardial delivery, which resulted in reduced collagen content and improved cardiac function (Schuh et al., 2012). Moreover, although CXCR4 overexpressing human MSCs were capable of increasing cytosolic Ca2+ and activating certain mitogen-activated protein kinases in response to SDF-1α stimulation, they were unable to demonstrate improvements in cell migration in vitro (Wiehe et al., 2013), which could be attributed to the strong basal chemokinesis properties already present in naïve MSCs.
MSCs overexpressing C-C motif chemokine receptor 1 (CCR1) were found to possess augmented migration and anti-apoptotic properties that led to the reinstatement of cardiac function and prevented cardiac remodeling post-MI (Huang et al., 2010). While CCR1-MSCs were delivered via intramyocardial injection, it will be interesting to assess if similar functional improvements could be obtained through intravenous/intracoronary delivery. Moreover, migration, survival, and proliferation of adipose-derived mesenchymal stem cells (ADSCs) via an extracellular signal-regulated kinase 1/2-MMP-9 axis may also be enhanced by the cardiokine c1q/tumor necrosis factor-related protein-9 (CTRP9) to aid engraftment in addition to guarding against oxidative stress-mediated apoptosis by elevating SOD3 (Yan et al., 2017).
In summary, achieving successful homing and engraftment of ASCs through less-invasive approaches is still a considerable challenge and hence, further studies are needed to identify other mediators that could facilitate homing. The application of intravenous/intracoronary delivery of ASCs should also be viewed with caution as it could heighten the risk of embolism and disrupt microcirculation (Fiarresga et al., 2015). Alternatively, to circumvent the unsuccessful engraftment of cells, CD34-CD42b platelet-targeting bispecific antibodies (PT-BsAbs) have been developed to concurrently recognize both CD34+ HSCs and CD42b+ platelets so as to redirect cells from the lung to the damaged heart as an intervention strategy for MI (Liu et al., 2020a). Other novel strategies also include the usage of multilineage-differentiating stress enduring (Muse) cells that home in via the sphingosine-1-phosphate/ sphingosine-1-phosphate receptor 2 axis to the infarcted heart with corresponding decreases in infarct size and increased LVEF (Yamada et al., 2018).
Anti-inflammatory modifications
Apart from engraftment and survival, another challenge faced by transplanted ASCs is their clearance by the host immune system and hence, measures have been taken to circumvent such events. The highly expressed angiopoietin-like 4 (ANGPTL4) in MSCs is critical for suppressing the inflammatory responses and instigating cardiac repair as evidenced by increases in anti-inflammatory macrophages and decreases in pro-inflammatory macrophages in the setting of MI (Cho et al., 2019). Consistently, ANGPTL4-deficient MSCs were found to be unsuccessful in inhibiting the inflammatory response, while treatment with ANGPTL4 led to improvements in cardiac function (Cho et al., 2019). The therapeutic efficacy of MSCs could also be bolstered by inhibiting Ras-proximate-1 or Ras-related protein 1 (Rap1), as Rap1-KO was found to decrease production of pro-inflammatory cytokines and prevent apoptosis of cardiomyocytes in vitro (Zhang et al., 2015). In other studies, MSC survival and proliferation following transplantation has been enhanced by overexpression of follistatin like 1 (a cardiokine), which led to improvements in cardiac function post-MI in addition to restricting scar formation, diminishing inflammatory response and augmenting neovascularization (Shen et al., 2019). Finally, transplantation of MSCs overexpressing TNFR (an anti-inflammatory mediator) was found to reduce production of inflammatory cytokines (TNF-, IL-1β and IL-6) as well as inhibited cardiomyocyte apoptosis, which culminated in improved cardiac function (Bao et al., 2008; Bao et al., 2010). In summary, transgenic ASCs that display modulated inflammatory signaling cascades, either through suppression of pro-inflammatory responses or by enhancing the action of anti-inflammatory mediators could be considered for cardiac therapy.
Alternate strategies to bolster ASC properties
In addition to enhancing survival, engraftment, homing, angiogenic potential, and anti-inflammatory properties, other modifications could also be considered to facilitate the development of ASCs with augmented therapeutic properties. These strategies include modulation of genes that regulate cell adhesion and cell-matrix communications. For instance, transplantation of MSCs overexpressing periostin (a protein assisting in cell adhesion) was found to enhance their survival in addition to preventing apoptosis of cardiomyocytes (Cho et al., 2012). Co-culture of cardiomyocyte with perisotin-MSCs or with their conditioned media led to better survival under hypoxic conditions where mechanistic studies attributed this to prevention of adhesion-related integrin reduction along with increases in PI3K and Akt phosphorylation. Since the loss of elastin is instrumental in cardiac remodeling, transplantation of elastin overexpressing bone marrow stromal cells into rats led to elastin deposits in the infarct, which in turn preserved myocardium structural integrity (Li et al., 2012a). In the same study, though reduction in infarct size was not observed, it was not found to expand either and more importantly, functional improvement was noted. Moreover, overexpression of fatty acid binding protein 3 (FABP3), a protein involved in early myocardial development and adult myocardial tissue repair has also been tested in human MSCs. However, despite improving MSC survival under hypoxic conditions, FABP3 expression unexpectedly had negative effects on cell growth and proliferation (Zhang et al., 2015), possibly by affecting the secretion of paracrine factors.
Injection of conditioned medium from integrin-linked kinase ,(ILK) overexpressing MSCs into MI hearts has been found to inhibit cardiac fibroblast proliferation, reduce collagen type I and III gene expression as well as TIMP-1, TIMP-2, α-SMA, and connective tissue growth factor (Mao et al., 2013). In the same study, increased gene expression of MMP-2 and MMP-9 was also documented and accompanied by improvements in contractility, decreased infarct size, and fibrosis. Similar observations have been made in large animal studies, where ILK-MSCs demonstrated increased proliferation and suppressed apoptosis, which led to reductions in cardiac remodeling and restored heart function (Mao et al., 2014), likely mediated by increased neovascularization as well as cardiomyocyte proliferation. In other studies, mouse Sca-1+ cells overexpressing ILK have also shown increased viability, proliferation, and survival in vitro through activation of Akt and cyclin D (Ling et al., 2013). Interestingly, when injected into the myocardium, though long-term assessment of ILK-Sca-1+ cells did not seem to indicate better engraftment than controls, they did seem to induce reductions in infarct size and improved cardiac function.
When mouse Sca-1+ cells overexpressed a cocktail of growth factors including human IGF-1, VEGF, SDF-1α, and HGF, they were found to up-regulate multiple angiogenic and survival factors that enhanced their survival in lethal hypoxic conditions (Li et al., 2014). In the same study, transplantation of growth factor-Sca-1+ cells were found to exhibit better survival with heightened mobilization of c-Kit+, multidrug resistance-1+, and CXCR4+ cells with histological analysis revealing extensive myofiber formation, increased gap junction proteins, and increased blood vessel density in both peri-infarct and infarct regions. Conversely, combined administration of MSCs overexpressing IGF-1 and HGF was unable to improve cardiac function in a porcine MI model (Kulandavelu et al., 2016). Although an increase in neovascularization was observed, this was also accompanied by an increase in fibrosis, suggesting that sustained exposure to growth factors could be beneficial as well as deleterious, while also highlighting the importance of validating initial findings in clinically relevant large animal models.
Future directions
The above studies support the use of transgenic ASCs for restoring cardiac function; however, careful consideration should be made when selecting a cell type for therapeutic intervention as patient age and cardiovascular risk factors are reported to weaken the effectiveness of ASCs (Zhao et al., 2018). Alternatively, human induced pluripotent stem cells (hiPSCs) that are derived from patient somatic cells can be considered, as they are capable of differentiating into virtually every cell type. In support, hiPSCs overexpressing cyclin D2 when differentiated into cardiomyocytes were found to induce LV muscle regeneration and increased angiogenesis at the border zone in MI mice (Zhu et al., 2018). In another study, overexpression of endothelial cell-specific molecule 1 in hiPSC-derived endothelial cells was found to improve angiogenesis and neovascularization (Vila-Gonzalez et al., 2019). The temporary overexpression of Ang-1 in hiPSC-derived cardiomyocytes has also been associated with better engraftment and improved heart structure and function post-MI (Tao et al., 2020). Similarly, overexpression of cadherin 2 (N-cadherin) in hiPSC-derived cardiomyocytes was found to improve their survival, engraftment, and reparative potency with elevated LVEF and reduced infarct size in MI mice (Lou et al., 2020). Interestingly, extracellular vesicles released by hiPSC-derived cardiac progenitor cells have also been found to be cardioprotective in an acute infarction model that was attributed to the down-regulation of pro-inflammatory (cytokines IL-1α, IL-2, and IL-6, M1 macrophages, pro-inflammatory monocytes) and up-regulation of anti-inflammatory responses (cytokines IL-10, M2 macrophages) (Lima Correa et al., 2021). Based on these findings, not only are hiPSCs an unlimited source of therapeutic cells but also provides the flexibility for implementing various cell types for different therapeutic purposes, should the need arise.
Implementation of exosomes derived from MSCs are rapidly emerging as a cell-free alternative as concurrent delivery of both exosomes and stem cells have been found to improve cardiac function post-MI (Huang et al., 2019). Although the exact mechanism through which exosomes exert beneficial effects is unclear, it could be speculated that exosomes mainly function through modulation of miRNAs (Luther et al., 2018; Sun et al., 2020b). In support, treatment of aged MSCs with miR-136 (exosome cargo) significantly attenuated apoptosis and senescence of aged MSCs by down-regulating apoptotic protease activating factor-1 (Zhang et al., 2020). Other studies have also demonstrated the use of exosomes overexpressing macrophage migration inhibitory factor for improving cardiac function and reducing cardiomyocyte apoptosis, underscoring the crucial role exosomes could play in the treatment of MI (Liu et al., 2020b).
Consistent with the beneficial effects associated with gene modulation, overexpression of hypoxia-inducible factor 1-alpha in exosomes from MSCs was found to restore the impaired phenotype of HUVECs exposed to hypoxic conditions, in addition to preserving cardiac function by up-regulating pro-angiogenic factors and inducing neovascularization in MI rats (Sun et al., 2020a). Moreover, as inhibition of MMPs by TIMP-2 is a critical mediator of cardiac remodeling post-MI, exosomes obtained from TIMP2 overexpressing hUCB-MSCs were found to improve cardiac function by alleviating MI-induced oxidative stress and ECM remodeling (Ni et al., 2019). In other studies, exosomes secreted by SIRT1 overexpressing ADSCs (ADSCs-SIRT1-Exos) were found to improve cardiac function, reduce infarct size, attenuate LV remodeling and inflammation, as well as promote vasculogenesis, likely mediated through the recruitment of endothelial progenitor cells (Huang et al., 2020). Finally, exosomes derived from miR-146a-modified ADSCs and miR-126 overexpressing ADSCs have been found to be associated with beneficial effects post-MI (Luo et al., 2017; Pan et al., 2019).
In summary, though the use of both iPSC-derived progenies and exosomes could potentially serve as effective alternatives to ASCs, the requirement for large quantities of cells/exosome could be a hindrance towards their clinical adoption. While these obstacles could be circumvented by devising efficient differentiation protocols (Mehta et al., 2014; Ramachandra et al., 2018) and by boosting exosome number through adiponectin treatment (Nakamura et al., 2020), further studies in large animal models are needed to draw conclusions on the therapeutic effectiveness of these approaches.
Conclusions
Several preclinical studies have reported the successful implementation of ASCs for restoring the failing heart. However, implementation of cardiac therapy in the clinical setting has been largely disappointing with only a few studies having been conducted with sufficient sample size and appropriate controls to demonstrate the effectiveness of ASCs for improving clinical outcomes. In reality, cardiac therapy faces several challenges; including (i) identifying the type/number of cell or combination of cells to be used, (ii) discerning the appropriate timing/frequency and route of cell delivery, (iii) the poor retention of injected cells or the low integration of cell sheets, and (iv) the limited effectiveness of the therapy. Recently, several experimental studies have revealed transgenic ASCs to be a viable alternative as it enables us to bolster key beneficial properties with the aim of augmenting their therapeutic potency for restoring the failing heart (Figure 2). These transgenic ASCs will probably face similar challenges as their naïve counterparts; however, their enhanced survival and engraftment, heightened angiogenic potential, increased homing, and reduced inflammatory responses are grounds for optimism. The increasing prevalence of HF and lack of restorative therapies does warrant an urgent need for novel intervention strategies and if transgenic ASCs are to be considered, they must be validated accordingly through standardized, large, multi-centered clinical trials.
Acknowledgements
Chrishan Ramachandra is supported by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Young Individual Research Grant (NMRC/OFYIRG/0073/2018), the National Health Innovation Centre Singapore under its Innovation to Develop Grant (NHIC-I2S-1811007) and the SingHealth Duke-NUS Academic Medical Centre under its SingHealth Duke-NUS Academic Medicine Research Grant (AM/TP033/2020 [SRDUKAMR2033]).
References
Abbate A, Biondi-Zoccai GG, Baldi A (2002) Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 193:145-153.
Abdalmula A, Dooley LM, Kaufman C, Washington EA, House JV, Blacklaws BA, Ghosh P, Itescu S, Bailey SR, Kimpton WG (2017) Immunoselected STRO-3(+) mesenchymal precursor cells reduce inflammation and improve clinical outcomes in a large animal model of monoarthritis. Stem Cell Res Ther 8:22.
Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M (2008) Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med 5:663-670.
Aonuma T, Takehara N, Maruyama K, Kabara M, Matsuki M, Yamauchi A, Kawabe J, Hasebe N (2016) Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction. Stem Cells Transl Med 5:1067-1078.
Bagno L, Hatzistergos KE, Balkan W, Hare JM (2018) Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther 26:1610-1623.
Banerjee MN, Bolli R, Hare JM (2018) Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ Res 123:266-287.
Bao C, Guo J, Lin G, Hu M, Hu Z (2008) TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J 42:56-62.
Bao C, Guo J, Zheng M, Chen Y, Lin G, Hu M (2010) Enhancement of the survival of engrafted mesenchymal stem cells in the ischemic heart by TNFR gene transfection. Biochem Cell Biol 88:629-634.
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763-776.
Bolli R et al. (2018) Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit(+) Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ Res 122:1703-1715.
Borow KM, Yaroshinsky A, Greenberg B, Perin EC (2019) Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ Res 125:265-281.
Braunwald E (2018) Cell-Based Therapy in Cardiac Regeneration: An Overview. Circ Res 123:132-137.
Broughton KM, Sussman MA (2018) Enhancement Strategies for Cardiac Regenerative Cell Therapy: Focus on Adult Stem Cells. Circ Res 123:177-187.
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076-1084.
Chen X, Gu M, Zhao X, Zheng X, Qin Y, You X (2013) Deterioration of cardiac function after acute myocardial infarction is prevented by transplantation of modified endothelial progenitor cells overexpressing endothelial NO synthases. Cell Physiol Biochem 31:355-365.
Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J, Chen Y, Lei W, Shen Z (2017) MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther 8:268.
Cheng M, Qin G (2012) Progenitor cell mobilization and recruitment: SDF-1, CXCR4, alpha4-integrin, and c-kit. Prog Mol Biol Transl Sci 111:243-264.
Cheng M, Huang K, Zhou J, Yan D, Tang YL, Zhao TC, Miller RJ, Kishore R, Losordo DW, Qin G (2015) A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol 81:49-53.
Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D (2008) Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 16:571-579.
Cho DI, Kang HJ, Jeon JH, Eom GH, Cho HH, Kim MR, Cho M, Jeong HY, Cho HC, Hong MH, Kim YS, Ahn Y (2019) Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight 4.
Cho HM, Kim PH, Chang HK, Shen YM, Bonsra K, Kang BJ, Yum SY, Kim JH, Lee SY, Choi MC, Kim HH, Jang G, Cho JY (2017) Targeted Genome Engineering to Control VEGF Expression in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells: Potential Implications for the Treatment of Myocardial Infarction. Stem Cells Transl Med 6:1040-1051.
Cho YH, Cha MJ, Song BW, Kim IK, Song H, Chang W, Lim S, Ham O, Lee SY, Choi E, Kwon HM, Hwang KC (2012) Enhancement of MSC adhesion and therapeutic efficiency in ischemic heart using lentivirus delivery with periostin. Biomaterials 33:1376-1385.
Cruz-Samperio R, Jordan M, Perriman A (2021) Cell augmentation strategies for cardiac stem cell therapies. Stem Cells Transl Med.
Desgres M, Menasche P (2019) Clinical Translation of Pluripotent Stem Cell Therapies: Challenges and Considerations. Cell Stem Cell 25:594-606.
Diederichsen AC, Moller JE, Thayssen P, Videbaek L, Saekmose SG, Barington T, Kassem M (2010) Changes in left ventricular filling patterns after repeated injection of autologous bone marrow cells in heart failure patients. Scand Cardiovasc J 44:139-145.
Durrani S, Haider KH, Ahmed RP, Jiang S, Ashraf M (2012) Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury. Stem Cells Dev 21:1769-1778.
Evans CH, Huard J (2015) Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol 11:234-242.
Evans CH, Ghivizzani SC, Robbins PD (2013) Arthritis gene therapy and its tortuous path into the clinic. Transl Res 161:205-216.
Fiarresga A, Mata MF, Cavaco-Goncalves S, Selas M, Simoes IN, Oliveira E, Carrapico B, Cardim N, Cabral JM, Ferreira RC, da Silva CL (2015) Intracoronary Delivery of Human Mesenchymal/Stromal Stem Cells: Insights from Coronary Microcirculation Invasive Assessment in a Swine Model. PLoS One 10:e0139870.
Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, Schachinger V, Tonn T, Martin H, Dimmeler S, Zeiher AM (2009) A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail 2:417-423.
Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL (2006) A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27:1114-1122.
Fukushima S, Coppen SR, Lee J, Yamahara K, Felkin LE, Terracciano CM, Barton PJ, Yacoub MH, Suzuki K (2008) Choice of cell-delivery route for skeletal myoblast transplantation for treating post-infarction chronic heart failure in rat. PLoS One 3:e3071.
Gao L, Bledsoe G, Yin H, Shen B, Chao L, Chao J (2013) Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction. Circ J 77:2134-2144.
Ghiroldi A, Piccoli M, Cirillo F, Monasky MM, Ciconte G, Pappone C, Anastasia L (2018) Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. Int J Mol Sci 19.
Ghodrat S, Hoseini SJ, Asadpour S, Nazarnezhad S, Alizadeh Eghtedar F, Kargozar S (2021) Stem cell-based therapies for cardiac diseases: The critical role of angiogenic exosomes. Biofactors.
Giraud MN, Liechti EF, Tchantchaleishvili V, Siepe M, Cook S, Carrel TP, Tevaearai HT (2010) Myocardial injection of skeletal myoblasts impairs contractility of host cardiomyocytes. Int J Cardiol 138:131-137.
Groenewegen A, Rutten FH, Mosterd A, Hoes AW (2020) Epidemiology of heart failure. Eur J Heart Fail 22:1342-1356.
Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska-Migaj E, Oko-Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M (2017) Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail 19:148-157.
Hare JM et al. (2012) Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308:2369-2379.
Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL (2006) Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation 114:I101-107.
Holzinger A, Barden M, Abken H (2016) The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother 65:1433-1450.
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, Zhou SH (2013) Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med 31:484-492.
Huang H, Xu Z, Qi Y, Zhang W, Zhang C, Jiang M, Deng S, Wang H (2020) Exosomes from SIRT1-Overexpressing ADSCs Restore Cardiac Function by Improving Angiogenic Function of EPCs. Mol Ther Nucleic Acids 21:737-750.
Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ (2010) Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106:1753-1762.
Huang P, Wang L, Li Q, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Liu J, Qian L, Yang Y (2019) Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther 10:300.
Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y, Hirai K, Fukushima Y, Baba K, Goto T, Horio N, Kobayashi J, Kuroko Y, Kotani Y, Arai S, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H (2017) Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ Res 120:1162-1173.
Janeczek A, Zimna A, Rozwadowska N, Fraczek M, Kucharzewska P, Rucinski M, Mietkiewski T, Kolanowski T, Malcher A, Kurpisz M (2013) Genetically modified human myoblasts with eNOS may improve regenerative ability of myogenic stem cells to infarcted heart. Kardiol Pol 71:1048-1058.
Jiang Y, Chen L, Tang Y, Ma G, Shen C, Qi C, Zhu Q, Yao Y, Liu N (2010) HO-1 gene overexpression enhances the beneficial effects of superparamagnetic iron oxide labeled bone marrow stromal cells transplantation in swine hearts underwent ischemia/reperfusion: an MRI study. Basic Res Cardiol 105:431-442.
Jimenez-Quevedo P et al. (2014) Selected CD133(+) progenitor cells to promote angiogenesis in patients with refractory angina: final results of the PROGENITOR randomized trial. Circ Res 115:950-960.
Joladarashi D, Garikipati VNS, Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj A, Youker KA, Uribe C, Suresh Babu S, Jeyabal P, Kishore R, Krishnamurthy P (2015) Enhanced Cardiac Regenerative Ability of Stem Cells After Ischemia-Reperfusion Injury: Role of Human CD34+ Cells Deficient in MicroRNA-377. J Am Coll Cardiol 66:2214-2226.
Karantalis V et al. (2015) Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J Am Coll Cardiol 66:1990-1999.
Kim SH, Moon HH, Kim HA, Hwang KC, Lee M, Choi D (2011) Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol Ther 19:741-750.
Kitabayashi K, Siltanen A, Patila T, Mahar MA, Tikkanen I, Koponen J, Ono M, Sawa Y, Kankuri E, Harjula A (2010) Bcl-2 expression enhances myoblast sheet transplantation therapy for acute myocardial infarction. Cell Transplant 19:573-588.
Konijnenberg LSF, Damman P, Duncker DJ, Kloner RA, Nijveldt R, van Geuns RM, Berry C, Riksen NP, Escaned J, van Royen N (2020) Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res 116:787-805.
Kulandavelu S, Karantalis V, Fritsch J, Hatzistergos KE, Loescher VY, McCall F, Wang B, Bagno L, Golpanian S, Wolf A, Grenet J, Williams A, Kupin A, Rosenfeld A, Mohsin S, Sussman MA, Morales A, Balkan W, Hare JM (2016) Pim1 Kinase Overexpression Enhances ckit(+) Cardiac Stem Cell Cardiac Repair Following Myocardial Infarction in Swine. J Am Coll Cardiol 68:2454-2464.
Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC (2006) Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation 114:I174-180.
Lancaster JJ, Koevary JW, Chinyere IR, Goldman S (2018) The Promise of Induced Pluripotent Stem Cell-Derived Cardiomyocytes to Treat Heart Failure. Circ Heart Fail 11:e005425.
Lee CY, Shin S, Lee J, Seo HH, Lim KH, Kim H, Choi JW, Kim SW, Lee S, Lim S, Hwang KC (2016) MicroRNA-Mediated Down-Regulation of Apoptosis Signal-Regulating Kinase 1 (ASK1) Attenuates the Apoptosis of Human Mesenchymal Stem Cells (MSCs) Transplanted into Infarcted Heart. Int J Mol Sci 17.
Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z (2009) Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep 36:725-731.
Li N, Pasha Z, Ashraf M (2014) Reversal of ischemic cardiomyopathy with Sca-1+ stem cells modified with multiple growth factors. PLoS One 9:e93645.
Li SH, Sun Z, Guo L, Han M, Wood MF, Ghosh N, Vitkin IA, Weisel RD, Li RK (2012a) Elastin overexpression by cell-based gene therapy preserves matrix and prevents cardiac dilation. J Cell Mol Med 16:2429-2439.
Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E (2012b) Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59:942-953.
Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G (2007) Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25:2118-2127.
Liang Y, Lin Q, Zhu J, Li X, Fu Y, Zou X, Liu X, Tan H, Deng C, Yu X, Shan Z, Yuan W (2014) The caspase-8 shRNA-modified mesenchymal stem cells improve the function of infarcted heart. Mol Cell Biochem 397:7-16.
Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC (2006) The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res 70:530-542.
Lima Correa B et al. (2021) Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc Res 117:292-307.
Linares GR, Leng Y, Maric D, Chuang DM (2020) Overexpression of fibroblast growth factor-21 (FGF-21) protects mesenchymal stem cells against caspase-dependent apoptosis induced by oxidative stress and inflammation. Cell Biol Int 44:2163-2169.
Ling L, Bai J, Gu R, Jiang C, Li R, Kang L, Ferro A, Xu B (2013) Sca-1+ cardiac progenitor cell therapy with cells overexpressing integrin-linked kinase improves cardiac function after myocardial infarction. Transplantation 95:1187-1196.
Lionetti V, Recchia FA (2010) New therapies for the failing heart: trans-genes versus trans-cells. Transl Res 156:130-135.
Liu M, Lutz H, Zhu D, Huang K, Li Z, Dinh PC, Gao J, Zhang Y, Cheng K (2020a) Bispecific Antibody Inhalation Therapy for Redirecting Stem Cells from the Lungs to Repair Heart Injury. Adv Sci (Weinh) 8:2002127.
Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, Fan B, Zhao H, He H, Zhang F (2020b) Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol 235:8010-8022.
Liu X et al. (2014) Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant 33:1083-1092.
Liu XH, Bai CG, Xu ZY, Huang SD, Yuan Y, Gong DJ, Zhang JR (2008) Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvasc Res 76:23-30.
Lou X, Zhao M, Fan C, Fast VG, Valarmathi MT, Zhu W, Zhang J (2020) N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovasc Res 116:671-685.
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M (2017) Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol Biochem 44:2105-2116.
Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A, Song Y, Shen Z, Gardner G, Kuffel G, Ren X, Zilliox MJ, Jones WK (2018) Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol 119:125-137.
Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW (2012) Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 111:312-321.
Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E (2012) Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379:895-904.
Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195-1201.
Manginas A, Goussetis E, Koutelou M, Karatasakis G, Peristeri I, Theodorakos A, Leontiadis E, Plessas N, Theodosaki M, Graphakos S, Cokkinos DV (2007) Pilot study to evaluate the safety and feasibility of intracoronary CD133(+) and CD133(-) CD34(+) cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv 69:773-781.
Mao Q, Lin CX, Liang XL, Gao JS, Xu B (2013) Mesenchymal stem cells overexpressing integrin-linked kinase attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. Mol Med Rep 7:1617-1623.
Mao Q, Lin C, Gao J, Liang X, Gao W, Shen L, Kang L, Xu B (2014) Mesenchymal stem cells overexpressing integrin-linked kinase attenuate left ventricular remodeling and improve cardiac function after myocardial infarction. Mol Cell Biochem 397:203-214.
Mathur A, Fernandez-Aviles F, Bartunek J, Belmans A, Crea F, Dowlut S, Galinanes M, Good MC, Hartikainen J, Hauskeller C, Janssens S, Kala P, Kastrup J, Martin J, Menasche P, Sanz-Ruiz R, Yla-Herttuala S, Zeiher A, Group B (2020) The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 41:3702-3710.
Mazo M, Gavira JJ, Abizanda G, Moreno C, Ecay M, Soriano M, Aranda P, Collantes M, Alegria E, Merino J, Penuelas I, Garcia Verdugo JM, Pelacho B, Prosper F (2010) Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant 19:313-328.
Mazo M, Planat-Benard V, Abizanda G, Pelacho B, Leobon B, Gavira JJ, Penuelas I, Cemborain A, Penicaud L, Laharrague P, Joffre C, Boisson M, Ecay M, Collantes M, Barba J, Casteilla L, Prosper F (2008) Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail 10:454-462.
Mehta A, Ramachandra CJ, Sequiera GL, Sudibyo Y, Nandihalli M, Yong PJ, Koh CH, Shim W (2014) Phasic modulation of Wnt signaling enhances cardiac differentiation in human pluripotent stem cells by recapitulating developmental ontogeny. Biochim Biophys Acta 1843:2394-2402.
Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D (2003) Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 41:1078-1083.
Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911-921.
Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12:459-465.
Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S (2005) Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 112:1128-1135.
Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, Nishida H, Takahashi Y, Kawachi Y, Tsugawa-Shimizu Y, Fujishima Y, Nishizawa H, Takakura Y, Miyagawa S, Sawa Y, Maeda N, Shimomura I (2020) Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol Ther 28:2203-2219.
Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R, Stamm C (2014) Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 35:1263-1274.
Ng AP, Alexander WS (2017) Haematopoietic stem cells: past, present and future. Cell Death Discov 3:17002.
Ni J, Liu X, Yin Y, Zhang P, Xu YW, Liu Z (2019) Exosomes Derived from TIMP2-Modified Human Umbilical Cord Mesenchymal Stem Cells Enhance the Repair Effect in Rat Model with Myocardial Infarction Possibly by the Akt/Sfrp2 Pathway. Oxid Med Cell Longev 2019:1958941.
Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 100:12313-12318.
Oskouei BN, Lamirault G, Joseph C, Treuer AV, Landa S, Da Silva J, Hatzistergos K, Dauer M, Balkan W, McNiece I, Hare JM (2012) Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl Med 1:116-124.
Pan J, Alimujiang M, Chen Q, Shi H, Luo X (2019) Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem 120:4433-4443.
Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr., Kormos R, Benetti F (2005) Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg 130:1631-1638.
Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, Recker D, DeMaria A, ix C-DCMI (2016) Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet 387:2412-2421.
Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL (2020) Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis 11:317.
Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD (2015) A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res 117:576-584.
Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT (2004) Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation 110:II213-218.
Perin EC, Sanz-Ruiz R, Sanchez PL, Lasso J, Perez-Cano R, Alonso-Farto JC, Perez-David E, Fernandez-Santos ME, Serruys PW, Duckers HJ, Kastrup J, Chamuleau S, Zheng Y, Silva GV, Willerson JT, Fernandez-Aviles F (2014) Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J 168:88-95 e82.
Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT (2003) Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 107:2294-2302.
Perin EC et al. (2012) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307:1717-1726.
Ramachandra CJA, Hernandez-Resendiz S, Crespo-Avilan GE, Lin YH, Hausenloy DJ (2020) Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 57:102884.
Ramachandra CJA, Mehta A, Wong P, Ja K, Fritsche-Danielson R, Bhat RV, Hausenloy DJ, Kovalik JP, Shim W (2018) Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes. Int J Cardiol 272:288-297.
Rossini A, Frati C, Lagrasta C, Graiani G, Scopece A, Cavalli S, Musso E, Baccarin M, Di Segni M, Fagnoni F, Germani A, Quaini E, Mayr M, Xu Q, Barbuti A, DiFrancesco D, Pompilio G, Quaini F, Gaetano C, Capogrossi MC (2011) Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res 89:650-660.
Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J (2008) Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res 103:107-116.
Schachinger V et al. (2006) Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 27:2775-2783.
Schuh A, Kroh A, Konschalla S, Liehn EA, Sobota RM, Biessen EA, Bot I, Sonmez TT, Schober A, Marx N, Weber C, Sasse A (2012) Myocardial regeneration by transplantation of modified endothelial progenitor cells expressing SDF-1 in a rat model. J Cell Mol Med 16:2311-2320.
Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM (2009) Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 30:2722-2732.
Sen S, Merchan J, Dean J, Ii M, Gavin M, Silver M, Tkebuchava T, Yoon YS, Rasko JE, Aikawa R (2010) Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor-1 gene after myocardial infarction. Hum Gene Ther 21:1327-1334.
Seth S, Narang R, Bhargava B, Ray R, Mohanty S, Gulati G, Kumar L, Reddy KS, Venugopal P, Group ACSCS (2006) Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial. J Am Coll Cardiol 48:2350-2351.
She Q, Xia S, Deng SB, Du JL, Li YQ, He L, Xiao J, Xiang YL (2012) Angiogenesis in a rat model following myocardial infarction induced by hypoxic regulation of VEGF(1)(6)(5) gene-transfected EPCs. Mol Med Rep 6:1281-1287.
Shen H, Cui G, Li Y, Ye W, Sun Y, Zhang Z, Li J, Xu G, Zeng X, Zhang Y, Zhang W, Huang Z, Chen W, Shen Z (2019) Follistatin-like 1 protects mesenchymal stem cells from hypoxic damage and enhances their therapeutic efficacy in a mouse myocardial infarction model. Stem Cell Res Ther 10:17.
Shu T, Zeng B, Ren X, Li Y (2010) HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell 42:217-222.
Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC (2005) Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111:150-156.
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E (2007) Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115:896-908.
Sterner RM, Sterner RC, Brenes-Salazar JA, Yu Ballard AC (2018) Cellular therapies for chronic ischemic heart failure. Hellenic J Cardiol 59:78-90.
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z (2020a) HIF-1alpha overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther 11:373.
Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, Kangtao M, Chen F, Zhou C (2007) Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun 357:779-784.
Sun L, Zhu W, Zhao P, Zhang J, Lu Y, Zhu Y, Zhao W, Liu Y, Chen Q, Zhang F (2020b) Down-Regulated Exosomal MicroRNA-221 - 3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair. Front Cell Dev Biol 8:263.
Sun T, Dong YH, Du W, Shi CY, Wang K, Tariq MA, Wang JX, Li PF (2017) The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int J Mol Sci 18.
Tao L, Bei Y, Zhou Y, Xiao J, Li X (2015) Non-coding RNAs in cardiac regeneration. Oncotarget 6:42613-42622.
Tao Z, Loo S, Su L, Tan S, Tee G, Gan SU, Zhang J, Chen X, Ye L (2020) Angiopoietin-1 enhanced myocyte mitosis, engraftment, and the reparability of hiPSC-CMs for treatment of myocardial infarction. Cardiovasc Res.
Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ (1999) Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100:II247-256.
Vazir A, Fox K, Westaby J, Evans MJ, Westaby S (2019) Can we remove scar and fibrosis from adult human myocardium? Eur Heart J 40:960-966.
Vila-Gonzalez M et al. (2019) Enhanced Function of Induced Pluripotent Stem Cell-Derived Endothelial Cells Through ESM1 Signaling. Stem Cells 37:226-239.
von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K (2012) Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30:1575-1578.
von Wattenwyl R, Blumenthal B, Heilmann C, Golsong P, Poppe A, Beyersdorf F, Siepe M (2012) Scaffold-based transplantation of vascular endothelial growth factor-overexpressing stem cells leads to neovascularization in ischemic myocardium but did not show a functional regenerative effect. ASAIO J 58:268-274.
Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, Haddad F, Torre-Amione G (2011) Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail 17:272-281.
Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC (2013) Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res 112:165-173.
Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC (2009) Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells 27:3021-3031.
Wang Y, Chen X, Cao W, Shi Y (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15:1009-1016.
Wiehe JM, Kaya Z, Homann JM, Wohrle J, Vogt K, Nguyen T, Rottbauer W, Torzewski J, Fekete N, Rojewski M, Schrezenmeier H, Moepps B, Zimmermann O (2013) GMP-adapted overexpression of CXCR4 in human mesenchymal stem cells for cardiac repair. Int J Cardiol 167:2073-2081.
Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM (2013) Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127:213-223.
Wojakowski W et al. (2017) Effects of Transendocardial Delivery of Bone Marrow-Derived CD133(+) Cells on Left Ventricle Perfusion and Function in Patients With Refractory Angina: Final Results of Randomized, Double-Blinded, Placebo-Controlled REGENT-VSEL Trial. Circ Res 120:670-680.
Xu C, Hu Y, Hou L, Ju J, Li X, Du N, Guan X, Liu Z, Zhang T, Qin W, Shen N, Bilal MU, Lu Y, Zhang Y, Shan H (2014) beta-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol 75:111-121.
Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, Baba S, Shigemoto T, Kuroda Y, Kanamori H, Amin M, Kawasaki M, Nishigaki K, Taoka M, Isobe T, Muramatsu C, Dezawa M, Minatoguchi S (2018) S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ Res 122:1069-1083.
Yan W, Guo Y, Tao L, Lau WB, Gan L, Yan Z, Guo R, Gao E, Wong GW, Koch WL, Wang Y, Ma XL (2017) C1q/Tumor Necrosis Factor-Related Protein-9 Regulates the Fate of Implanted Mesenchymal Stem Cells and Mobilizes Their Protective Effects Against Ischemic Heart Injury via Multiple Novel Signaling Pathways. Circulation 136:2162-2177.
Yang PC (2018) Induced Pluripotent Stem Cell (iPSC)-Derived Exosomes for Precision Medicine in Heart Failure. Circ Res 122:661-663.
Yao Y, Sheng Z, Li Y, Fu C, Ma G, Liu N, Chao J, Chao L (2013) Tissue kallikrein-modified human endothelial progenitor cell implantation improves cardiac function via enhanced activation of akt and increased angiogenesis. Lab Invest 93:577-591.
Yau TM et al. (2019) Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial. JAMA 321:1176-1186.
Yu K, Zeng Z, Cheng S, Hu W, Gao C, Liu F, Chen J, Qian Y, Xu D, Zhao J, Liu X, Wang J (2020) TPP1 Enhances the Therapeutic Effects of Transplanted Aged Mesenchymal Stem Cells in Infarcted Hearts via the MRE11/AKT Pathway. Front Cell Dev Biol 8:588023.
Yu YS, Shen ZY, Ye WX, Huang HY, Hua F, Chen YH, Chen K, Lao WJ, Tao L (2010) AKT-modified autologous intracoronary mesenchymal stem cells prevent remodeling and repair in swine infarcted myocardium. Chin Med J (Engl) 123:1702-1708.
Zeng B, Ren X, Lin G, Zhu C, Chen H, Yin J, Jiang H, Yang B, Ding D (2008) Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol Int 32:1256-1264.
Zhang DY, Zhang CF, Fu BC, Sun L, Wang XQ, Chen W, Liu W, Liu KY, Du GQ, Ma CY, Jiang SL, Li RK, Tian H (2018) Sirtuin3 protects aged human mesenchymal stem cells against oxidative stress and enhances efficacy of cell therapy for ischaemic heart diseases. J Cell Mol Med 22:5504-5517.
Zhang L, Wu Y, Li Y, Xu C, Li X, Zhu D, Zhang Y, Xing S, Wang H, Zhang Z, Shan H (2012) Tanshinone IIA improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell Physiol Biochem 30:843-852.
Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H (2020) Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther 11:273.
Zhang Y, Chiu S, Liang X, Gao F, Zhang Z, Liao S, Liang Y, Chai YH, Low DJ, Tse HF, Tergaonkar V, Lian Q (2015) Rap1-mediated nuclear factor-kappaB (NF-kappaB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov 1:15007.
Zhao ZA, Han X, Lei W, Li J, Yang Z, Wu J, Yao M, Lu XA, He L, Chen Y, Zhou B, Hu S (2018) Lack of Cardiac Improvement After Cardiosphere-Derived Cell Transplantation in Aging Mouse Hearts. Circ Res 123:e21-e31.
Zheng SX, Weng YL, Zhou CQ, Wen ZZ, Huang H, Wu W, Wang JF, Wang T (2013) Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev Rep 9:339-349.
Zhu W, Zhao M, Mattapally S, Chen S, Zhang J (2018) CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ Res 122:88-96.
Zimna A, Janeczek A, Rozwadowska N, Fraczek M, Kucharzewska P, Rucinski M, Mietkiewski T, Kurpisz M (2014) Biological properties of human skeletal myoblasts genetically modified to simultaneously overexpress the pro-angiogenic factors vascular endothelial growth factor-A and fibroblast growth factor-4. J Physiol Pharmacol 65:193-207.
References
En Ping Yap1-3†
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Xavier Chan1,2,4†
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 4Faculty of Science, National University of Singapore, Singapore.
Fan Yu1-3
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Shuo Cong1-3
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Chrishan J. A. Ramachandra1,2*
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.
†These authors contributed equally to this article.
*Corresponding author:
Dr. Chrishan J. A. Ramachandra
Email: chrishan.ramachandra@nhcs.com.sg

In a new window | Download PPT
Figure 1: Schematic highlighting the types of adult stem cells that can be used to potentially restore the failing heart by attenuatiing adverse cardiac remodeling and improving cardiac function.

In a new window | Download PPT
Figure 2: Schematic overview of transgenes that can bolster the therapeutic properties of adult stem cells (Up and down arrows indicate overexpression and suppression respectively).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6245 | 23 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA