International bi-monthly journal of cell signaling, tissue protection, and translational research.
Ischemia ± exercise as a means to promote peripheral as well as central organ preconditioning
Kristian Vissing1, Benjamin F. Miller2, Kim Ryun Drasbek3, Hans Erik Bøtker4
Author Affiliations


- 1Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark.
- 2Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, United States.
- 3Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
- 4Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Abstract
Exposing tissues to brief periods of ischemia confers resistance to subsequent prolonged ischemia (local ischemic preconditioning). Though first described in the tissue undergoing ischemia, a systemic response with protection of remote tissues (remote ischemic conditioning, RIC) can be induced by repeated brief ischemia of a limb. RIC can be applied clinically to reduce infarct size and/or improve outcomes in patients admitted with acute myocardial infarction or stroke. A resembling stimulus and systemic protective effect may be achievable from intermittent occlusion-reperfusion elicited by the contraction-relaxation phases during resistance exercise. Moreover, this stimulus may be further enhanced when practiced with simultaneous blood flow restriction, referred to as blood flow-restricted resistance exercise (BFRRE). While the preconditioning effects of BFRRE and the detailed mechanisms by which RIC and BFRRE may exert protection remain to be defined, RIC has been shown to induce organ protection via systemic mediators acting through direct cytoprotection in the target organ. The protective processes appear to be partly facilitated by extracellular vesicles (EV) carrying micro RNAs (miRNAs) from the site of occlusion to sites of organ damage. Moreover, RIC and exercise regimens may promote tissue rebuilding upon repeated application. However, little is known on the effects of RIC versus BFRRE on acute EV-derived responses and on chronic peripheral skeletal muscle responses. In the current review, with background in the literature and recent experimental discoveries, we discuss the potential of ischemia and/or exercise regimens in promoting peripheral and central organ preconditioning.
Keywords: Remote ischemic conditioning, blood flow-restricted exercise, chronic heart failure, stroke, skeletal muscle, extracellular vesicles
Abstract
Exposing tissues to brief periods of ischemia confers resistance to subsequent prolonged ischemia (local ischemic preconditioning). Though first described in the tissue undergoing ischemia, a systemic response with protection of remote tissues (remote ischemic conditioning, RIC) can be induced by repeated brief ischemia of a limb. RIC can be applied clinically to reduce infarct size and/or improve outcomes in patients admitted with acute myocardial infarction or stroke. A resembling stimulus and systemic protective effect may be achievable from intermittent occlusion-reperfusion elicited by the contraction-relaxation phases during resistance exercise. Moreover, this stimulus may be further enhanced when practiced with simultaneous blood flow restriction, referred to as blood flow-restricted resistance exercise (BFRRE). While the preconditioning effects of BFRRE and the detailed mechanisms by which RIC and BFRRE may exert protection remain to be defined, RIC has been shown to induce organ protection via systemic mediators acting through direct cytoprotection in the target organ. The protective processes appear to be partly facilitated by extracellular vesicles (EV) carrying micro RNAs (miRNAs) from the site of occlusion to sites of organ damage. Moreover, RIC and exercise regimens may promote tissue rebuilding upon repeated application. However, little is known on the effects of RIC versus BFRRE on acute EV-derived responses and on chronic peripheral skeletal muscle responses. In the current review, with background in the literature and recent experimental discoveries, we discuss the potential of ischemia and/or exercise regimens in promoting peripheral and central organ preconditioning.
Keywords: Remote ischemic conditioning, blood flow-restricted exercise, chronic heart failure, stroke, skeletal muscle, extracellular vesicles
Introduction
Cardiovascular disease has immense negative effects on global population health and life expectancy (Roger et al., 2012). The most detrimental acute ischemic events are myocardial infarction and stroke. Acute myocardial infarction is often caused by atherosclerotic rupture, leading to an inflammatory response, thrombosis, and platelet aggregation, further resulting in decreased oxygenation of the myocardium and ultimately cell death (Heusch and Gersh, 2017). Acute ischemic stroke is caused by occlusion of a cerebral artery, resulting in oxidative stress, excitotoxicity, immune responses, and cerebrovascular dysfunction, which similarly lead to hypoxia and cell death (Kuriakose and Xiao, 2020). Organ failure caused by such acute ischemic complications collectively constitutes the leading cause of death globally and carry a vast socio-economic burden. In accordance, 17.6 million people worldwide are estimated to die from cardiovascular diseases (which are primarily attributed to the 9.5 million from ischemic heart disease and the 5.5 million from stroke) (The Global Burden of Disease Study, 2017). Annually, 15 million people suffer a stroke of whom 1/3 die and 1/3 are left disabled, while the 30-day mortality risk is as high as 15% (Feigin et al., 2018). Treatment of cardiovascular disease is therefore crucial to alleviate the disease burden.
In acute ischemic conditions, early and successful restoration of tissue perfusion following an ischemic event is the most effective strategy to reduce tissue injury and improve clinical outcome (Heusch and Gersh, 2017). However, reperfusion treatment strategies may itself inflict further tissue damage, referred to as reperfusion injury (Heusch, 2020). Thus, although reperfusion treatment strategies continue to progress by improved logistics and medical treatment, even optimized acute reperfusion treatment may leave patients with chronic tissue damage (Heusch, 2020). Yet, the development of effective drugs to target the detrimental effects of reperfusion injury has proven to be a challenge. In accordance, several pharmacological strategies showing convincing effects in animal models of ischemia-reperfusion injury have failed to translate into clinical benefit (Hausenloy et al., 2019). Consequently, while the developments in acute reperfusion therapy has improved initial survival from ischemic events (Schmidt et al., 2012), the number of patients with chronic ischemic heart disease is growing globally. Accordingly, the chronic ischemic conditions carry vast disease burdens with more than 35 million people worldwide suffering from heart failure, most frequently as a consequence of ischemic heart disease (Hofmann and Frantz, 2013; Heusch et al., 2014). Despite increased survival, heart failure patients exhibit a poorer 5-year survival rate than patients with most types of cancer and a 1-year mortality of nearly 50% (Chen et al., 2013). In addition to the affected central organ, chronic ischemia may also trigger activation of unfortunate compensatory metabolic and remodeling processes in peripheral organs such as skeletal muscle. For instance, N-terminal pro- brain natriuretic peptide (NT-proBNP) elicited from stress imposed on the myocardium is considered a plasma marker of cardiovascular disease severity. In accordance, NT-proBNP is associated with loss of muscle mass (Martins et al., 2014; Ikeda et al., 2016) potentially partly explaining why symptoms of cardiovascular complications include intolerance towards physical effort and increased fatigue development (Clark et al., 1996; Dhakal et al., 2015). Loss of skeletal muscle mass can severely deteriorate muscle contractile and metabolic properties to negatively affect locomotion and whole body metabolism and loss of muscle mass and strength constitute strong predictors of all-cause mortality (Hülsmann et al., 2004; Szulc et al., 2010; Srikanthan and Karlamangla, 2014). Consequently, there is therefore great perspective in identi cation of practical strategies to counteract the detrimental consequences of chronic ischemic conditions in central and peripheral organs.
Intriguingly, the application of transient sub-lethal ischemia- reperfusion under resting conditions (i.e., remote ischemic conditioning, RIC) seems to preserve against tissue degradation of acute lethal ischemia. On the other hand, whereas this ability of RIC to counteract acute ischemic complications in the heart and the brain has received considerable attention (Heusch et al., 2015; Kleinbongard et al., 2017), the potential exploitation of transient occlusion-reperfusion inherent of muscle contraction during activity (i.e., exercise conditioning) has so far received much less attention. However, evidence exist that RIC and exercise conditioning may share signals to preserve cellular health. Among such signals, recent findings suggest that both RIC and exercise regimens can promote release of extracellular vesicles (EVs) containing a cargo of miRNA associated with cellular survival and/or protein turnover (Frey et al., 2019; Just et al., 2020; Lassen et al., 2021) to potentially engage in preconditioning processes.
In the current narrative review, we will first address how ischemia ± exercise stimulation (collectively referred to by us as conditioning-based strategies) can be conducted to potentially produce effects. Next, we will then address how these stimulators may share inducing cues and mechanisms, with special emphasis on the ability of EV-carried miRNA. Finally, we will address some recent ndings that support the effect of ischemia ± exercise on EV-carried tissue effects and/ or functional effects. This narrative is visualized as an event scheme in Figure 1.

In a new window | Download PPT
Figure 1: Event scheme of ischemia ± exercise preconditioning. Stimulus: transient sub-lethal remote ischemia and/or occlusion-reperfusion evoked from remote ischemic conditioning (RIC), low-load blood flow restricted resistance exercise (BFRRE), or high-load resistance exercise (HLRE). Signal release: signals consisting of extracellular vesicle (EV) carried miRNA released from host cells at the origin of ischemia- reperfusion to remote organ. Effects: the EV-derived miRNA engage in target cell regulatory mechanisms to counteract cellular degradation or to stimulate healthy function.
Conditioning-based intervention strategies
Ischemic stimulus - RIC
RIC constitutes a new treatment modality that has been shown to exert powerful protection against ischemia-reperfusion injury in the heart and brain as well as other tissues in animal models, and RIC has successfully been translated into clinical use (Heusch et al., 2015; Bøtker et al., 2018). Organ protection by RIC can be achieved simply by conducting 3-4 ve-minute cycles of limb ischemia and reperfusion using a tourniquet, a simple blood pressure cuff, or an automated programmable pressure cuff. In this procedure, the occlusion pressure is set to completely obstruct both arterial supply as well as venous return (Kharbanda et al., 2002). A specific advantage of RIC is its easy application during ongoing ischemia of the target organ. This has been exploited in animal models and humans to show that RIC reduces injury during evolving myocardial infarction (Bøtker et al., 2010; Sloth et al., 2014; Kleinbongard et al., 2017) and stroke (Hougaard et al., 2014), with the magnitude of tissue damage (infarct size) being the main prognostic determinant (Hausenloy and Yellon, 2013). While the mediators and mechanisms of RIC are yet to be firmly established, RIC has been shown to induce cytoprotection, to improve endothelial function and microcirculation, and to modify inflammation, suggesting a potentially powerful tool to simultaneously counteract detrimental biological processes involved in the development of tissue damage. RIC may have further potential because continued RIC after myocardial infarction seems to induce sustained benefits during the following adverse remodeling of the heart (Wei et al., 2011).
Exercise stimulus. High load resistance exercise (HLRE)
Locomotion is partly reliant on skeletal muscle contractile capacity as dictated by neuromuscular properties, including muscle myofibrillar mass and function. To stimulate muscle accretion and strength development traditional HLRE principles employ high mechanical loading (> 75%, which for most individuals corresponds to a 8-12 repetition range) through use of resistance training equipment and relatively long recovery time (typically 3-5 minutes of interset recovery) between 3-5 sets (American College of Sports Medicine, 2009). During HLRE, the muscle produces a large increase in tissue pressure during contraction immediately followed by a large decrease in tissue pressure upon cessation of contraction, leading to brief occlusion-reperfusion during each movement cycle. Single- session HLRE has been found to stimulate acute transient increases in net muscle protein synthesis (Phillips et al., 1997; Miller et al., 2005) and repeated HLRE training has been found to produce cumulative increases alongside muscle hypertrophy (Brook et al., 2015; Damas et al., 2016). Increased ribosomal activity (Stec et al., 2016; Brook et al., 2017) and satellite cell-mediated addition of myonuclei to existing muscle fibers (Petrella et al., 2008) has been suggested to contribute to muscle hypertrophy and remodeling. Furthermore, HLRE has recently been reported to increase mitochondrial function (Porter et al., 2015) and stimulate angiogenesis (Holloway et al., 2018), which may partly serve to support metabolic costs related to anabolic or remodeling events. HLRE is regarded feasible as well as efficient in improving functional recovery in chronic heart failure patients as well as patient suffering from ischemic stroke, provided that disease severity is considered low to moderate (Selig et al., 2010; Severinsen et al., 2014).
Ischemic ± exercise stimulus. Low-load blood ow-restricted resistance exercise (BFRRE)
Chronic ischemic disease severity as well as some other speci c clinical conditions (e.g. rheumatoid arthritis or recovery from orthopedic surgery) may impede the use of high mechanical loading. Interestingly, low-load traditional resistance exercise regimens have proven effective in stimulating muscle protein synthesis as well as in promoting muscle hypertrophy and strength gains (Burd et al., 2010; Mitchell et al., 2012), provided that much greater work volumes are conducted with low-load regimes compared to HLRE regimes. Interestingly, BFRRE can offer low work volume as well as low loading. BFRRE accomplish this by using inflatable cuffs to achieve partial occlusion of arterial blood in ow and near-complete/complete occlusion of venous outflow. Standardization of the relative degree of occlusion of arterial in ow (most often approximately 50%) can be established from initial individualized assessment of the cuff pressure required to promote complete obstruction of arterial inflow (Sieljacks et al., 2018). The blood flow restriction is combined with loading as low as 20%-50% of predetermined maximum dynamic strength. Usually, 3-4 sets of resistance exercise repetitions are conducted to a state of near or complete volitional failure with very short interset recovery (most often 30-60 seconds) during which time the occlusion is maintained. Using such principles has been demonstrated to accelerate fatigue development and increase muscle pressure due to metabolic build up and muscle water retention (Fahs et al., 2015). A single bout of BFRRE has been reported to stimulate muscle protein synthesis (Fujita et al., 2007; Fry et al., 1985) and ≤ 6 weeks of BFRRE training has been reported to produce strength gains as well as both whole-muscle and ber hypertrophy (Nielsen et al., 2012; Farup et al., 2015).
Underlying cues and mechanisms
Similarities and differences between RIC and BFRRE
Originally developed to augment muscle accretion, BFRRE research has predominantly been directed towards improvements in exercise performance and musculoskeletal rehabilitation. The mechanisms underlying the muscle hypertrophic effects of BFRRE have been suggested to be related to heightened growth hormone signaling, mechanical stress adhering to cell swelling from venous pooling on cell pressure or length changes from loaded contractions, as well as altered patterns of neuromuscular recruitment (Pearson and Hussain, 2015; Rindom and Vissing, 2016; Rindom et al., 2019; Wernbom and Aagaard, 2020). Its parallels to RIC are not thoroughly investigated, but many common features exist in the cardioprotective signaling initiated by exercise and RIC. Thus, release of endogenous opioids (Michelsen et al., 2012), cytokines (Cai et al., 2012; Dorneles et al., 2016) , nitric oxide (Calvert et al., 2011) and most recently EVs (Giricz et al., 2014; Vicencio et al., 2015; Just et al., 2020; Lassen et al., 2021), support that RIC and BFRRE share a variety of hallmarks that may induce cytoprotection, improve endothelial function and microcirculation, and modify inflammation, and suggest that they constitute at least partially analogous modalities (Paradis- Deschênes et al., 2016).
In contrast to RIC, the restriction strategy by BFRRE does not completely abolish, but only limits arterial blood flow, while the venous return is completely obstructed (Loenneke et al., 2012). Hence, it can be argued that ischemia, de ned as insuf cient blood ow required to meet metabolic needs, may not constitute a similarly crucial prerequisite for stimulating either tissue protection or musculoskeletal rehabilitation. Rather intramuscular metabolic stress, de ned as decreases in phosphocreatine and intramuscular pH independent of blood ow, seems to be a contributing factor although it still remains to be determined how such metabolic stress responses relate to tissue protection as well as musculoskeletal rehabilitation (Okita et al., 2019). On the other hand, BFRRE has been reported to produce substantial reductions in myocellular oxygen tension (as a result of vascular occlusion and exercise combined) as well as shifts in hemodynamics due to occlusion-reperfusion and reactive hyperemia upon cuff release (Ganesan et al., 2015; Lauver et al., 2017; Reis et al., 2019), which suggest that BFRRE to some extent resemble the ischemia and reperfusion of RIC.
Alternative triggers, including peripheral nociception (Jones et al., 2009; Gross et al., 2011; Gross et al., 2013), direct peripheral nerve stimulation (Redington et al., 2012), and noninvasive transcutaneous nerve stimulation (Merlocco et al., 2014), and electroacupuncture (Redington et al., 2013) are capable of recapitulating the infarct-sparing effect of RIC by stimulating known cardioprotective signaling pathways (Hausenloy et al., 2016).
Regardless, if distinct from RIC, which clearly induces intermittent ischemia, the hypoxia induced by BFRRE seems capable of inducing sufficient metabolic stress to mobilize endogenous protective mechanisms resembling RIC by yet- unidenti ed similar mechanisms (Pearson and Hussain, 2015). The mechanisms may include muscle stem cells (Nielsen et al., 2012; Wernbom et al., 2013; Aguayo et al., 2016), secretion of cytokines or growth factors (Wernbom et al., 2013; Layne et al., 2017), and potentially miRNAs delivered to remote locations by extravesicular transport (Giricz et al., 2014; Li et al., 2014). Noteworthy, with regards to safety during physical rehabilitation in risk populations, BFRRE, similar to RIC, seems to attenuate the amplification of the exercise pressor re ex seen during conventional exercise (Sprick and Rickards, 2017b, a).
Signaling between remote tissues via EV carried miRNA
While several mechanisms have been proposed to explain RIC induced cytoprotection (Schmidt et al., 2015; Kleinbongard et al., 2017; Heusch, 2020), these have so far failed to translate into pharmaceutically based clinical practice. EVs have more recently been proposed to constitute systemic mediators able to affect remote cellular events, leading to remote tissue protection. EVs characteristics and their potential role in RIC have recently been exhaustively reviewed (Chong et al., 2019; Frey et al., 2019). In brief, EVs are released from a variety of different cell types and detected in most body uids including blood, urine, and saliva (Raposo and Stoorvogel, 2013). Their surface is composed of a lipid bilayer membrane and ranges in size from 30-1000 nm in diameter. In the host cell, EVs are formed and released in several different ways including being released from multi-vesicular bodies and budding from the cell membrane (Février and Raposo, 2004). The secreted EVs may carry speci c components that affect the function and identity of the host cell. Furthermore, the magnitude of EV release, EV content and/or EV surface marker profile can vary by physiological condition of the organ and re ect the immediate state of the secreting cell (Jakobsen et al., 2015; French et al., 2017). The released EVs can home in on local and/or distant tissue by targeted cell receptor-ligand interactions. Uptake of EV cargo in the forms of proteins, lipids, and nucleic acids by the target cells can occur through e.g. endocytosis, direct plasma membrane fusion, or phagocytosis (Mulcahy et al., 2014; Pitt et al., 2016; French et al., 2017).
Noteworthy, the EV cargo, among signaling substances, is observed to be widely enriched with different RNAs, including small non-coding miRNAs (Bartel, 2004; Bartekova et al., 2019). Characterization of miRNA and their potential role in RIC has also recently been exhaustively reviewed (Shvedova et al., 2016; Nazari-Shafti et al., 2020). In brief, miRNAs are endogenous ~22 nucleotides non-coding RNAs that are believed to play an important role in directing the degradation of messenger RNA (mRNA) or the silencing of mRNA translation in the cytoplasm as well in nuclear compartments (Bartel, 2004). To this end, miRNA expression and release from the host cell upon a given physiological cue and uptake in remote target cells (via EVs or otherwise) provide an avenue to interfere with the translation of degradative components in target cells experiencing e.g. ischemia-reperfusion damage. Extracellular release of miRNA expressed in host cells are not outright dependent on EVs. However, although miRNAs are assumed to exhibit greater extracellular resistance to RNase- mediated degradation than mRNAs, greater stability is ensured by packaging and transporting in EVs (Shvedova et al., 2016).
Signals and effects with ischemia ± exercise
Some common underlying mechanistic traits of RIC and exercise appear to adhere to EVs and/or miRNA. With regards to EVs, plasma EV concentrations have previously been reported to increase immediately following RIC as well as traditional exercise interventions. Furthermore, plasma EVs secreted after RIC have been reported to reduce infarct size after myocardial ischemic reperfusion injury (Minghua et al., 2018; Lassen et al., 2021).
With regards to miRNAs e.g. myocardial miRNA144- 3p (miR144-3p) levels have been reported to be reduced by IR injury, with this reduction attenuated by RIC (Bøtker et al., 2018). RIC has also been reported to produce increased circulatory exosome levels, while exosomes enriched with miR22 and miR451a from anoxic cultured mesenchymal stem cells and cardiomycyte progenitor cells have been reported to mitigate cardiac injury. This invites the idea that treatment with specific miRNA loaded into exosomes may provide protection against acute and chronic effects of myocardial ischemia-reperfusion injury in patients (Emanueli et al., 2015). Several studies using traditional exercise regimes have observed exercise-induced release of miRNAs circulating free in the plasma or EV-carried. As reported in a previous review (Polakovičová et al., 2016), most of these studies primarily describe expression changes in muscle-associated miRNA (myo-miRs), while the direct physiological impact of EV- derived miRNA on exercise adaptations is so far lacking. However, miRNAs have been shown to be involved in multiple processes, including stem cell regulation (Cheung et al., 2012). Interestingly, high-frequency BFRRE, has previously been reported to substantially induce muscle stem cell proliferation, which could relate to ongoing muscle regenerative or remodeling events as well as muscle growth (Nielsen et al., 2012). Noteworthy, muscle damage has also been observed to induce proliferation of bro-adipogenic progenitors (FAPs) in muscle tissue and FAP have been suggested to be regulated by EVs released from muscle stem cells (Fry et al., 2017).
Acute effects of ischemia ± exercise
Since occlusion-reperfusion of BFRRE resemble that of RIC, we recently investigated the acute effects of single-bout BFRRE on plasma EV characteristics and impact on skeletal muscle stem cell and FAP proliferation. Moreover, we investigated the EV miRNA cargo pro le including pathway association analysis (Just et al., 2020). To our knowledge, no other studies have investigated the impact of BFRRE on EV characteristics and its potential conditioning effects at present. Although BFRRE did not produce changes in plasma EV content, pro ling of EV- carried miRNAs revealed a number of miRNA changes, which showed pathway association to canonical pathways involved in muscle protein synthesis and muscle protein degradation respectively. Moreover, when exposing primary muscle stem cells and FAPs to the BFRRE-induced EVs, proliferation was activated. Finally, EV surface marker analysis suggested that BFRRE stimulates EV release from blood cells (Just et al., 2020). Interestingly, miR451a (which has been tied to mitigation of cardiac injury) and hypoxia-inducible miR- 182–5p (which has been shown to enhance HIF1α signaling, protect cardiomyocytes from hypoxia-induced apoptosis) were observed to be upregulated with BFRRE (Just et al., 2020). This suggests that both RIC and BFRRE may partly function through these EV-carried miRNA to engage in preconditioning processes, but this requires further investigation. Whereas these findings are interesting in their own right, some limitations of our study design should be emphasized. Firstly, the abovementioned study served as a proof of concept, but did not include comparative experiments that allowed us to distinguish the effects of ischemia per se from the effects of exercise per se (i.e., RIC versus BFRRE). Therefore we can merely claim that our results support the hypothesis that BFRRE possesses the ability to affect skeletal muscle remodeling through EV- carried miRNAs. In ongoing follow-up studies (yet published) we compared the acute EV and EV-carried miRNA responses between RIC, BFRRE, and HLRE in healthy individuals as well as in congestive heart failure patients (CHF). Furthermore, in these follow-up studies we included comparison to non- intervention controls and age-matched controls to distinguish true effects of intervention from the effects of confounding factors such as dietary conditions or stress related to tissue collection (Vissing et al., 2005) and age. Secondly, in the study by Just et al. (2020) we demonstrated that BFRRE was con ned to localized muscle, while not extending to remote tissues of central organs such as the heart or the brain. In follow-up studies in cellular and animal models we have investigated the acute effects of human plasma-derived EVs stimulated by RIC, BFRRE, HLRE, and non-exercise control conditions in ischemic tissue.
Chronic effects of ischemia ± exercise
In a previous study, we observed that prolonged RIC treatment did not improve left ventricular ejection fraction, but did promote increased skeletal muscle function and reductions in blood pressure and NT-proBNP in patients with chronic ischemic heart failure (Pryds et al., 2017). In continuation of these findings, in the abovementioned collaborative research initiative, we investigated the effects of prolonged (6 weeks) RIC, BFRRE, HLRE, and non-exercise control intervention in heart, brain, and muscle (see Figure 2). During the 6-week intervention period, deuterium oxide (D2O) was continuously administered. This approach of labeling of newly synthesized skeletal muscle proteins combined with skeletal muscle biopsy collection and mass spectrometry allowed the assessment of new cumulative myofibrillar and mitochondrial protein as well as RNA synthesis (Miller et al., 2020). One advantage of this approach is that it reflects genuine individual habitual preferences for activity and diet over the full course of the intervention period, as opposed to the frequently arbitrary standardization inherent of experimental approaches in studies on acute synthetic effects.
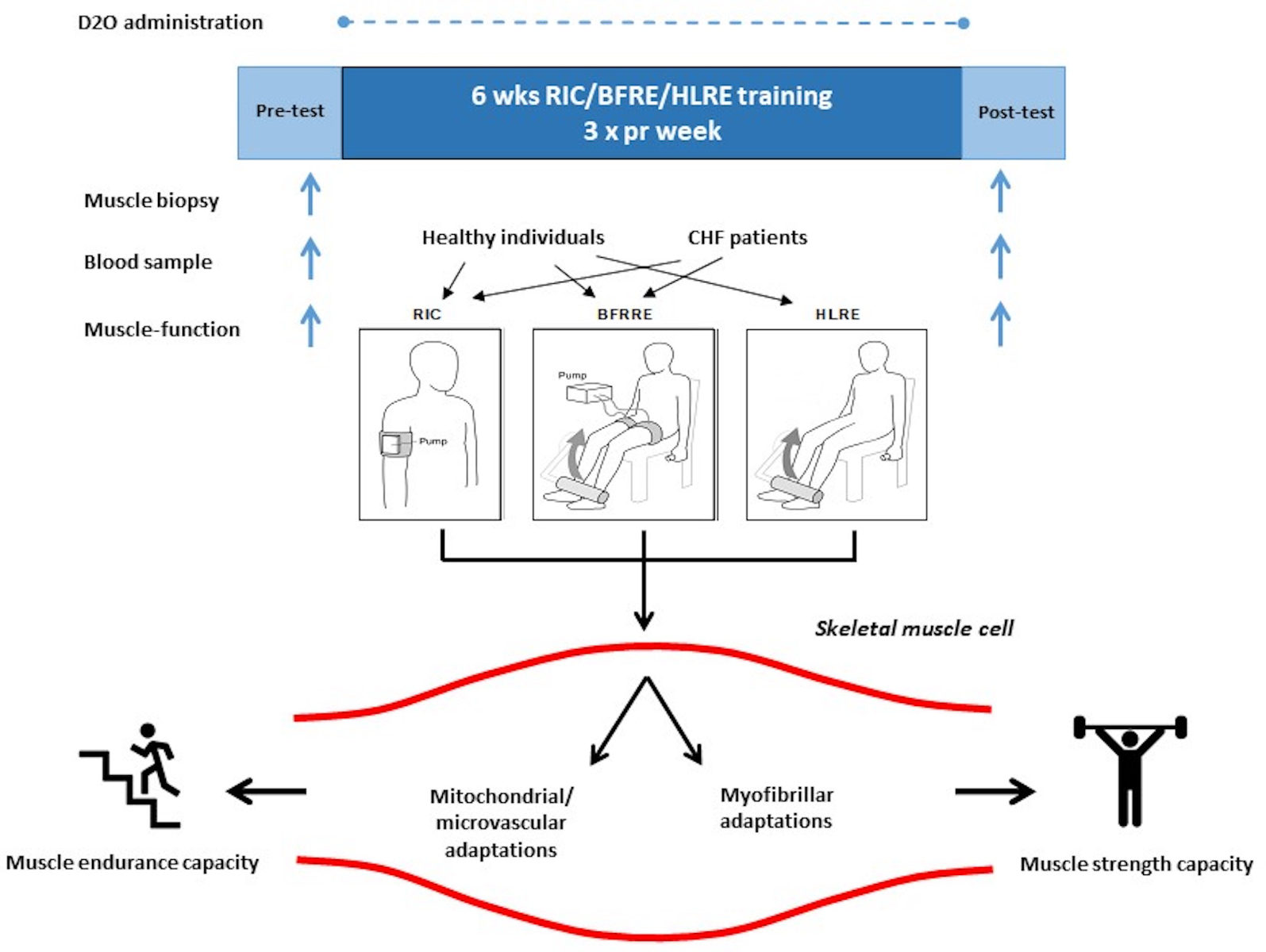
In a new window | Download PPT
Figure 2: Overview of comparative study on 6 weeks of differentiated conditioning intervention. Basal muscle and blood samples were collected and functional capacity was tested before and after a 6-week intervention period consisting of RIC, BFRRE, and HLRE stimulation 3 times per week for healthy young individuals and congestive heart failure (CHF) patients (in separate studies), respectively. The studies also included non-exercise control subjects (not shown). Throughout the 6-week intervention period, D2O was orally administered to the participants with blood collected every second week to measure tracer enrichment. Myocellular adaptations relating to metabolism and contraction as well as endurance-type and resistance-type capacity, was measured. RIC, remote ischemic conditioning; BFRRE, blood flow restricted resistance exercise; HLRE, high-load resistance exercise; D2O, deuterium oxide.
While data analysis related to plasma characterization and biological effects are still ongoing, we have recently published results on chronic adaptions in skeletal muscle in healthy individuals and in CHF patients, respectively. In healthy individuals, we demonstrated that BFRRE and HLRE were equally capable of stimulating cumulative mitochondrial and myofibrillar protein synthesis as well as cumulative RNA synthesis compared to non-exercise controls, collectively suggesting that myofibrillar growth, mitochondrial biogenesis and ribosomal biogenesis were stimulated (Groennebaek et al., 2018; Sieljacks et al., 2019). Moreover, we observed increased mitochondrial respiratory function and increases in both endurance exercise and resistance exercise capacity. Consequently, BFRRE and HLRE both seem very capable of promoting dual potentially health beneficial effects on properties relating to contractile and metabolic properties and suggest that the low loading of BFRRE is feasible and effective to counteract myopathy or decay in skeletal muscle health in various clinical settings (Vissing et al., 2020).
In a follow-up study in CHF patients, we therefore consequently employed a quite similar design to study the effects, except in this study we compared prolonged RIC and BFRRE intervention (Groennebaek et al., 2019). The results of this study showed that BFRRE is feasible for CHF patients (i.e., no records of adverse effects and almost full compliance). Moreover, the results showed that BFRRE, but not RIC, was able to promote improvements in skeletal muscle mitochondrial function and in functional capacity to the extent that it was clinically relevant. The lack of functional improvement with RIC is somewhat in contrast to our previous findings (Pryds et al., 2017). Possible explanations include differences in magnitude of RIC stimulation (i.e., more high- frequent stimulation in the Pryds-study) or differences in the methodological approach to assess muscle strength. Moreover, although, ribosomal biogenesis was observed to be higher with BFRRE than non-exercise control, myofibrillar and mitochondrial protein synthesis was not. We speculate that a degree of anabolic resistance can explain these results in CHF patients compared to the young healthy subjects described above. To obtain further insight on the extent to which anabolic resistance is a consequence of disease or related to the advanced age of the CHF patient requires a comparison to age-matched healthy individuals and identification of the magnitude of stimulation to overcome anabolic resistance. We are currently investigating this. Nonetheless, BFRRE seems to be both feasible and effectual in improving important features of skeletal muscle.
Summary and perspectives
The resemblance in some ischemic cues and mechanisms adhering to EV-carried miRNAs between RIC and BFRRE suggest that they both possess the capability to infer remote tissue conditioning in central organs. Moreover, low-load BFRRE possess similar capability as HLRE in producing peripheral skeletal muscle health bene cial adaptations, which also seem to involve EV-carried miRNA. Consequently, BFRRE may possess simultaneously dual ability to promote central as well as peripheral conditioning, but this awaits further information by ourselves and others on the comparative effects of differentiated interventions. This approach entail great perspective as it may serve to elucidate ef cient low-intensity practical regimes to counteract acute and chronic effects of ischemic disease and serve as platform for using naturally secreted EVs as carriers of nucleic acid based therapeutics.
Conflicts of interest statement
The authors declare that they have no con icts of interest.
Acknowledgements
Benjamin F. Miller is supported by National Institutes of Health grants R01AG064951, R56AG067754 and R21AR077387. Kristian Vissing, Kim Ryun Drasbek and Hans Erik Bøtker is supported by the Novo Nordisk Foundation Synergy program (NNF15OC0016674) and the Novo Nordisk Foundation Tandem program (NNF20OC0060998). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
Kristian Vissing1*
1Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark.
Benjamin F. Miller2
2Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, United States.
Kim Ryun Drasbek3
3Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Hans Erik Bøtker4
4Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Correspondening author: Kristian Vissing
Email: vissing@ph.au.dk

In a new window | Download PPT
Figure 1: Event scheme of ischemia ± exercise preconditioning. Stimulus: transient sub-lethal remote ischemia and/or occlusion-reperfusion evoked from remote ischemic conditioning (RIC), low-load blood flow restricted resistance exercise (BFRRE), or high-load resistance exercise (HLRE). Signal release: signals consisting of extracellular vesicle (EV) carried miRNA released from host cells at the origin of ischemia- reperfusion to remote organ. Effects: the EV-derived miRNA engage in target cell regulatory mechanisms to counteract cellular degradation or to stimulate healthy function.

In a new window | Download PPT
Figure 2: Overview of comparative study on 6 weeks of differentiated conditioning intervention. Basal muscle and blood samples were collected and functional capacity was tested before and after a 6-week intervention period consisting of RIC, BFRRE, and HLRE stimulation 3 times per week for healthy young individuals and congestive heart failure (CHF) patients (in separate studies), respectively. The studies also included non-exercise control subjects (not shown). Throughout the 6-week intervention period, D2O was orally administered to the participants with blood collected every second week to measure tracer enrichment. Myocellular adaptations relating to metabolism and contraction as well as endurance-type and resistance-type capacity, was measured. RIC, remote ischemic conditioning; BFRRE, blood flow restricted resistance exercise; HLRE, high-load resistance exercise; D2O, deuterium oxide.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8310 | 30 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA