Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Anesthetic conditioning for ischemic stroke: from bench to bedside
Time:2021-08-21
Number:5345
Yunlu Guo1, Peiying Li1
Author Affiliations


- 1Department of Anesthesiology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine Shanghai Jiao Tong University, Shanghai, China.
Conditioning Medicine 2021. 4(3): 135-140.
Abstract
Ischemic stroke is a leading cause of cognitive dysfunction, morbidity and death, causing an ever-increasing burden to society. It is therefore of great clinical value and significance to find medications that are efficacious outside the therapeutic window of current treatments for long-term neuroprotection. Anesthetic postconditioning (PostC) is a promising strategy against ischemic stroke. In this mini-review, we summarized the protective mechanisms underlying anesthetic PostC, including excitotoxicity inhibition, anti-inflammation, anti-oxidation, signal transduction, and cell death inhibition. We also raised a few issues that need to be resolved before successful translation into the clinic, such as the time window, the risks, and the impact of gender, age, and comorbidities. We conclude that prospective randomized controlled trials are highly warranted to pave the way for the clinical translation of anesthetic PostC in treating ischemic stroke patients for long-term neuroprotection.
Keywords: ischemic stroke, anesthetics, postconditioning, neuroprotection
Abstract
Ischemic stroke is a leading cause of cognitive dysfunction, morbidity and death, causing an ever-increasing burden to society. It is therefore of great clinical value and significance to find medications that are efficacious outside the therapeutic window of current treatments for long-term neuroprotection. Anesthetic postconditioning (PostC) is a promising strategy against ischemic stroke. In this mini-review, we summarized the protective mechanisms underlying anesthetic PostC, including excitotoxicity inhibition, anti-inflammation, anti-oxidation, signal transduction, and cell death inhibition. We also raised a few issues that need to be resolved before successful translation into the clinic, such as the time window, the risks, and the impact of gender, age, and comorbidities. We conclude that prospective randomized controlled trials are highly warranted to pave the way for the clinical translation of anesthetic PostC in treating ischemic stroke patients for long-term neuroprotection.
Keywords: ischemic stroke, anesthetics, postconditioning, neuroprotection
Ischemic stroke is a leading cause of cognitive dysfunction, morbidity and death. With a global incidence of 9.5 million events and a lifetime risk of 18.3% in 2016 (Feigin et al., 2018; 2019), ischemic stroke is causing a great and increasing burden to society. So far, only thrombolysis with tissue plasminogen activator (t-PA) and mechanical thrombectomy have been approved by the Food and Drug Administration to treat acute ischemic stroke. However, the above treatments are still limited by the short therapeutic time window (Wechsler and Jovin, 2012) and the increased risk of hemorrhagic transformation (Camara et al., 2021). It is of great clinical value and signi cance to nd medications outside the therapeutic window of the above acute treatments for long-term neuroprotection.
Ischemic conditioning refers to one or more brief episodes of non-lethal hypoperfusion to protect organs against a lethal ischemic insult (Murry et al., 1986; Hausenloy and Yellon, 2011). It can be classified into ischemic pre-conditioning (IPreC), per-conditioning (IPeC), or post-conditioning (IPostC) according to whether the treatment precedes, occurs during, or after the stroke ischemia or reperfusion time points (Wang et al., 2015c). The concept of conventional IPostC has been extended to the more feasible remote IPostC (Weir et al., 2021) and chemical PostC with carbon dioxide and anesthetics, for example (Fan et al., 2017). Notably, several anesthetic agents can reduce cerebral blood flow (CBF), decrease the cerebral metabolic rate of oxygen (CMRO2) and show neuroprotective effects, such as intravenous anesthetics (propofol, etomidate, dexmedetomidine, benzodiazepines) and volatile anesthetics (isoflurane, sevoflurane and desflurane) (Slupe and Kirsch, 2018). These are thought to be capable of inducing conditioning, which is termed “anesthetic conditioning” (Wang et al., 2016a). Since most cerebrovascular events are unpredictable, anesthetic PostC could provide more opportunities for therapeutic intervention and is more applicable compared to anesthetic pre- conditioning (Zhao, 2007). In this short review, we will focus on the translation of anesthetic PostC from bench to bedside, review laboratory and clinical studies to evaluate the effects of anesthetics after reperfusion of ischemic stroke, and discuss limitations and feasibility of anesthetic PostC.
In the rodent ischemic stroke model using lament occlusion, anesthetic treatment was reported to reduce neurological injury (infarct volume and neurological deficit score combined) by 28% with considerable heterogeneity according to a systemic review of 80 studies (Archer et al., 2017). Isoflurane and sevoflurane PostC, which were the most studied agents, have been shown to alleviate ischemia/reperfusion (I/R) injury and improve short and long-term neurological outcomes in the middle cerebral artery occlusion (MCAO) model (Lee et al., 2008; Li et al., 2013; Dong et al., 2014; Li et al., 2014; Wang et al., 2015b; Wang et al., 2016b; Zhu et al., 2017; Yuan et al., 2018; Peng et al., 2019a; Peng et al., 2019b; Yin et al., 2020). In addition, propofol and dexmedetomidine injection can attenuate cerebral I/R injury and induce neuroprotection in focal ischemia (Wang et al., 2009; Wang et al., 2011; Liang et al., 2013; Ji et al., 2015; Wang et al., 2015a; Luo et al., 2017). Similar to other kinds of PostC, neuroprotection by anesthetic PostC is concentration-dependent and time-dependent. For example, 1.5- 3.0%, i.e. 1-2 minimal alveolar concentration (MAC) iso urane posttreatment for 60 min at the start of reperfusion can protect against brain injury, while 4.5% isoflurane failed to show similar effects (Wang et al., 2016b). The protective effects of propofol on neurological de cits, infarct volume, and neuronal apoptosis are dose-dependent, which all reach statistical significance at a dose of 20 mg/kg/h (Liang et al., 2013). However, the therapeutic time window of these anesthetics against ischemic stroke should be further investigated as the treatments were generally only given at the onset of reperfusion in the above studies (Table 1).
Table 1. Summary of representative studies on anesthetic PostC for ischemic stroke
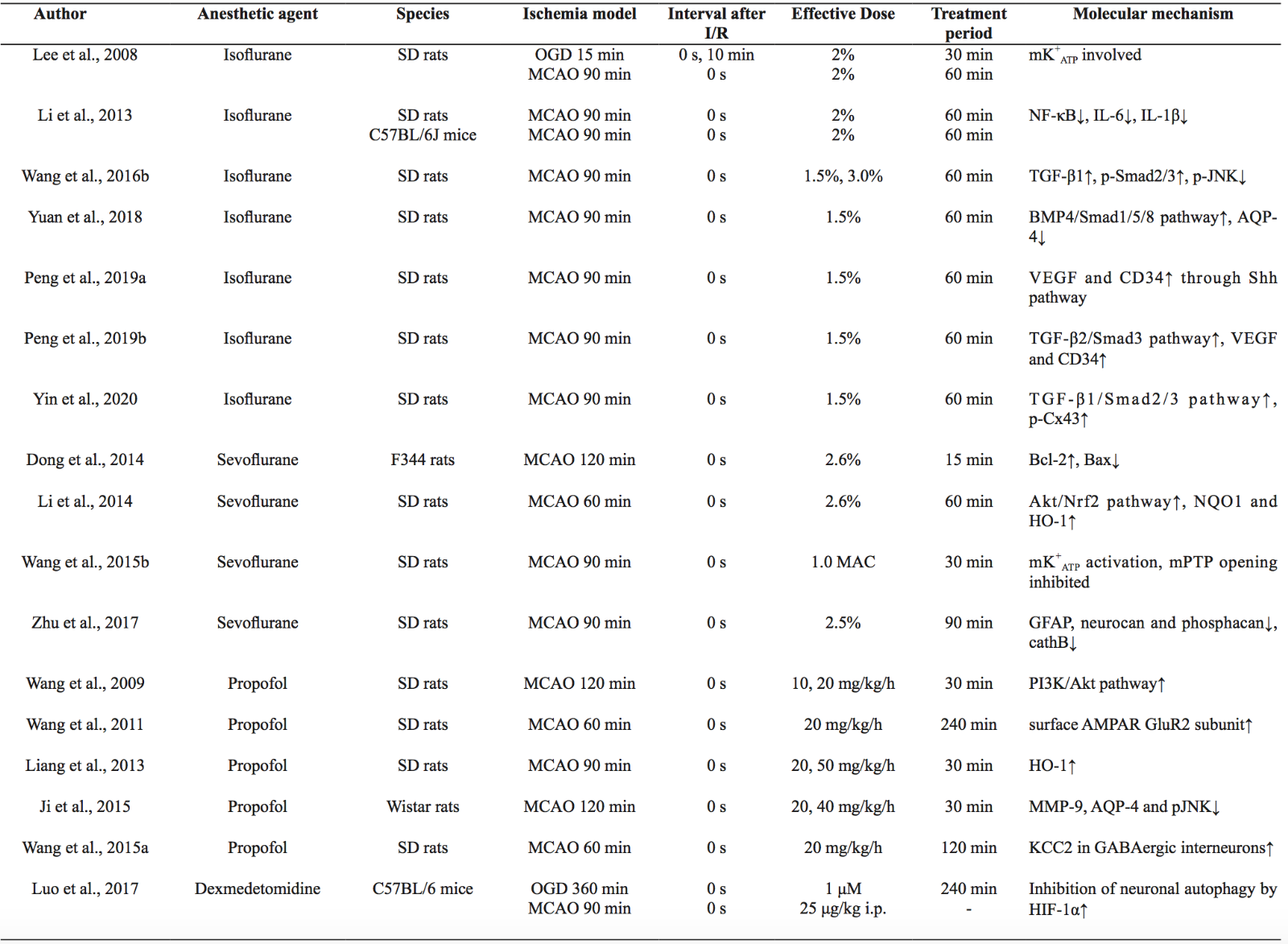
I/R, ischemia/reperfusion; OGD, oxygen-glucose deprivation; MCAO, middle cerebral artery occlusion; MAC, minimal alveolar concentration; i.p., intraperitoneal injection; mK+ATP, mitochondrial ATP-dependent potassium channels; NF-κB, nuclear factor kappa-B; TGF-β, transforming growth factor beta; p-JNK, phosphorylated c-Jun N-terminal kinase; BMP4, bone morphogenetic protein 4; AQP-4, aquaporin-4; VEGF, vascular endothelial growth factor; Shh, sonic hedgehog; Cx43, connexin 43; Bcl-2, B-cell lymphoma-2; Bax, BCL-associated X protein; Akt, protein kinase B; Nrf2, nuclear factor-erythroid 2-related factor-2; NQO1, quinine oxidoreductase1; HO-1, hemeoxygenase-1; mPTP, mitochondrial permeability transition pore; GFAP, glial fibrillary acidic protein; CathB, cathepsin B; PI3K, phosphoinositide-3-kinase; AMPAR, α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid receptor; MMP-9, matrix metalloproteinase-9; KCC2, K+-Cl--co-transporter 2; HIF-1α, hypoxia inducible factor-1α
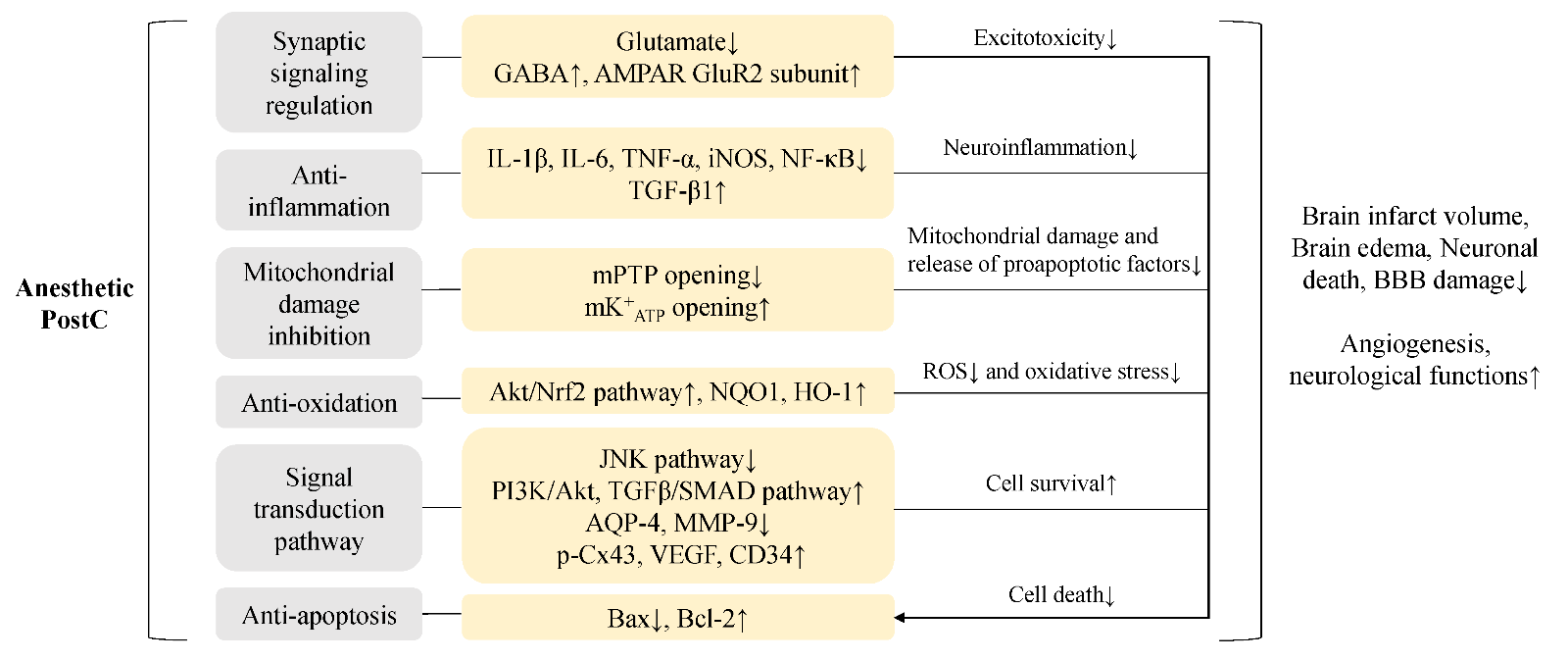
In terms of protective mechanisms, anesthetic PostC can decrease brain infarct volume, reduce neuronal death, attenuate reactive astrogliosis (Zhu et al., 2017), inhibit brain edema (Yuan et al., 2018), protect the blood brain barrier (Ji et al., 2015), enhance angiogenesis (Peng et al., 2019a), and improve neurological function in experimental stroke models in rodents (Table 1). However, although the protective mechanisms of multi-target anesthetic PostC are not completely understood, there are some molecular mechanisms frequently studied, including regulation of synaptic signaling, anti-inflammation, inhibition of mitochondrial damage, anti-oxidation, activation of the phosphoinositide-3-kinase/ protein kinase B (PI3K/Akt) pathway, and anti-apoptosis (Archer et al., 2017; Fan et al., 2017). We summarize the interrelated protective mechanisms underlying the anesthetic PostC as follows (Figure 1):
In a new window | Download PPT
Figure 1: The schematic represents major neuroprotective mechanisms of anesthetic PostC. The 6 protective mechanisms underlying anesthetic PostC and the corresponding molecules and pathways are listed in the scheme. The above 5 mechanisms (synaptic signaling regulation, anti-inflammation, mitochondrial damage inhibition, anti-oxidation, signal transduction pathway) can indirectly reduce neuronal apoptosis. Together, these molecular mechanisms lead to inhibition of brain infarction, brain edema, neuronal death and BBB damage, with promotion of neurological functions. GABA, γ-aminobutyric acid; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa-B; TGF-β, transforming growth factor beta 1; mPTP, mitochondrial permeability transition pore; mK+ATP, mitochondrial ATP-dependent potassium channels; Akt, protein kinase B; Nrf2, nuclear factor-erythroid 2-related factor-2; NQO1, quinine oxidoreductase1; HO-1, hemeoxygenase-1; JNK, c-Jun N-terminal kinase; PI3K, phosphoinositide-3-kinase; AQP-4, aquaporin-4; MMP-9, matrix metalloproteinase-9; Cx43, connexin 43; VEGF, vascular endothelial growth factor; Bcl-2, B-cell lymphoma-2; Bax, BCL-associated X protein; ROS, reactive oxygen species; BBB, blood brain barrier.
a) Anesthetics protect synaptic function by regulating the content of neurotransmitters to prevent excitotoxicity. PostC with sevoflurane was shown to reduce neuronal injury after oxygen-glucose deprivation (OGD)-reperfusion in rat hippocampal slices, in vitro, with a decrease in excitatory amino acids (aspartate, glutamate) and a signi cant increase in the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (Peng et al., 2011). Propofol PostC can provide short-term (24 h) neuroprotection by increasing K+-Cl--co-transporter 2 (KCC2) expression in GABAergic interneurons (Wang et al., 2015a), and induce long-term (up to 28 days) neuroprotection by inhibiting internalization of the α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) glutamate receptor GluR2 subunit (Wang et al., 2011).
b) Anesthetics can reduce secondary neuroinflammation. As shown in volatile anesthetics, the proinflammatory factors interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and nuclear factor kappa-B (NF-κB) are down-regulated (Li et al., 2013), whereas the anti-inflammatory transforming growth factor beta 1 (TGF-β1) is up-regulated (Archer et al., 2017).
c) Mitochondria-based mechanisms may play an important role in the neuroprotection against stroke. The process of mitochondrial damage causes mitochondrial swelling, reactive oxygen species (ROS) overproduction, and release of proapoptotic molecules. Isoflurane or sevoflurane PostC can inhibit the opening of mitochondrial permeability transition pore (mPTP) located in the inner mitochondrial membrane, which may be related to mitochondrial ATP-dependent potassium channels (mK+ATP) opening (Wang et al., 2015b).
d) Anti-oxidation against ROS generated after stroke could inhibit oxidative stress. Inhibition of mitochondrial damage and anti-oxidation mechanisms both reduce ROS the content of neurotransmitters to prevent excitotoxicity. PostC with sevoflurane was shown to reduce neuronal injury after oxygen-glucose deprivation (OGD)-reperfusion in rat hippocampal slices, in vitro, with a decrease in excitatory amino acids (aspartate, glutamate) and a signi cant increase in the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (Peng et al., 2011). Propofol PostC can provide short-term (24 h) neuroprotection by increasing K+-Cl--co-transporter 2 (KCC2) expression in GABAergic interneurons (Wang et al., 2015a), and induce long-term (up to 28 days) neuroprotection by inhibiting internalization of the α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) glutamate receptor GluR2 subunit (Wang et al., 2011).
e) PI3K/Akt and other pathways are important in signal transduction and cell survival. The propofol PostC effect is mediated partly by maintaining the activity of the PI3K/Akt pathway (Wang et al., 2009). Besides PI3K/Akt pathway, the mitogen-activated protein kinase (MAPK) and transforming growth factor beta (TGFβ)/SMAD pathways have also been studied. The MAPK pathways include c-Jun N-terminal kinase (JNK) and extracellular signal-regulated protein kinase (ERK) 1/2. Isoflurane at 1.5% can upregulate the expression of TGF-β1 and downregulate phosphorylated JNK to mitigate cerebral injury (Wang et al., 2016b). The underlying mechanism of propofol PostC involves inhibition of aquaporin-4 (AQP- 4), matrix metalloproteinase-9 (MMP-9), and phosphorylation of JNK (Ji et al., 2015). Dexmedetomidine PostC also inhibits JNK pathway activation to reduce the inflammatory response and autophagy effect (Zhu et al., 2019). For the TGFβ/SMAD pathways, isoflurane PostC can upregulate phosphorylated connexin 43 in gap junctions through the TGF-β1/Smad2/3 signaling pathway (Yin et al., 2020), downregulate AQP-4 to inhibit brain edema through the bone morphogenetic protein 4 (BMP4)/Smad1/5/8 pathway (Yuan et al., 2018), and can increase the expression of vascular endothelial growth factor (VEGF) and CD34 related to angiogenesis by activating the TGF-β2/Smad3 pathway (Peng et al., 2019b). In addition, isoflurane PostC reduced cerebral injury by enhancing angiogenesis through the sonic hedgehog (Shh)/Gli signaling pathway (Peng et al., 2019a).
f) Anti-apoptosis of neurons is related to the rescue of ischemic penumbra areas. Anesthetic PostC could reduce apoptosis by up-regulating the anti-apoptosis protein B-cell lymphoma-2 (Bcl-2), which is a downstream protein of the PI3K/Akt pathway, and by down-regulating the pro-apoptotic BCL-associated X protein (Bax) (Dong et al., 2014). The above 5 mechanisms (inhibition of excitotoxicity, anti-in ammation, inhibition of mitochondrial damage, anti-oxidation, and signal transduction) can also indirectly reduce neuronal apoptosis.
Among the above neuroprotective mechanisms, signal transduction is the most frequently studied one. Alteration in signal transduction could also influence other molecular mechanisms of anesthetic PostC. The key mechanisms shown to be causal related to PostC were con rmed in some pre-clinical studies using a selective PI3K inhibitor (Wang et al., 2009), JNK pathway blocker or activator (Wang et al., 2016b; Zhu et al., 2019), TGF-β1 or TGF-β2 inhibitor (Peng et al., 2019b; Yin et al., 2020), and Shh pathway inhibitor (Peng et al., 2019a). In addition, IL-1β deficient mice (Li et al., 2013), a mK+ATP blocker with mPTP opener (Wang et al., 2015b), and HO-1 inhibitor (Liang et al., 2013) were used to identify the causality of anti-inflammation, mitochondrial damage inhibition, and anti-oxidation mechanisms, respectively. The above evidence suggest that signal transduction, anti-inflammation, mitochondrial damage inhibition, and anti-oxidation may represent the key mechanisms that underlie the neuroprotection of PostC, while others may warrant further investigation.
Although several anesthetics have shown high neuroprotective potential in animal studies, there is still a lack of clinical data to support that PostC is protective in stroke patients at present. In nonrandomized studies, general anesthesia during thrombectomy for acute ischemic stroke was thought to be associated with poor outcome and high mortality (Abou-Chebl et al., 2015; van den Berg et al., 2015; Brinjikji et al., 2017; Campbell et al., 2018). However, a few relevant randomized controlled trials (RCT) showed different results. For instance, in the Sedation vs. Intubation for Endovascular Stroke Treatment (SIESTA; NCT02126085) trial, among 150 patients with acute ischemic stroke in the anterior circulation undergoing endovascular thrombectomy, conscious sedation (CS) compared with general anesthesia (GA) did not result in greater neurological improvement at 24 h or long-term functional recovery after 3 months. The peri-interventional feasibility and safety of GA were also comparable to that of sedation (Schönenberger et al., 2016). Furthermore, analysis of stroke patients in the General or Local Anesthesia in Intra Arterial Therapy (GOLIATH; NCT02317237) trial found that GA did not result in larger infarct growth or worse clinical outcomes compared to CS during endovascular therapy (Simonsen et al., 2018). In a meta-analysis of 3 single-center randomized clinical trials (SIESTA, Anesthesia During Stroke [ANSTROKE; NCT01872884] and GOLIATH), protocol-based GA was shown to be signi cantly associated with lower disability at 3 months (Schönenberger et al., 2019). A pooled analysis of three large prospective multi-center studies (SOLITAIRETM FR With the Intention For Thrombectomy [SWIFT; NCT01054560], Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment [SWIFT PRIME; NCT0165746] Solitaire FR Thrombectomy for Acute Revascularization [STAR; NCT01327989] trials) also showed that GA was related to a lower risk for hemorrhagic transformation after thrombectomy (Raychev et al., 2020). More high-quality clinical trials on the posttreatment of anesthetics are needed, especially in stroke patients undergoing thrombolysis.
As a clinically available treatment in hospitals, anesthetic PostC has several advantages. For example, it can be performed simultaneously with thrombolysis and mechanical thrombectomy and the safety and accessibility of these widely used medications are guaranteed. In addition, timely anesthetic PostC may be suitable for perioperative stroke. To successfully translate laboratory results to the bedside, some issues or limitations need to be considered. 1) Comorbidities have influence on PostC mechanisms. Anesthetic neuroprotection failed in females, aged animals, and animals with comorbidities (diabetes, diet-induced obesity, high-fat diets) as the specific neuroprotective mechanisms are impaired (Kitano et al., 2007; Dong et al., 2014; Yang et al., 2014; Yu et al., 2014). Thus, young healthy male animals may not be an ideal model for human stroke. Replication of PostC effects in animals with comorbidities may significantly improve the translation of laboratory findings to the clinic (Archer et al., 2017). 2) The differences between the traditional MCAO model and human stroke are relatively large. As the reperfusion is performed with thrombolytics in the clinic, it’s of more value to study in the embolic stroke model with t-PA (Fan et al., 2017; Wu et al., 2020), which is closer to ischemic stroke in human. 3) The therapeutic time window, choice of anesthetic agents, effective dose, duration, and the risks of anesthetic PostC need to be clari ed in animal and human stroke studies.
As reviewed above, anesthetic PostC is a promising strategy against ischemic stroke. The protective mechanisms mainly include excitotoxicity inhibition, anti-inflammation, anti-oxidation, signal transduction, and cell death inhibition. However, a few issues should be resolved before successful translation into the clinic, such as the time window, risks, and the impact of gender, age, and comorbidities. Prospective randomized controlled trials are highly warranted to pave the way for the clinical translation of anesthetic PostC in treating ischemic stroke patients for long-term neuroprotection.
Acknowledgements
P.L. is supported by the National Natural Science Foundation of China (NSFC, 81722017, 91957111, 81971096, 82061130224), New Frontier Technology Joint Research sponsored by Shanghai Shenkang Hospital Development Center (SHDC12019102), Shanghai Municipal Education Commission- Gaofeng Clinical Medical Grant Support (20181805), Shuguang Program”supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (20SG17), and Shanghai Outstanding Academic Leaders’ Program from Shanghai Municipal Science and Technology Committee (20XD1422400), Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the authorship, and/or publication of this article.
References
Yunlu Guo1
1Department of Anesthesiology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine Shanghai Jiao Tong University, Shanghai, China.
Peiying Li1
1Department of Anesthesiology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine Shanghai Jiao Tong University, Shanghai, China.
Corresponding author:
Dr. Peiying Li
Email: peiyingli.md@gmail.com

In a new window | Download PPT
Figure 1: The schematic represents major neuroprotective mechanisms of anesthetic PostC. The 6 protective mechanisms underlying anesthetic PostC and the corresponding molecules and pathways are listed in the scheme. The above 5 mechanisms (synaptic signaling regulation, anti-inflammation, mitochondrial damage inhibition, anti-oxidation, signal transduction pathway) can indirectly reduce neuronal apoptosis. Together, these molecular mechanisms lead to inhibition of brain infarction, brain edema, neuronal death and BBB damage, with promotion of neurological functions. GABA, γ-aminobutyric acid; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa-B; TGF-β, transforming growth factor beta 1; mPTP, mitochondrial permeability transition pore; mK+ATP, mitochondrial ATP-dependent potassium channels; Akt, protein kinase B; Nrf2, nuclear factor-erythroid 2-related factor-2; NQO1, quinine oxidoreductase1; HO-1, hemeoxygenase-1; JNK, c-Jun N-terminal kinase; PI3K, phosphoinositide-3-kinase; AQP-4, aquaporin-4; MMP-9, matrix metalloproteinase-9; Cx43, connexin 43; VEGF, vascular endothelial growth factor; Bcl-2, B-cell lymphoma-2; Bax, BCL-associated X protein; ROS, reactive oxygen species; BBB, blood brain barrier.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5345 | 3 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA