Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Conditioning medicine for ischemic and hemorrhagic stroke
Time:2021-08-21
Number:5393
David C Hess1, Mohammad Badruzzaman Khan1, Pradip Kamat1, Kumar Vaibhav2,3, Krishnan M. Dhandapani2, Babak Baban1,3, Jennifer L. Waller4, Md Nasrul Hoda5, Rolf Ankerlund Blauenfeldt6, Grethe Andersen6
Author Affiliations


- 1Departments of Neurology, Medical College of Georgia, Augusta, GA.
- 2Departments of Neurosurgery, Medical College of Georgia, Augusta, GA.
- 3Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta, GA.
- 4Population Health Sciences4 Medical College of Georgia, Augusta, GA.
- 5Department of Neurology, Henry Ford Hospital, Detroit, MI.
- 6Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Conditioning Medicine 2021. 4(3): 124-129.
Abstract
Remote ischemic conditioning (RIC) is a promising safe, feasible, and inexpensive treatment for acute stroke, both ischemic and hemorrhagic. It is applied with a blood pressure cuff on the limbs and is ideal for the prehospital setting. RIC is a form of preconditioning with similarities to physical exercise. Its mechanisms of action are multiple and include improvement of collateral cerebral blood flow (CBF) and RIC acts as a “collateral therapeutic”. The increased CBF is likely related to nitric oxide synthase 3 in the endothelium and more importantly in circulating blood cells like the red blood cell. The RESIST clinical trial is a 1500 subject multicenter, randomized, sham-controlled trial of RIC in the prehospital setting in Denmark and should address the questions of whether RIC is safe and effective in acute stroke and whether the effect is mediated by an effect on nitric oxide/nitrite metabolism.
Keywords: preconditioning, remote ischemic conditioning, nitric oxide synthase 3, red blood cell
Abstract
Remote ischemic conditioning (RIC) is a promising safe, feasible, and inexpensive treatment for acute stroke, both ischemic and hemorrhagic. It is applied with a blood pressure cuff on the limbs and is ideal for the prehospital setting. RIC is a form of preconditioning with similarities to physical exercise. Its mechanisms of action are multiple and include improvement of collateral cerebral blood flow (CBF) and RIC acts as a “collateral therapeutic”. The increased CBF is likely related to nitric oxide synthase 3 in the endothelium and more importantly in circulating blood cells like the red blood cell. The RESIST clinical trial is a 1500 subject multicenter, randomized, sham-controlled trial of RIC in the prehospital setting in Denmark and should address the questions of whether RIC is safe and effective in acute stroke and whether the effect is mediated by an effect on nitric oxide/nitrite metabolism.
Keywords: preconditioning, remote ischemic conditioning, nitric oxide synthase 3, red blood cell
Thrombolysis and endovascular thrombectomy (ET) remain the only two Food and Drug Administration (FDA)-approved treatments for acute ischemic stroke (Goyal et al., 2016). Despite the efficacy of ET, 50% of stroke patients remain disabled three months after therapy (Goyal et al., 2016; Albers et al., 2018). Despite extensive preclinical studies and many clinical trials, clinical translation has failed as no neuroprotective agents have been approved by the FDA for stroke. Targeting single cascades and molecular pathways have not met with success. Since presence of collaterals is a major predictor of outcome with ET, a new therapeutic avenue is development of “collateral therapeutics” (Leng et al., 2016; Berkhemer et al., 2016). Adjunctive therapies to thrombolysis and ET are needed that provide “bridging neuroprotection” and improve collateral blood flow.
A promising avenue in acute stroke therapy is “preconditioning”. Preconditioning harnesses endogenous protective pathways and induces a protective phenotype (Hess et al., 2015a). Physical exercise is a form of preconditioning. In the 1950s, J,N Morris (1953a; 1953b) showed that conductors in the London Transit Authority who climbed up and down steps and were physically active on the job had a much lower incidence of sudden cardiac death than the sedentary bus drivers. This was one of the first demonstrations of the efficacy of physical exercise. Subsequent studies demonstrate there is no better protection against the occurrence and severity of stroke and cardiovascular events than physical exercise (Chave et al., 1978; Krarup et al., 2007; 2008; Armstrong et al., 2015).
Acute exercise preconditioning
While physical exercise reduces the risk and severity of stroke, it was not clear whether a single bout of exercise lessens the severity of stroke and acutely preconditions the brain. We tested whether acute exercise “preconditioning” was neuroprotective in a rat thromboembolic stroke model.(Hafez et al., 2019). Thirty minutes of forced high-intensity interval (HHI) treadmill exercise reduced infarct size and improved functional outcomes when the interval between exercise and stroke was one hour. This effect remained, but was attenuated with an interval of 24 hrs, but was lost when the interval was extended to 72 hrs. A pharmacological inhibitor of nitric oxide synthase 3 (NOS3), N5-(1-Iminoethyl)-L-ornithine, dihydrochloride (L-NIO) blocked the protective effect of exercise.
Remote ischemic conditioning
While physical exercise should be encouraged to prevent stroke and myocardial infarction, physical exercise is not clinically practical as an acute stroke therapy as it cannot be implemented hyperacutely during stroke. Remote ischemic conditioning (RIC), the repeated inflation and deflation of a blood pressure cuff on the limbs, is a form of preconditioning that is highly translatable. RIC is based on the principle that a small sub-lethal dose protects against a later lethal dose (Hess et al., 2015a). RIC triggers adaptive, endogenous, protective responses in the brain, an “ischemia tolerant” state. A major advantage of RIC is that it has multiple mechanisms of action. Therapies targeted at one pathway or one injury cascade have failed in stroke.
We and others have shown that RIC is effective in preclinical stroke models (Hess et al., 2013). We tested RIC in an autologous thromboembolic model in mice, with and without “late” intravenous tissue plasminogen activator (tPA) at 4 hrs, and found RIC to be effective alone and in combination with tPA in both male and ovariectomized female mice (Hoda et al., 2012; 2014),. RIC improved cerebral blood flow (CBF) as measured by laser speckle contrast imaging. An increase of CBF and enhancement of collateral flow is one of the mechanisms of action of RIC. Ma and colleagues (2017; 2020) showed that RIC prevented collapse of pial collaterals and improved collateral blood flow to the penumbra and reduced brain infarct size in young and aged mice. A consistent finding in all our pre-clinical studies is that RIC increases CBF. We have tested RIC in mouse models of acute ischemic stroke (thromboembolic clot model), traumatic brain injury, intracranial hemorrhage, and bilateral carotid stenosis (BCAS), and in all these models, RIC increases CBF as measured by laser speckle contrast imaging (Hess et al., 2016).
In response to the failure of the clinical translation of so many neuroprotective agents that appeared promising in preclinical development, the National Institute of Neurological Diseases and Stroke (NINDS) funded the Stroke Preclinical Assessment Network (SPAN Network) (Anon, n.d.). SPAN is a multicenter, randomized preclinical trial network modeled on randomized clinical trials. RIC is one of the six promising interventions/drugs being tested in the SPAN Network (https://www.spannetwork.org).
RIC and nitric oxide synthase 3 (NOS3)
The mechanisms of RIC are pleiotropic but include dependence upon NOS3 and nitrite. In cardiac models, RIC-induced cardioprotection is lost in NOS3 knockout mice (Rassaf et al., 2014). There are 3 isoforms of NOS: NOS1, NOS2, and NOS3. NOS3 is vasculo-protective in the brain while partial NOS3 deficiency is associated with microinfarcts (Katusic and Austin, 2014; 2016; Tan et al., 2015)., Moreover, inhibition of NOS3 with L-NIO is a commonly used experimental model for subcortical white matter stroke (Nunez et al., 2016).
Although initially identified in endothelium, NOS3 is expressed in red blood cells (RBCs) (Kleinbongard et al., 2006; Cortese-Krott and Kelm, 2014) and in most circulating blood cells including all main leukocyte subpopulations (Mühl and Pfeilschifter, 2003), platelets (Radomski et al., 1990), and circulating blood microparticles (Horn et al., 2013). NOS3 from both endothelial and circulating blood cells contribute significantly to blood pressure and systemic nitrite levels, the latter being a major component of the circulating NO reservoir (Wood et al., 2013). RBC NOS3 (erythrocine (ery) NOS3) plays a key role in cardioprotection. Patients with coronary artery disease (CAD) have reduced eryNOS3 activity compared to healthy controls (Eligini et al., 2013). In a myocardial ischemia model, circulating blood cell NOS3 (presumably RBC) plays an important role in myocardial reperfusion injury as depleting bone marrow NOS3 in a chimera model increases the size of myocardial infarction and worsens left ventricular function (Merx et al., 2014). Since endothelium-resident NOS3 (endNOS3) expression decreases with decreasing lumen size, vasodilation in microvessels is primarily regulated by eryNOS3 activation and NO carried by RBCs (Cortese-Krott and Kelm, 2014). A unique safety feature of RBC-mediated NO delivery is that it is dependent on a low oxygen-gradient, produces hypoxic vasodilation, and delivers NO to improve CBF in ischemic regions (Stamler et al., 1997).
RIC is an exercise mimetic and shares common mechanisms of action with exercise, namely dependence on NOS3 (Hess et al., 2015b). While the mechanisms of the beneficial effect of exercise are multiple, there is strong evidence for a role for NOS3. Daily exercise for a month reduces cerebral infarct size in mice and the induced neuroprotection is dependent upon NOS3 (Endres et al., 2003). We showed that NOS3 inhibition abrogates the neuroprotective effect of short term exercise in acute stroke (Hafez et al., 2019). Conventional wisdom has thought this to be dependent upon endothelial cell (EC) NOS3 but blood cell and RBC NOS3 may play a larger role. RIC and exercise also affect RBC deformability. Exercise (HHI) improves RBC deformability in hypertensive subjects (Soltani et al., 2020). Grau (2016) showed that RIC increases RBC deformability and eryNOS3 in healthy humans.
RBC
Despite comprising 45- 50% of blood volume and over 80% of the total cells in the body the RBC has been largely ignored in stroke research (Sender et al., 2016). Each RBC circulates 3000 to 4000 times/day. RBCs are biconcave discs, 6-8 μm in diameter, and must pass through the smallest microvasculature (2-3 μm) under very high shear stress to deliver oxygen. To aid in passage through the microvasculature, healthy RBCs are highly deformable. This deformability can be measured and quantified by ektacytometry at the bedside.
CBF changes depend on two critical phenomena: 1) Vasodilation in major vessels by endothelial NOS3, which primarily maintains the “local” nitric oxide/nitrite (NO/NO2−) pool. However, the endothelial NOS3 is “uncoupled” during stroke, generating superoxide/peroxynitrite, which depletes the vasculo-protective NO/NO2− pool and impairs microvascular perfusion (Endres et al., 2004; Garry et al., 2015); 2) Adequate rheo-erythrocrine function, permitting reversible deformation of RBCs and their passage through microvessels under various shear stresses to oxygenate the tissue (Özüyaman et al., 2008; Cortese-Krott and Kelm, 2014). More than 50% of RBCs undergo severe morphological changes within 48 hrs after stroke in humans, likely contributing to poor deformability, impaired microcirculation, and resistance of clots to lysis (Swanepoel and Pretorius, 2012; Pretorius and Lipinski, 2013; Van Der Spuy and Pretorius, 2013).
RBC dysfunction in human stroke
We conducted a pilot study measuring RBC deformability by ektacytometry in patients with ischemic stroke and measured Elongation index (EI) at 24 hrs, 48 hrs, and 90 days. Using a two-sample t-test, we compared the EI at 24 hrs (mean ± SD) in stroke patients (N = 20, 0.29 ± 0.08) to control patients (n = 7 0.36 ± 0.01) and found that stroke patients had significantly lower EI (p = 0.0015) at 24 hrs. Using a mixed model we examined whether the change over time in EI among stroke patients was different due to race, sex, diabetes, tobacco use, tPA, ET, and hypertension. Hypertension was the only factor showing a significant interaction over time (p = 0.0688). Controlling for age, race, sex, diabetes, tobacco, tPA, and ET the change in EI over time [24 hrs (n = 18), 48 hrs (n = 16), 90 days (n = 7)] was different by hypertension with hypertensive stroke patients (n = 14) showing no change in EI from 24 hrs (adjusted mean ± SE 0.33±0.02) to 48 hrs (0.34 ± 0.02) to 90 days (0.36 ± 0.04) (all p > 0.42), but non-hypertensive stroke patients (n = 4) saw an increase in EI from 24 hrs (0.20 ± 0.04) to 90 days (0.34 ± 0.05, p = 0.0033) and 48 hrs (0.20 ± 0.05) to 90 days (p = 0.0043), but no change from 24 hrs to 48 hrs (p = 0.9810). There was a strong trend for EI to rise over time returning near the EI of control subjects at 90 days suggesting that the stroke itself may cause the decrease in deformability and rheo-erythrocine dysfunction. These data suggest that human stroke is associated with a decrease in RBC deformability.
Intracerebral hemorrhage (ICH)
Because of its ease of use, RIC is an ideal prehospital intervention that can be started in the ambulance or helicopter. However, any prehospital drug needs to be safe in patients with ICH. A concern raised by the FDA in submission for an Investigational New Device (IND) for RIC in acute stroke was the risk of exacerbation of ICH as RIC increases CBF. Geng and colleagues (2012) showed that RIC did not exacerbate ICH in a collagenase rat model when administered one time acutely. We undertook a study of RIC in a murine ICH model (Vaibhav et al., 2018). RIC started at 2 hrs post ICH and continued daily for five days reduced hematoma volume at five days and improved functional outcome. RIC was effective in both a collagenase and direct blood injection model in mice. Experiments with parabiotic pairs demonstrated the effect of RIC was humoral and mediated by circulating monocytes/macrophages. RIC treatment induced an increase in the ratio of anti-inflammatory/pro-inflammatory macrophages and was dependent upon AMP-activated protein kinas α1 activation in myeloid cells. Bone marrow chimera transplantation experiments showed that the RIC effect on hematoma clearance was dependent on bone marrow derived CD36. These studies demonstrate that RIC is also effective in a murine ICH model (if continued for 5 days post ICH) and supports the testing of RIC in the prehospital setting for stroke and as a treatment for ICH.
RIC trials in ischemic stroke and ICH
There have been a number of small clinical trials of RIC in acute ischemic stroke. The Remote Ischemic Conditioning After Stroke Trial (RECAST) (NCT 86672015) was a randomized blinded trial of 26 subjects with acute stroke treated within 24 hrs with RIC (England et al., 2017). Thirteen subjects were randomized to RIC and 13 to sham (4 cycles of 5 minutes) within a mean of 15.8 hrs post onset of stroke. RIC was well tolerated, safe and feasible. Subjects treated with RIC had significantly lower National Institute of Health stroke scale (NIHSS) scores at 90 days suggesting RIC improved long term outcome.
The Remote Ischemic Conditioning Paired with Endovascular Treatment Acute Ischemic Stroke (REVISE-1) trial was a single-arm, open-label, safety, and feasibility trial of RIC in 20 subjects with acute stroke undergoing ET (NCT03210051). There were no serious adverse events related to RIC. RIC was safe, well tolerated, and feasible when used on combination with ET (Zhao et al., 2018).
The Remote Ischemic Conditioning in Acute Brain Infarction (RESCUE BRAIN) trial (Pico et al., 2020) was a multicenter, randomized open-label, blinded endpoint trial (PROBE design). In this in-hospital trial, 188 subjects were randomized to RIC (n = 93) or usual care (n = 95). RIC was administered once with a thigh cuff within 6 hrs (mean 3 hrs 42 minutes). The primary outcome was growth in brain infarct size at 24 hrs. No significant difference was seen in infarct growth at 24 hrs but excellent outcome (modified Rankin scale 0,1) at 90 days was improved non-significantly in the RIC group (51% to 41%, p = 0.11). Most of the patients were treated with tPA (87%) and 34% underwent mechanical thrombectomy.
In the Remote Ischemic Conditioning for Intracerebral Hemorrhage (RICH) trial (NCT03930940) (Zhao et al., 2021), 40 subjects with ICH were randomized to RIC within 24-48 hrs of onset and treated daily for 7 days or usual care. There was no significant difference in hematoma volume at 7 days but the perihematomal resolution rate was higher in the RIC group. There was no difference in favorable functional outcomes at 90 days.
Prehospital clinical trials of RIC in stroke
Prehospital trials allow patients to receive the intervention earlier than in-hospital trials. One of the problems in stroke clinical trials is late administration of agents and interventions. A group led by Grethe Andersen and colleagues (Hougaard et al., 2014) in Aarhus Denmark completed a randomized clinical trial of RIC in acute stroke patients in the prehospital setting. The primary endpoint was penumbral salvage defined as perfusion-diffusion mismatch not progressing to infarction after 1 month. While there was no difference between the RIC and control group in the primary outcome and there was no difference in 3-month functional outcome, there was suggestive evidence that RIC showed “activity”. Transient ischemic attack was more frequent in patients who received RIC than in patients who did not (42 of 247 versus 16 of 196, p = 0.006), and patients who received RIC had lower baseline NIHSS scores than patients who did not (median 4 versus 5, P = 0.016). MRI data showed no significant effect of RIC between the intervention and control group on penumbral salvage, final infarct size, or infarct growth. However, after adjustment for baseline perfusion and diffusion lesion severity, voxel-wise logistic analysis showed that RIC reduced tissue risk of infarction (p = 0.0003). RIC was safe and well tolerated in the prehospital setting.
A number of methodological and operational issues impacted the trial. First, the trial enrolled a mild stroke population with a baseline mean hospital NIHSS of 5. Second, many subjects did not receive the full dose of RIC. Since RIC was discontinued when the patients arrived at the emergency department, only 41% of the subjects received the full RIC dose of 4 cycles. Lessons learned include the need to include a prehospital severity scale and to use an automated RIC device so that the RIC can be continued once the patient arrives at the emergency department for the full dose.
This experience led to the design of Denmark Remote Ischemic Conditioning in Patients with Acute Stroke (RESIST) Trial (Blauenfeldt et al., 2020). The RESIST trial (NCT03481777) in Aarhus, Denmark is a 1500 subject multicenter, randomized, sham-controlled of an automated RIC device in acute stroke in the prehospital setting (Blauenfeldt et al., 2020). Aarhus, Denmark is an ideal environment for prehospital stroke trials. One of the earliest prehospital RIC trials in ST-segment elevated myocardial infarction was conducted in Aarhus (Botker et al., 2010). Our group at the Medical College of Georgia has collaborated with Grethe Andersen’s group in Aarhus on an NIH funded biomarker study (R01NS112511) to examine the effects of stroke and RIC on rheo-erythrocrine markers including RBC deformability, RBC nitric oxide, and plasma nitrite. In this trial, RIC or sham RIC is started in the ambulance and repeated at 6 hrs and then twice per day for 7 days so that per- and postconditioning will be tested. Subjects will have blood drawn at three different time points (Fig 1) prior to RIC/sham RIC treatment, upon arrival at the hospital, and again at 24 hrs (Fig 2). Most RIC trials have tested only one RIC treatment acutely. About 10% of the subjects will have ICH so this is an opportunity to test whether RIC reduces hematoma size and improves outcome in ICH patients. ICH patients will have follow up CT scans at 24 hrs and 7 days, and functional outcome measured at 90 days.

In a new window | Download PPT
Figure 1: 1) RIC (arm) in the ambulance. 2) Shear stress increases RBC or eryNOS3 and improves deformability. 3) Increase in eryNOS3 “rescues” endothelial NOS3 and improved RBC deformability improves collateral flow. 4) Biomarker analysis in blood samples drawn pre and post RIC.
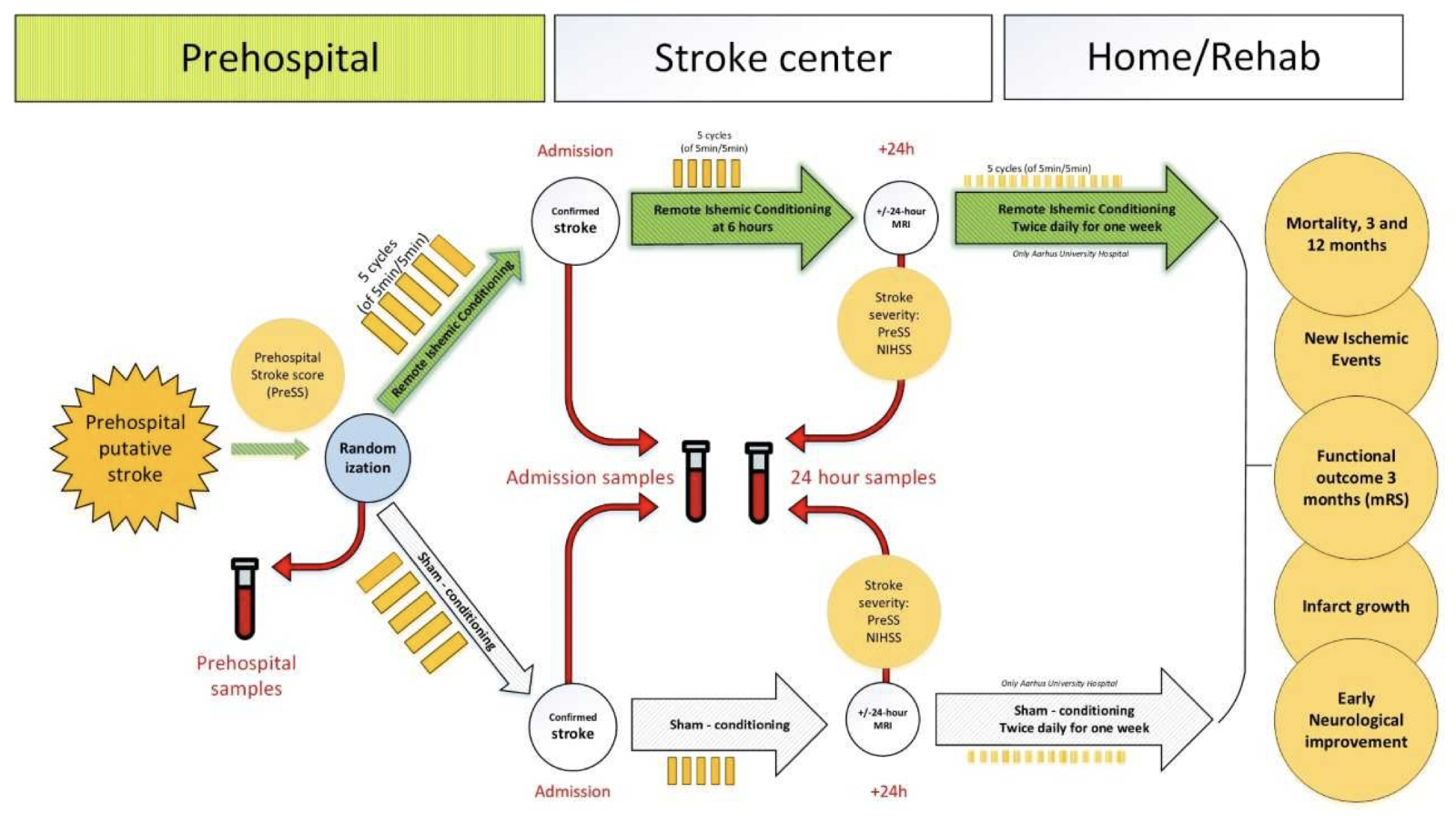
In a new window | Download PPT
Figure 2: RESIST Trial Biomarker substudy Blood samples are drawn at the 3 time points shown by the red arrows: 1.) in the ambulance immediately after randomization to RIC or sham RIC; 2.) after arrival at the stroke center and 3.) at 24 hrs after randomization.
This well designed and powered randomized clinical trial is meeting its recruitment targets and will answer the question of whether per-and postconditioning with RIC is effective in acute stroke. We will also be able to address the question of whether rheo-erythrocrine biomarkers predict outcome in acute stroke and response to RIC.
Acknowledgements
NIH funding; R01 NS114560 (KV); R01 NS110378 (KD/BB); R01 NS17565 (KD); R01 NS099455, 1UO1Ns113356, and R01 NS112511 (DCH).
References
David C Hess1
1Departments of Neurology, Medical College of Georgia, Augusta, GA.
Mohammad Badruzzaman Khan1
1Departments of Neurology, Medical College of Georgia, Augusta, GA.
Pradip Kamat1
1Departments of Neurology, Medical College of Georgia, Augusta, GA.
Kumar Vaibhav2,3
2Departments Neurosurgery, Medical College of Georgia, Augusta, GA. 3Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta, GA.
Krishnan M. Dhandapani2
2Departments Neurosurgery, Medical College of Georgia, Augusta, GA.
Babak Baban1,3
1Departments of Neurology, Medical College of Georgia, Augusta, GA. 3Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta, GA.
Jennifer L. Waller4
4Population Health Sciences4 Medical College of Georgia, Augusta, GA.
Md Nasrul Hoda5
5Department of Neurology, Henry Ford Hospital, Detroit, MI.
Rolf Ankerlund Blauenfeldt6
6Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Grethe Andersen6
6Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Corresponding author:
David C. Hess
Email: dhess@augusta.edu

In a new window | Download PPT
Figure 1: 1) RIC (arm) in the ambulance. 2) Shear stress increases RBC or eryNOS3 and improves deformability. 3) Increase in eryNOS3 “rescues” endothelial NOS3 and improved RBC deformability improves collateral flow. 4) Biomarker analysis in blood samples drawn pre and post RIC.

In a new window | Download PPT
Figure 2: RESIST Trial Biomarker substudy Blood samples are drawn at the 3 time points shown by the red arrows: 1.) in the ambulance immediately after randomization to RIC or sham RIC; 2.) after arrival at the stroke center and 3.) at 24 hrs after randomization.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5393 | 26 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA