Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Cardioprotection at the Bedside: an Exploration of Conditioning Phenomena and their Clinical Applications
Time:2018-02-21
Number:8186
Robert L. Yellon1, Derek M. Yellon1
Author Affiliations


- 1The Hatter Cardiovascular Institute, University College London, London, UK
Conditioning Medicine, 2018. 1(2):80-84.
Abstract
Ischemic conditioning is the use of short, repeated episodes of non-lethal ischemia to protect the myocardium from lethal ischemia-reperfusion injury. This term may also refer to the use of pharmacologic agents to mimic this mechanical protection. It is currently the only known method of protecting against ischemia-reperfusion injury. This review aims to investigate the various forms of conditioning as well as past, current, and future trials to explore their potential clinical applications.
Abstract
Ischemic conditioning is the use of short, repeated episodes of non-lethal ischemia to protect the myocardium from lethal ischemia-reperfusion injury. This term may also refer to the use of pharmacologic agents to mimic this mechanical protection. It is currently the only known method of protecting against ischemia-reperfusion injury. This review aims to investigate the various forms of conditioning as well as past, current, and future trials to explore their potential clinical applications.
Introduction
The most effective method of minimizing myocardial infarct-associated cardiomyocyte death is rapid reperfusion. With the introduction of fibrinolytics in the mid-80s, and then primary percutaneous coronary intervention (PPCI) in the early 21st century, myocardial infarction (MI) survival rates increased worldwide. However, coronary reperfusion is associated with a paradoxical cellular injury known as ischemia-reperfusion injury (IRI). It has been suggested that this injury may contribute up to 50% of final MI size and can also directly contribute to an increased incidence of long-term heart failure (Hausenloy and Yellon, 2016). As such, IRI is a valuable target for modern therapeutics. Unfortunately, to date there exists no established clinical therapy to prevent this mode of injury. This review explores the clinical potential of the so-called “ischemic conditioning” phenomena and how they may ultimately prove crucial in limiting IRI.
The principles of conditioning phenomena
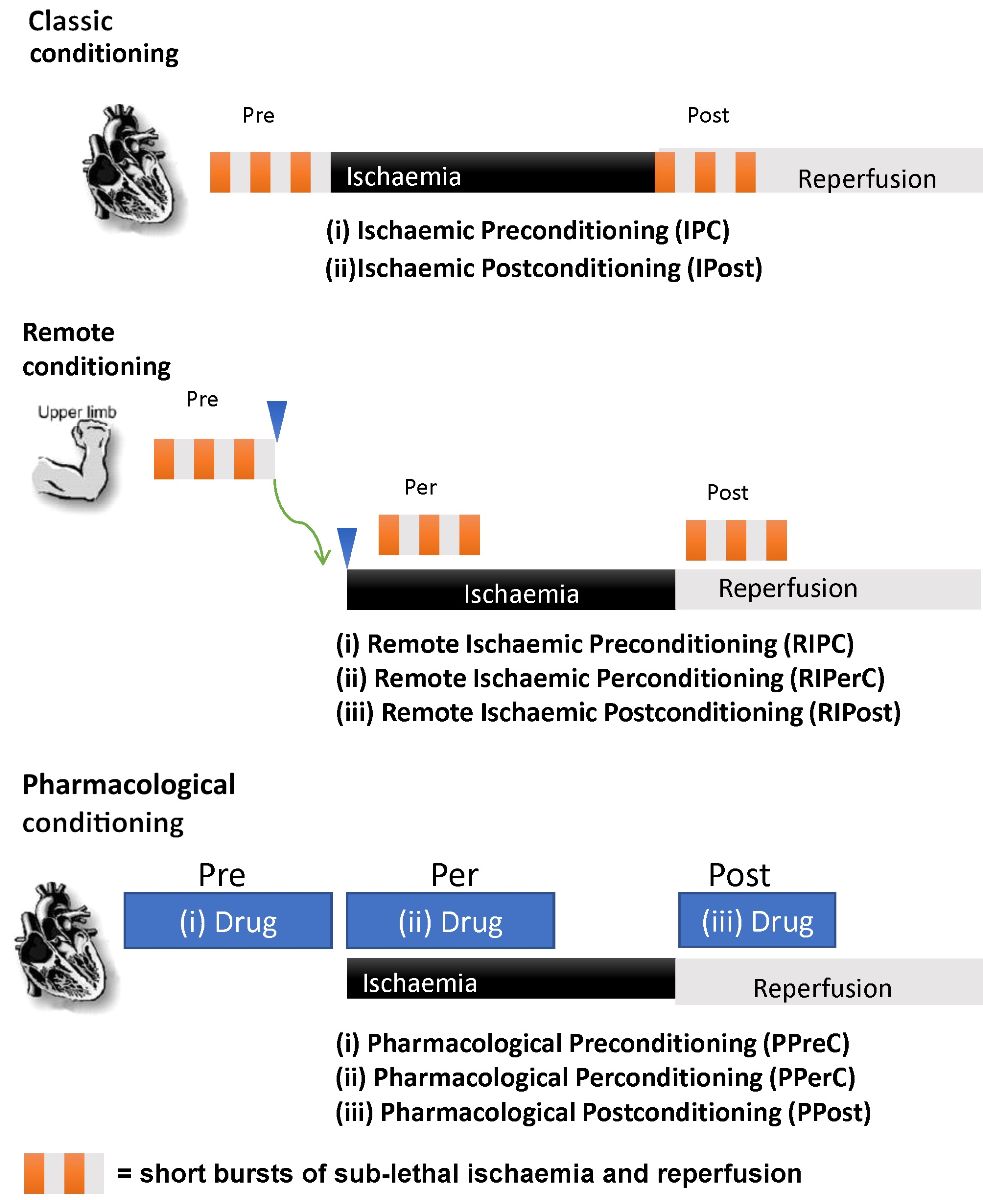
Ischemic conditioning is defined as the protection conveyed to the ischemic myocardium by short, repeated periods of non-injurious ischemia either before, during, or after a lethal ischemic insult (Hausenloy and Yellon, 2016).
Ischemic conditioning may be classified in a variety of ways. From a temporal point of view, ischemic conditioning may occur prior to the injurious episode (pre-conditioning), during the injurious episode (per-conditioning), or following the injurious episode (post-conditioning). From a spatial/anatomical point of view, ischemic conditioning may occur at the site of injurious ischemia (classical conditioning), or distal to the site of injurious ischemia (remote conditioning) (Hausenloy and Yellon, 2016).
In addition, the physiological mechanisms underlying ischemic conditioning may be mimicked using pharmacological agents in what is known as “pharmacological conditioning” (Hausenloy and Yellon, 2016).
Classical ischemic pre-conditioning (IPC) may be defined as brief, repeated periods of non-injurious ischemia induced locally, e.g. via aortic clamping during cardiac surgery, prior to a lethal ischemic episode (Hausenloy and Yellon, 2016). This conditioning method has been shown to reduce infarct size in animals in which a MI is subsequently induced. Small-scale human studies have shown that aortic clamping prior to coronary artery bypass graft (CABG) surgery reduces post-surgical markers of cardiovascular damage, e.g. adenosine triphosphate (Yellon et al., 1993); troponin T (Jenkins et al., 1997; Teoh et al., 2002); and cardiac index (IIlles and Swoyer, 1998). However, the embolic risk inherent to aortic clamping related to co-existing aortic atheromatous disease restricts classical pre-conditioning to small-scale trials and prevents clinical implementation.
Remote ischemic pre-conditioning (RIPC) may be defined as brief, repeated periods of non-injurious ischemia applied via a blood pressure cuff to one or more limbs prior to an injurious episode of cardiac ischemia (Hausenloy and Yellon, 2016). This conditioning method has been shown to reduce infarct size in animal models (Birnbaum et al., 1997). Proof-of-concept studies in humans have shown that, when implemented prior to CABG surgery, remote pre-conditioning can reduce post-surgical troponin-T levels (Hausenloy et al., 2007; Venugopal et al., 2009). The non-invasive nature of this intervention has made it a prime candidate for large-scale human trials. However, to date such large-scale trials in the setting of cardiac bypass surgery have shown no benefit, including the RIPHeart and ERICCA trials (Meybohm et al., 2015; Hausenloy et al., 2015); these will be explored further in the next section.
Pharmacological pre-conditioning (PPreC) may be defined as the use of pharmacological agents to mimic a pre-conditioning stimulus to protect the myocardium from IRI prior to an injurious ischemic insult (Hausenloy and Yellon, 2016). Examples of such a pharmacological approach include studies on rabbits where volatile anaesthesia has led to smaller infarct sizes prior to induced infarction (Cason et al., 1997; Cope et al., 1997). A proof-of-concept, double-blinded, controlled study showed that a 10-minute dose of 4% sevoflurane prior to CABG resulted in reduced post-operative serum brain natriuretic peptide (a marker of myocardial contractile dysfunction) but no difference in levels of creatine kinase-MB or cardiac troponin T (Julier et al., 2003). To date, no large-scale studies on the use of PPreC prior to cardiac surgery have been undertaken.
Remote ischemic per-conditioning (RIPerC) may be defined as brief, repeated periods of non-injurious ischemia, applied via a blood pressure cuff to one or multiple limbs during an injurious episode of cardiac ischemia (Hausenloy and Yellon, 2016). Animal studies have shown that RIPerC can result in reduced infarct size and a reduced risk of reperfusion arrhythmias (Schmidt et al., 2007; Zhu et al., 2003). The proof-of-concept ERIC-LYSIS trial (Yellon et al., 2015) examined the impact of RIPerC in patients presenting with ST-elevated myocardial infarction (STEMI) and who were treated with thrombolysis. In this study, individuals were randomized to receive thrombolysis either with or without RIPerC on immediate arrival to the hospital. Those who received RIPerC had a statistically significant reduction in MI size as assessed by serum troponin-T and creatinine kinase levels 24 hours after thrombolysis. Median MI size was 32% smaller according to troponin-T levels (P = 0.020), and 19% smaller according to creatinine kinase levels (P = 0.026) in the RIPerC cohort. The proof-of-concept CONDI trial (Sloth et al., 2014) examined the impact of RIPerC in patients presenting with STEMI and who were treated with PPCI. In this study, individuals were randomized to receive PPCI either with or without RIPerC during ambulance transfer. Those who received RIPerC had a statistically significant reduction in all-cause mortality (P = 0.027) on follow-up (median = 3.8 years). Large-scale human trials of RIPerC in STEMI patients undergoing PPCI are currently underway, including CONDI2/ERIC-PPCI (Hausenloy et al., 2015), and are explored further on in this review.
Pharmacological per-conditioning (PPerC) may be defined as the use of pharmacological agents to mimic a conditioning stimulus for the purposes of protecting the myocardium from reperfusion injury during an injurious ischemic insult (Hausenloy and Yellon, 2016). Multiple drugs have shown promise in this capacity, including cyclosporine (Piot et al., 2008) and metoprolol (Ibanez et al., 2013), both of which have been associated with a significant reduction in infarct size in small-scale human studies. However, the recent large-scale CIRCUS (Mewton et al., 2015) and CYCLE (Ottani et al., 2016) studies exploring cyclosporine A (CsA) as a PPerC agent have showed no benefit to clinical outcomes and are discussed in more detail below.
Classical ischemic post-conditioning (IPost) may be defined as brief, repeated periods of non-injurious ischemia applied via balloon inflation to a previously ischemic coronary artery immediately following STEMI-indicated stent implantation (Hausenloy and Yellon, 2016). While IPost has shown the capability to reduce infarct size in animal models (Zhao et al., 2003), proof-of-concept human studies have produced mixed results (Lønborg et al., 2010; Freixa et al., 2012). The large DANAMI-3-iPOST trial showed no clinical benefit and is also discussed below (Engstrøm et al., 2017).
Remote ischaemic-postconditioning (RIPost) may be defined as brief, repeated periods of non-injurious ischemia applied via a blood pressure cuff to one or multiple limbs immediately following stent implantation (Hausenloy and Yellon, 2016). RIPost has been shown to reduce infarct size in animal models (Li et al., 2006) and in proof-of-concept human studies (Crimi et al., 2013). Importantly, however, as of yet there have been no large-scale clinical outcome trials exploring the impact of RIPost in humans.
Pharmacological postconditioning (PPost) may be defined as the use of pharmacological agents to mimic a post-conditioning stimulus in order to protect the myocardium from reperfusion injury immediately following reperfusion (Hausenloy and Yellon, 2016). The most promising drug in this case has been atrial natriuretic peptide (ANP). A recent randomized clinical trial (RCT) (Kitakaze et al., 2007), showed that ANP, when given as an infusion following reperfusion treatment for myocardial infarction, resulted in a statistically significant reduced infarct size of 14.7% (P = 0.019), and a reduced incidence of readmission (HR = 0.267; P = 0.011) for heart failure relative to the control group over the follow-up period (median = 2.7 years). Another promising compound is the GLP-1 analogue exenatide. A study of 172 STEMI patients (Woo et al., 2013) showed that those individuals given exenatide at the time of reperfusion therapy had on average a 15% larger salvage index (i.e., the difference between actual and potential infarct size) compared to those treated with classical reperfusion (P = 0.003). No significant difference in clinical outcomes was measured.
Finally, multiple trials involving a combination of pharmacological conditioning subtypes have also been undertaken. A small 58-patient RCT demonstrated that a combination of per- and post-conditioning with exenatide, a GLP-1 analogue, was associated with a reduction in infarct size (Woo et al., 2013). Another notable RCT of 96 STEMI patients demonstrated that treatment with another GLP-1 analogue, liraglutide, for 30 minutes before intervention and for 7 days afterwards was associated with improved myocardial salvage and infarct size (Chen et al., 2016).
The conundrum of ischemic conditioning
Though the various conditioning methods have shown promise in animal studies and small-scale proof-of-concept human trials, large-scale trials have yet to show any benefit in limiting IRI. This section will explore these trials in further detail and ask the question, “Why, despite the replicable ability of conditioning techniques to limit IRI in the laboratory, are these phenomena not yet transferable to the clinical setting in terms of patient outcome benefit?”.
With regards to RIPC, in 2015 the RIPHeart study (“A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery) (Meybohm et al., 2015) was published. RIPHeart was a 1403-patient, multicenter, double-blinded RCT exploring the use of RIPC in patients undergoing elective cardiovascular surgery necessitating cardiac bypass. The primary endpoint was a composite of death from any cause, non-fatal MI, new stroke, or acute renal failure up to the time of hospital discharge (or a maximum of 14 days). Following anesthetic induction and prior to surgical incision, patients underwent 4 cycles of either true or sham RIPC. The trial results showed no statistically significant difference in the primary outcomes of either intervention. Concomitant with RIPHeart was the ERICCA study (“The Effect of Remote Ischemic Conditioning on Clinical Outcomes in Coronary Artery Bypass Graft Surgery”) (Hausenloy et al., 2015). ERICCA was a 1612-patient, multicenter, double-blinded RCT exploring the use of RIPC in patients undergoing CABG surgery. The primary endpoint was the rate of major adverse cardiac and cerebral events 12 months post-intervention. Following anesthetic induction and prior to surgical incision, patients underwent 4 cycles of either true or sham RIPC. As with the RIPHeart study, there was no statistically significant difference in the primary outcomes of either intervention. Why did these trials fail to link RIPC to improved outcomes? It is notable that these trials both examined RIPC in the setting of CABG surgery. It may be that the injury induced during CABG is too insignificant for the RIPC stimulus to produce a substantial effect. RIPC may yet still prove useful in the setting of PPCI as discussed in the next section. It is also important to note the induction agent propofol was used in all patients in RIPHeart, and >90% of patients in ERICCA. Propofol has been shown to abolish RIPC protection in humans (Kottenberg et al., 2012).
With regards to PPerC, there are two major human trials of note: the so-called CIRCUS trial (“Cyclosporine to Improve Clinical Outcome in ST-elevation Myocardial Infarction Patients”) (Mewton et al., 2015), and the CYCLE trial (“Cyclosporine A in Reperfused Acute Myocardial Infarction”) (Ottani et al., 2016). CIRCUS was a 970-patient, multicenter, double-blinded RCT exploring the perconditioning potential of CsA in patients undergoing PPCI to treat STEMI. The primary endpoint was a 1- and 3-year composite of death from any cause, progression of heart failure during initial hospitalization, rehospitalization for heart failure, or adverse left ventricular modelling (as measured by an increase in left ventricular [LV] end-diastolic volume). Patients were randomized to an IV infusion of either CsA or a placebo immediately prior to PPCI. At 1 year (Cung et al., 2015), no statistically significant differences in primary outcomes were detected between the two cohorts. This neutral data could potentially be explained by one of the primary endpoint components. Data for LV end-diastolic volume were missing in 17% of patients. This missing data, combined with the high incidence of adverse remodeling in both the CsA cohort and the control (42.8% to 40.7%, respectively) would have made it difficult to detect a significant difference in the other components of the primary endpoint composite. At one point it was thought that the neutral results could have been put down to the formulation of CsA. The formulation of CsA used in this trial (CicloMulsion; an ethanol and polyoxyethylated castor oil carrier vehicle) was different from that used in the initial proof-of-concept study (Piot et al., 2008) (Sandimmune; a lipid emulsion carrier vehicle) that prompted CIRCUS. However, this hypothesis was put to rest by the neutral results of the CYCLE trial. CYCLE was a 410-patient, multicenter, Prospective/Randomized/Open-label/Blinded-Endpoint (PROBE) trial which also explored the perconditioning potential of CsA in patients undergoing PPCI to treat STEMI. The primary endpoint was the incidence of ≥70% ST-segment resolution 60 min after thrombolysis in myocardial infarction (TIMI) flow grade 3. STEMI patients were randomized to an IV injection of either 2.5mg/kg CsA or a control injection prior to PPCI. No statistically significant difference between the CsA and the control cohorts was detectable. The CYCLE trial may therefore prove the end of cyclosporine as a potential perconditioning agent.
DANAMI 3-iPost (Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction) (Engstrøm et al., 2017) was a treatment subgroup of a trial program comparing treatment strategies in STEMI patients. The IPost arm of the trial was a 1234-patient, multicenter, PROBE trial comparing standard PCI to PCI plus IPost. The primary endpoint was a composite of death from any cause and hospitalization for heart failure within 2 years of the procedure. Patients were randomized to standard PCI, or PCI plus four 30-second balloon inflations within the reperfused coronary artery following initial reperfusion. The study showed no statistically significant difference between the two cohorts. The study authors hypothesized that this result may reflect a general improvement in STEMI outcomes concealing any benefit of IPost. In their discussion, the authors suggested that the 30 seconds of reperfusion prior to IPost might have been too long, with reperfusion injury having taken place before initiation of the IPost itself. As such, a modified protocol of 15 seconds of reperfusion prior to IPost may be more effective (though this runs counter to the more traditional IPost protocol of 60 seconds of reperfusion (Freixa et al., 2012). Further large-scale studies are needed to investigate the effect that different protocol times may have on the efficacy of IPost.
Upcoming clinical trials
In this final section, we shall explore the near future of conditioning-related clinical trials. This section will focus on studies that are currently underway and which provide hope for the future of conditioning methods as clinical interventions.
The CONDI2 (Effect of RIC on Clinical Outcomes in STEMI Patients Undergoing PPCI) and ERIC-PPCI (Effect of Remote Ischemic Conditioning on Clinical Outcomes in STEMI Patients Undergoing PPCI) are two collaborative studies investigating the use of RIPerC in STEMI patients (Hausenloy et al., 2015). Both CONDI2 and ERIC-PPCI are multicenter, multinational, double-blinded RCTs with 2600 patients at each center (total 5200). The primary endpoints of both trials are cardiovascular mortality at 1 year and hospitalization for heart failure at 1 year. In these trials, individuals will be randomized to receive PPCI either with or without RIPerC. In countries where transit times allow (Denmark/Spain), the RIPerC will be delivered in the ambulance prior to arrival for PPCI. In countries where transit times are noticeably shorter (Serbia/United Kingdom) the RIPerC will be delivered on arrival to the PPCI center prior to the PPCI itself.
Although no large-scale trials exploring the potential of RIPerC in STEMI treated by thrombolysis are currently underway, the ERIC-LYSIS trial (Yellon et al., 2015) provides an incentive to do so. While thrombolysis has been superseded by PPCI in much of the Western world, its expense means that thrombolysis remains an important cornerstone for the management of acute STEMI in much of the world. If RIPerC could be exploited in this setting, it has the potential to be a cost-neutral, life-saving adjunct to thrombolytic intervention in countries where PPCI is not available.
Finally, when exploring future evidence for conditioning methods as clinical interventions, it is important to return to the aforementioned CIRCUS study. Whilst the CIRCUS study of PPerC showed no statistically significant effect at 1 year (Mewton et al., 2015), clinical outcomes at 3 years remain to be seen in the follow-up study, or “CIRCUS II” (Clinicaltrials.gov, 2018). The question of cyclosporine A’s potential as a preconditioning agent will not be settled until these results have been announced.
The inadequacy of preclinical models and clinical study design
Though explanations have been offered above for the neutral outcome of individual trials, the repeated failure to translate preclinical models into the clinical setting is concerning. The translation failure has been labelled “the disconnection paradigm” (Rossello and Yellon, 2016). In their commentary on the topic, the authors discussed the many factors responsible for this disconnect. A significant proportion of preclinical studies into conditioning models utilize animals that do not adequately represent the general population exposed to reperfusion injury (Hausenloy et al., 2017). It is common for young, healthy animals to be utilized in preclinical studies. This grossly misrepresents the average MI patient, i.e. diabetic, obese, hypertensive and exposed to multiple cardioprotective pharmacological agents such as statins, beta-blockers and anti-platelet drugs (Hausenloy et al., 2017). As such, it is important that future preclinical and clinical trials take this into account during design, utilizing animal cohorts that more appropriately fit the model of the metabolic syndrome and attempting to standardize as best as possible the patient population being studied.
Conclusion
Ischemic conditioning is a recognized phenomenon in animal models. If it could be harnessed in the clinical setting, its potential to reduce infarct size through limitation of IRI could prove extremely valuable to patients. Not only could it reduce overall cardiovascular mortality, but it also could combat the burgeoning epidemic of ischemic cardiomyopathy associated with improved clinical practice and thus improved MI survival rates. As of yet, large clinical trials have provided neutral results for a variety of reasons unique to each study. It is important to note that translation of animal studies to clinical outcomes in this field is deeply flawed due to the common practice of utilizing animals that do not adequately reflect the clinical population. Although this might appear to be a pessimistic view, it is important to strive forward in our exploration of these phenomena, while taking these lessons into account. The book has not yet been closed on ischemic conditioning, and the CONDI2/ERIC-PPCI trials may yet prove to be ischemic conditioning’s saving grace.
In a new window | Download PPT
Figure 1: This figure shows the various forms of conditioning that are associated with this phenomenon.
References
Robert L. Yellon
1The Hatter Cardiovascular Institute, University College London, London, UK
Derek M. Yellon
1The Hatter Cardiovascular Institute, University College London, London, UK
Corresponding author
Derek M. Yellon
Email: d.yellon@ucl.ac.uk

In a new window | Download PPT
Figure 1: This figure shows the various forms of conditioning that are associated with this phenomenon.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8186 | 24 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA