Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Searching for new stroke therapeutics with a mindset drawn from the preconditioning field: the example of a recent discovery related to the targeting of the polyamine pathway
Time:2021-11-15
Number:6711
Nicolas Blondeau1, Michel Tauc2
Author Affiliations


- 1Université Côte d’Azur, CNRS UMR-7275, IPMC, Sophia Antipolis, F-06560, France.
- 2Université Côte d’Azur, CNRS UMR-7370, LP2M, Nice, F-06107, France.
Conditioning Medicine 2021. 4(4): 192-197.
Abstract
Stroke, by its frequency and severity, represents an economic burden and a public health issue for our societies. To limit the neuronal and functional damage induced by ischemic stroke, the only option in clinical practice is to restore cerebral perfusion as quickly and completely as possible. Unfortunately, most individuals with stroke do not benefit from drug or mechanical reperfusion, therefore it is important to seek alternative anti-ischemic therapies. One of the options for identifying new target/drug pairs with increased predictive clinical translation is to take advantage of the conservation of molecular mechanisms involved in cellular protection between species, organs, and cells. Therefore, starting from the discovery of the link between polyamine synthesis and hypoxia tolerance in the Drosophila animal model, this review describes how the several results obtained in different experimental setups of ischemia in various animals, organs, and cell cultures contribute to the identification of anti-ischemic drugs targeting the polyamine pathway. Focusing on the recent example of the inhibitory targeting of eIF5A hypusination, this review describes how lessons learned in the field of preconditioning have changed the mindset by drawing attention to the intersection of how different conditioning paradigms to protect organs from ischemic death, leading to unexpected biological targets and insights against stroke.
Keywords: Brain ischemia, Polyamine, eIF5A, Mitochondria, Oxidative Stress, Neuroprotection
Abstract
Stroke, by its frequency and severity, represents an economic burden and a public health issue for our societies. To limit the neuronal and functional damage induced by ischemic stroke, the only option in clinical practice is to restore cerebral perfusion as quickly and completely as possible. Unfortunately, most individuals with stroke do not benefit from drug or mechanical reperfusion, therefore it is important to seek alternative anti-ischemic therapies. One of the options for identifying new target/drug pairs with increased predictive clinical translation is to take advantage of the conservation of molecular mechanisms involved in cellular protection between species, organs, and cells. Therefore, starting from the discovery of the link between polyamine synthesis and hypoxia tolerance in the Drosophila animal model, this review describes how the several results obtained in different experimental setups of ischemia in various animals, organs, and cell cultures contribute to the identification of anti-ischemic drugs targeting the polyamine pathway. Focusing on the recent example of the inhibitory targeting of eIF5A hypusination, this review describes how lessons learned in the field of preconditioning have changed the mindset by drawing attention to the intersection of how different conditioning paradigms to protect organs from ischemic death, leading to unexpected biological targets and insights against stroke.
Keywords: Brain ischemia, Polyamine, eIF5A, Mitochondria, Oxidative Stress, Neuroprotection
The actual context of stroke research
Stroke is one of the most devastating diseases in the world. It places an enormous economic and human burden on our modern societies (Moskowitz et al., 2010). The ischemic form of stroke, which is the most common (more than 70%), is caused by occlusion of an intracranial artery by a thrombus that usually originates in the bloodstream but it can also be formed in situ. To limit neuronal damage and functional sequels, the primary goal of current stroke management is to restore cerebral perfusion as quickly and completely as possible to stop the spread of ischemic necrosis. Unfortunately, most people are not eligible for reperfusion therapies, which aim to restore blood flow to obstructed arteries, either with drugs like recombinant tissue plasminogen activator (rtPA) (Wardlaw et al., 2003) or mechanically (endovascular thrombectomy) (Leo and Sharma, 2013; Shi et al., 2018). Moreover, among the limited number of patients who receive this type of treatment, few make a full recovery. Thus, not only are stroke patients faced with an extremely limited repertoire of treatment options, but stroke-related incidence, death, and disability continue to rise. Over the past decades, more than 200 clinical trials have evaluated neuroprotective candidates for ischemic stroke, but to date nearly all have failed (O’Collins et al., 2006). This has led to a massive and lasting exodus of pharmaceutical companies from clinical trials and preclinical stroke research. Therefore, now more than ever, new concepts and targets are needed to reverse this trend, re-engage stroke researchers, and accelerate the development of new anti-ischemic therapies (Tauskela and Blondeau, 2009; 2018; Tauskela et al., 2017).
How lessons learned from the preconditioning field may lead to an innovative concept to discover new therapeutic target for stroke
The concept may seem simple at first: rather than looking for a specific therapeutic target in neurons to protect them from stroke, experience with preconditioning is driving a shift in perspective toward common targets at the intersection of different research paradigms that have independently been useful to identify novel target/drug combinations, but whose clinical implications are unfortunately still pending. This has been envisioned since the discovery of the preconditioning phenomenon - more than 35 years ago (Murry et al., 1986), and as repeatedly emphasized in this journal, it requires the development of an integrative and cross-sectional view of conditioning research. To this end, it is necessary to consider all available options, such as using model organisms like Drosophila, as well as different experimental setups of pre-, post-, repeated, and remote preconditioning in different organs or cell types suffering from ischemia. In several experimental settings, conditioning, also referred as ischemic tolerance, has been achieved in multiple organs, including heart, kidney, and brain, as well as their respective cell types (Gidday, 2006; Pignataro et al., 2008; Iadecola and Anrather, 2011; Chen et al., 2020; Schmidt et al., 2021) indicating that they possess one or more extremely efficient systems for self-protection against ischemia. Tolerance to ischemia has also been the focus of investigation in Drosophila, known as the invertebrate model primarily used to study developmental genetics. While the anatomy of its organs, particularly the brain, is obviously very different from that of humans, the cellular system and physiological pathways for using oxygen as an energy source, as well as the response of this system from oxygen management to hypoxia are highly conserved during evolution (Zhou and Haddad, 2013). We therefore sought to identify, through the results obtained by these strategies, a common molecular target that would meet the selection criteria favored in drug R&D, i.e. a molecular target easy to inhibit, such as a receptor or an enzyme, which together represents more than 70% of current pharmacological targets (Imming et al., 2006; Landry and Gies, 2008).
While it has never been considered a potential target in the treatment of ischemic pathologies, eIF5A is at the intersection of several conditioning paradigms.
Using Drosophila as a model organism, Michel Tauc and his collaborators have identified a link between hypoxia resistance and the polyamine synthesis pathway (Vigne and Frelin, 2008; Vigne et al., 2009), suggesting that targeting this pathway could improve hypoxic tolerance of an organism and, by extension, of an organ. Based on the demonstration that dietary protein reduction significantly improved the longevity of Drosophila subjected to hypoxic conditions (Vigne and Frelin, 2006; 2007), they identified that the toxic effect of proteins during hypoxia was not associated with a specific amino acid, but rather with their metabolic derivatives related to the urea cycle, L-citrulline and L-ornithine. Finally, they were able to demonstrate that the toxic effect of proteins was due to their ability to fuel the polyamine synthesis pathway trough the urea cycle, generating putrescine, spermine, and spermidine, with these polyamines also being toxic to longevity the of Drosophila under hypoxic condition. Moreover, a pharmacological approach validated the efficacy of targeting the polyamine pathway to counter hypoxia in Drosophila. The pharmacological inhibition of ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, by α-difluoromethylornithine (DFMO) that specifically inhibits ODC (LoGuidice et al., 2018) (Figure 1) extended up to 3-fold the survival of Drosophila subjected to chronic hypoxic conditions (Vigne and Frelin, 2008; Tauc et al., 2019).

In a new window | Download PPT
Figure 1: Schema of the urea cycle involved in amino acid catabolism and leading to the synthesis of polyamines by sequential enzymatic catalysis and the activation by hypusination of the translation initiation factor eI5A (modified from Tauc et al., 2019). Pharmacological inhibition of ornithine decarboxylase (ODC), an enzyme in the first step of polyamine biosynthesis, by DFMO (α-difluoromethylornithine) increases Drosophila resistance to hypoxia, and also protects cardiomyoblast or neuronal cell cultures from ischemia. Downstream, GC7 (N1-guanyl-1,7-diaminoheptane) inhibition of the enzyme deoxyhypusine hydroxylase (DOHH), a crucial step in eIF5A activation/hypusination increases Drosophila resistance to hypoxia, as well as protects renal and neuronal cells in several in vitro and in vivo models of hypoxia/ischemia.
Interestingly, a link between high polyamine levels and cardiac and cerebral ischemia has also been reported. First, in 1993, Longo et al. (1993) have reported that acute hypoxia rapidly and transiently increased ODC activity as well as polyamine concentrations in the fetal rat brain. At that time, the increase in polyamine concentrations was even proposed as a potential enzymatic marker of fetal brain hypoxia, and although not experimentally addressed in the study, as a potential protective mechanism of the brain in response to hypoxia. More recently, a similar increase in ODC activity and putrescine accumulation was observed in Langendorff perfused rat hearts subjected to 40 min of ischemia without reperfusion (Zhao et al., 2007) and following transient focal cerebral ischemia induced by 2h of middle cerebral artery occlusion (MCAo) in the rat (Babu et al., 2003), suggesting that hypoxia/ischemia affect polyamine metabolism and that putrescine may be involved in ischemic death. This was confirmed by the observation that, like in Drosophila, DFMO reduces ischemic-induced death in neuronal cultures. Interestingly, the protective effect of DFMO was also observed against apoptosis of H9c2 cardiomyoblasts in a model of in vitro ischemia by oxygen and serum deprivation (Tantini et al., 2006). Again, in this model, ischemia rapidly and transiently induced an increase in ODC activity and in polyamine levels that were counteracted by DFMO application. Interestingly, the anti-ischemic effect of ODC pharmacological inhibition, thought to be related to the preservation of the mitochondria, was lost in the presence of exogenous putrescine, indicating the involvement of a downstream event in the development of apoptosis in this in vitro model of ischemia. Thus, ischemic tolerance has been achieved by targeting the polyamine pathway through ODC inhibition in several experimental settings, including in a model organism such as Drosophila, in multiple organs, including heart, kidney, and brain, as well as their respective cell types, demonstrating that strong anti-ischemic potential lies downstream of this pathway.
The polyamine synthesis pathway generates spermidine, one of the main functions of which is to activate the eukaryotic translation initiation factor eIF5A by hypusination (i.e. addition of hypusine) (Park, 2006). The hypusination reaction consists of the addition of a 4-aminobutyl residue derived from the cleavage of spermidine onto the ε-amine group of a specific lysine (Lys50), followed by the hydroxylation of this residue, and is catalyzed by two enzymes: deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH). eIF5A activation/hypusination that was originally described as being crucial in modulating RNA translation initiation, elongation, and termination (Scjuller et al., 2017) was recently shown to modulate mitochondrial function. By regulating the expression of a subset of mitochondrial proteins involved in the tricarboxylic acid (TCA or Krebs) cycle and oxidative phosphorylation (OXPHOS) (Puleston et al., 2019), which are the two important sets of reactions to meet most of the total energy needs of eukaryotic cells, activation/hypusination of eIF5A may control metabolic switching between OXPHOS and glycolysis supports. During ischemia, energy production is compromised by the alteration of both the aerobic and anaerobic pathway culminating in the inability of mitochondria to regenerate chemical energy in the form of ATP (Vannucci et al., 2016). Moreover, recent evidence conferred new roles for metabolites generated during energy production, including those arising from the TCA cycle, in controlling cellular function and fate in different physiologic and disease conditions (Martinez-Reyes and Chandel, 2020). Mitochondria and cerebral metabolism thus play central roles in injury and recovery after stroke. The possible involvement of eIF5A in these mechanisms, combined with the fact that it is the only known substrate of DHS in Drosophila and mammals (Park et al., 1984), underscores the interest in its therapeutic targeting, whereas it has never been considered a potential pharmacological target in the treatment of ischemic pathologies. However, the first indications of its potential had been highlighted in Drosophila using the specific competitive inhibitor of DHS named N1-guanyl-1,7-diaminoheptane, or GC7. GC7-induced inhibition of eIF5A significantly increased survival of Drosophila subjected to hypoxia, including those fed a diet enriched in amino acids or polyamines (Vigne and Frelin, 2008).
Keeping in mind our starting idea from our experience in the field of preconditioning, namely the need to ensure an anti-ischemic effectiveness of the drug/target in different models, organs, and cell types before investigating the effect against stroke, we first investigated a possible anti-ischemic effect of eIF5A hypusination inhibition in renal ischemia. As with the brain, renal function depends on a high renal oxygen supply via adaptive blood flow, and hypoxia/ischemia is now recognized as a unifying pathway to both chronic and acute kidney diseases (Hansell et al., 2013). In cultured cells from mouse proximal tubule, GC7 treatment, as well as siRNA targeting DHS or DOHH, reduced anoxia-induced cell death (Melis et al., 2017; Tauc et al., 2019). GC7-induced protection was associated with a reversible reprogramming of glucose handling and oxygen dependence in kidney cells (Cougnon et al., 2021). Moreover, in vivo preconditioning of rats with GC7 that decreases the hypusinated form of eIF5A protected renal function from unilateral ischemia/reperfusion challenge of kidney (for review see Tauc et al., 2019).
Because EIF5A is a highly conserved protein also found in neuronal cells and because this target was a common element meeting the different selection criteria we had set, we investigated whether inhibition of GC7 and its associated DHS could lead to new therapeutic opportunities in ischemic stroke.
The prospect of a novel anti-ischemic target applicable to the brain and ischemic stroke
We first sought to provide proof of concept of the anti-ischemic effect of GC7 in vitro. The effect of DHS inhibition by GC7 was studied in primary cultures of cortical neurons subjected to oxygen and glucose deprivation (the OGD model is accepted as the gold standard model of stroke in vitro (Goldberg and Choi, 1993; Tauskela et al., 2016), as well as against two neurotoxic mechanisms involved in stroke: glutamate excitotoxicity induced by uncontrolled stimulation of receptors for this excitatory neurotransmitter, and oxidative stress induced by the overproduction of free radicals (Iadecola and Anrather, 2011). In all experimental paradigms, treatment with GC7 provided significant protection of neurons in culture (Bourourou et al., 2021). We then evaluated the anti-ischemic effect of GC7 in different in vivo protocols to assess the translational potential on neuronal survival (Figure 2A) and spontaneous functional recovery (Figure 2B) after stroke in mice. A beneficial effect on these two parameters was obtained even when GC7 was administered after stroke onset within a time window compatible with the management of stroke patients (Bourourou et al., 2021).
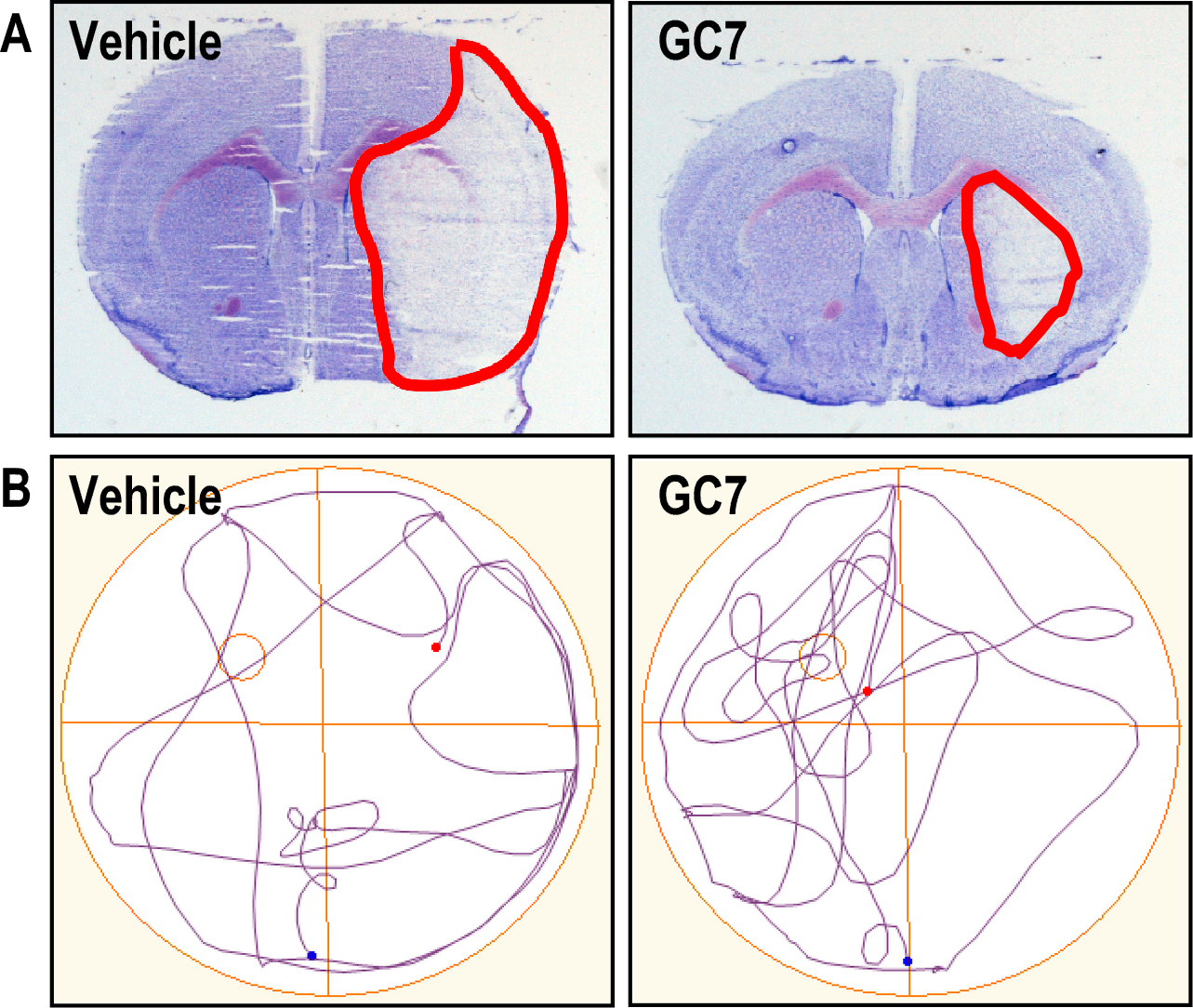
In a new window | Download PPT
Figure 2: Inhibition of deoxyhypusine synthase by GC7 reduces brain injury and improves spontaneous functional recovery after ischemic stroke. A. GC7 treatment reduces cerebral infarction (outlined in red) induced by focal ischemic stroke. B. GC7-treated mice show improved functional (cognitive) recovery after stroke. In rodents, the study of cognition is usually limited to an assessment of learning and spatial memory. To assess the effect of post-treatment on cognitive recovery in animals after stroke, the most commonly used test is the Morris water maze test, a circular pool in which a platform is hidden by opaque water. The animal is trained to learn to find the platform through external visual cues placed around the pool. Then, 18 days after the stroke, placing them again in the pool from which the platform had been removed tests the spatial memory of the ischemic mice. A recording of the swimming trajectories shows the ability of the mice to recall the location of the platform during the post-stroke learning phase. When the animal remembers the position of the platform, its search focused on the corresponding quadrant. In this case, GC7-treated animals show a better post-stroke memory capacity than control animals.
To close the loop from a concept originating from a preconditioning-drawn mindset ending in an evident model of preconditioning, the anti-ischemic effect of GC7 preconditioning has also been observed in a preclinical porcine kidney transplantation model (Giraud et al., 2020). Transplantation represents the near-unique clinical situation resulting from ischemia-reperfusion injury that can be anticipated, and thus be appropriated for being protected by preconditioning (Veighey, 2018). In this preconditioning paradigm, GC7 was administrated five minutes after porcine brain death at the beginning of the 4h-donor management, after which kidneys were collected and subjected to 18h cold-storage before being allotransplanted. A 90 day follow-up after allotransplantation revealed a reduced level of creatininemia and fibrosis, and thereby a better outcome of the GC7-preconditioned kidney (Giraud et al., 2020).
The opening of new research axes in linking GC7-preconditioning, mitochondria, and glucose metabolism.
Beyond the success in identifying a new target/drug pair against ischemia, these studies provide new mechanistic insights identifying mitochondria as the ultimate target of the anti-ischemic effects of GC7 and thereby of inhibition of eIF5A hypusination. A recent study suggests that the level of eIF5A activation would direct cellular metabolism towards oxidative phosphorylation or glycolysis and thus may regulate cellular activity (Puleston et al., 2019). This demonstration of a strong link between eIF5A and the mitochondrial respiratory chain is supported by several recent studies showing that GC7 inhibits the Krebs cycle while preserving glycolytic ATP synthesis (Melis et al., 2017; Cougnon et al., 2021). This production of ATP outside the Krebs cycle would allow the cell to meet its essential energy needs when the oxygen supply is reduced, which could in turn be beneficial in hypoxic/ischemic conditions like stroke. Moreover, the mitochondria, through its respiratory chain, is responsible for almost all of the cellular production of reactive oxygen species (ROS), especially those that include an oxygen radical (O2•-, OH•) or from which free radicals (H2O2) are derived (Balaban et al., 2005). These chemical species are highly reactive and contribute to damage to lipids (peroxidation), proteins (oxidation), and DNA in the ischemic brain (Chan, 2016). Their excessive and deregulated production during stroke leads to cellular alterations ranging from loss of function and integrity to cell death. In the murine stroke model, GC7 increases mitochondrial resistance to excitotoxicity (Bourourou et al., 2021) and reduces cytotoxic overproduction of ROS during stroke (Figure 3). In the rat model of renal ischemia, as well as in the porcine kidney transplantation model, GC7-preconditioning appeared to preserve antioxidant defenses and mitochondria homeostasis (Melis et al., 2017; Giraud et al., 2020). Overall, all the effects of GC7 on mitochondria, an organelle acknowledged as an attractive target for the development of innovative pharmacological interventions against several diseases including stroke (Galluzzi et al., 2009; Perez-Pinzon, 2012; Balog et al., 2016; Kalkhoran et al., 2020), contrive to increase resistance to ischemia in different cell types, and several other studies are needed to explore the mechanisms involved.

In a new window | Download PPT
Figure 3: Anti-ischemic effect of GC7 in an in vitro oxygen-glucose deprivation (OGD) model of stroke in neurons. GC7 reduces OGD-induced mitochondrial overproduction of reactive oxygen species (MitoSOX Red, in red), which is toxic to the neurons. Cell nuclei are stained blue. Scale bar = 20 μm.
Conclusion
The first lesson to be learned from this review is perfectly in line with the objective of the journal Conditioning Medicine and is conceptual in nature: it is to highlight the importance of de-compartmentalizing the field and strategies of therapeutic research. It is at the intersection of the different research areas, the different conditioning paradigms, and overall efforts to save other organs from ischemic death, that unexpected biological targets will most likely be found and could offer new perspectives against stroke.
The second lesson is a "proof of concept" that targeting the polyamine-eIF5A-hypusine axis with GC7 may be effective against ischemia-related diseases, and more particular to fight stroke. Not only does this discovery open up a whole new avenue for the search for innovative molecular targets around eIF5A activation, but it is also of obvious interest to the pharmaceutical world. Although this therapeutic target has never been considered in stroke before, it belongs to a pathway that has been the subject of development of pharmacological modulators for other diseases. The perspective of an anti-ischemic effect of these modulators should lead to increasing interest in developing and testing other inhibitors of eIF5A and should rapidly translate to patient care. Considering the well-documented safety and tolerability of the compounds targeting the polyamine biosynthetic pathway (that for some are extensively studied in cancer (Thomas and Thomas, 2001; Park, 2006), demonstrating the potential of targeting this pathway against stroke should be considered as an advantage for future “drug repurposing/repositioning” studies. Thus, our review indicates that inhibitors of this pathway, which are already available to the pharmaceutical industry, should be studied for therapeutic indication in the prevention or treatment of stroke.
Acknowledgements
The authors thank Drs. Heurteaux and Mazella and all their past and present team members and collaborators who have contributed to the data discussed in the review. This work was supported by the Centre National de la Recherche Scientifique – CNRS, the Fondation pour la recherche médicale (FRM, DPM 20121125559), the Agence Nationale de la Recherche (ANR-19-CE18-0029 KIRI) and the French Government for the “Investments for the Future” LabEx ICST #ANR-11 LabEx 0015".
Disclosures/Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
Nicolas Blondeau1
1Université Côte d’Azur, CNRS UMR-7275, IPMC, Sophia Antipolis, F-06560, France.
Michel Tauc2
2Université Côte d’Azur, CNRS UMR-7370, LP2M, Nice, F-06107, France.
Corresponding author:
Nicolas Blondeau
Email: Blondeau@ipmc.cnrs.fr

In a new window | Download PPT
Figure 1: Schema of the urea cycle involved in amino acid catabolism and leading to the synthesis of polyamines by sequential enzymatic catalysis and the activation by hypusination of the translation initiation factor eI5A (modified from Tauc et al., 2019). Pharmacological inhibition of ornithine decarboxylase (ODC), an enzyme in the first step of polyamine biosynthesis, by DFMO (α-difluoromethylornithine) increases Drosophila resistance to hypoxia, and also protects cardiomyoblast or neuronal cell cultures from ischemia. Downstream, GC7 (N1-guanyl-1,7-diaminoheptane) inhibition of the enzyme deoxyhypusine hydroxylase (DOHH), a crucial step in eIF5A activation/hypusination increases Drosophila resistance to hypoxia, as well as protects renal and neuronal cells in several in vitro and in vivo models of hypoxia/ischemia.

In a new window | Download PPT
Figure 2: Inhibition of deoxyhypusine synthase by GC7 reduces brain injury and improves spontaneous functional recovery after ischemic stroke. A. GC7 treatment reduces cerebral infarction (outlined in red) induced by focal ischemic stroke. B. GC7-treated mice show improved functional (cognitive) recovery after stroke. In rodents, the study of cognition is usually limited to an assessment of learning and spatial memory. To assess the effect of post-treatment on cognitive recovery in animals after stroke, the most commonly used test is the Morris water maze test, a circular pool in which a platform is hidden by opaque water. The animal is trained to learn to find the platform through external visual cues placed around the pool. Then, 18 days after the stroke, placing them again in the pool from which the platform had been removed tests the spatial memory of the ischemic mice. A recording of the swimming trajectories shows the ability of the mice to recall the location of the platform during the post-stroke learning phase. When the animal remembers the position of the platform, its search focused on the corresponding quadrant. In this case, GC7-treated animals show a better post-stroke memory capacity than control animals.

In a new window | Download PPT
Figure 3: Anti-ischemic effect of GC7 in an in vitro oxygen-glucose deprivation (OGD) model of stroke in neurons. GC7 reduces OGD-induced mitochondrial overproduction of reactive oxygen species (MitoSOX Red, in red), which is toxic to the neurons. Cell nuclei are stained blue. Scale bar = 20 μm.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6711 | 4 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA