International bi-monthly journal of cell signaling, tissue protection, and translational research.
Remote Ischemic Conditioning for Stroke: Where We Are and Where to Go
Wenbo Zhao1,2,3, Wantong Yu1, Sijie Li3, Changhong Ren2,3, Xunming Ji2,4
Author Affiliations


- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
- 2Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China.
- 3Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China. Author affiliation.
- 4Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Abstract
Stroke is the second leading cause of death and the third leading cause of disability worldwide. Current therapies for acute ischemic stroke remain have large room left to improve; moreover, no effective therapy for intracerebral hemorrhage has been established to date. Neuroprotection has been considered a promising adjunctive therapy for stroke, but few strategies have been applied clinically for patients with stroke. Remote ischemic conditioning, a physical therapy that works through multiple mechanisms, has been widely investigated in acute ischemic stroke and intracerebral hemorrhage with inconsistent results. However, most clinical trials of remote ischemic conditioning for stroke are single-center studies with only a small sample size and limited corroborating evidence, and research in this field is still in its early stages. This review summarizes previous studies on remote ischemic conditioning for stroke, with an emphasis on the results and recommendations for future research.
Keywords: Remote ischemic conditioning; Acute ischemic stroke; Reperfusion therapy; Intracerebral hemorrhage; Neuroprotection
Abstract
Stroke is the second leading cause of death and the third leading cause of disability worldwide. Current therapies for acute ischemic stroke remain have large room left to improve; moreover, no effective therapy for intracerebral hemorrhage has been established to date. Neuroprotection has been considered a promising adjunctive therapy for stroke, but few strategies have been applied clinically for patients with stroke. Remote ischemic conditioning, a physical therapy that works through multiple mechanisms, has been widely investigated in acute ischemic stroke and intracerebral hemorrhage with inconsistent results. However, most clinical trials of remote ischemic conditioning for stroke are single-center studies with only a small sample size and limited corroborating evidence, and research in this field is still in its early stages. This review summarizes previous studies on remote ischemic conditioning for stroke, with an emphasis on the results and recommendations for future research.
Keywords: Remote ischemic conditioning; Acute ischemic stroke; Reperfusion therapy; Intracerebral hemorrhage; Neuroprotection
Introduction
Stroke, including ischemic and hemorrhagic strokes, has become the second leading cause of death and the third leading cause of disability worldwide, and its disease burden continues to increase (Collaborators, 2020). For acute ischemic stroke (AIS), recanalization of the occluded artery by intravenous thrombolysis or endovascular therapy has proven to be the most effective strategy (Powers et al., 2019). However, even with highly successful recanalization rates of over 88%, functional independence at 90 d is typically observed between 50% to 55% with a mortality rate of >10% (Campbell et al., 2016; Goyal et al., 2016; Leng and Xiong, 2019). This counterintuitive mismatch between the rate of recanalization and poor prognosis calls for adjunctive neuroprotection (Zhao et al., 2020b). Intracerebral hemorrhage (ICH) is another main type of stroke, and its incidence is less than that of ischemic stroke, however, ICH is characterized by high morbidity and mortality (van Asch et al., 2010). Many strategies have been investigated for the treatment of ICH, but only a few of these strategies have demonstrated clinical benefits. Therefore, ICH is considered the least treatable type of stroke, and new therapies are warranted (Zhao et al., 2020a).
Remote ischemic conditioning (RIC) is a systemic protective strategy in which one or more cycles of brief focal ischemia followed by reperfusion confers protection against subsequent, more severe ischemia in distant organs. At present, this is generally performed on limbs with blood pressure cuffs inflated to a pressure that blocks limb blood perfusion for several minutes followed by deflation for several minutes (Gidday, 2015; Al Kasab et al., 2016). Compared to conventional pharmacological and surgical treatments, RIC is an easy-to-use, cost-effective, and noninvasive therapy with few adverse events, which makes it promising for clinical investigation and application (Heusch et al., 2015). Although the mechanisms by which RIC protects the brain are not yet fully understood, it was demonstrated to increase cerebral tolerance to ischemic injury, improve the cerebral perfusion status, reduce perihematomal edema, and promote hematoma clearance (Zhao et al., 2019). RIC can be applied in various clinical scenarios that have been widely investigated, with patients with intracranial atherosclerosis, AIS, subarachnoid hemorrhage, ICH, and other types of cerebrovascular diseases. These results suggest broad future prospects.
Compared with clinical investigations of RIC for the heart, the majority of clinical trials of RIC for the brain have only a small sample size with limited corroborating evidence, and only few single-center small-scale studies have demonstrated the effects of RIC on clinical outcomes. Therefore, to add to the limited existing literature in this regard, we have provided a summary of previous studies on RIC for AIS and ICH, and highlighted discussion of the results and the recommendations for future research.
Studies on AIS treated with reperfusion therapy
Upon onset, arterial occlusion initiates the ischemic cascade of AIS. It is now widely recognized that not all ischemic tissue is lost immediately after arterial occlusion, rather the ischemic penumbra surrounding the ischemic core consists of salvageable brain tissue, which rapidly evolves into irreversibly infarcted tissue (Astrup et al., 1981). The therapeutic target of reperfusion therapies is the salvageable brain tissue that presents as a diffusion-perfusion mismatch on imaging; thus, recanalization of the occlusion must be achieved as early as possible to maximized the salvageable penumbra and minimize the ischemic core (Knecht et al., 2018). However, many patients have a large ischemic core before receiving reperfusion therapy, which calls for neuroprotection to prevent ischemic core enlargement and preserve more salvageable brain tissue for reperfusion therapy (Lyden, 2021). Neuroprotective strategies to slow down the enlargement of the ischemic core should be used as early as possible during the prehospital phase and throughout the inter-hospital transfer to maximize their ability to inhibit ischemic core growth (Fisher and Saver, 2015; Savitz et al., 2017). Most of the previous preclinical studies investigating the beneficial associations of remote ischemic perconditioning with slowing the enlargement of the ischemic core were performed in animal models of transient ischemia with complete reperfusion (Ji, 2015). Due to the easy-to-use profile of RIC, it has been investigated in pre-hospital scenarios for the treatment of AIS (Zhao et al., 2019). Remote ischemic perconditioning appears to hold promise in reducing brain infarction growth in patients who have salvageable brain tissue before reperfusion therapy and who could achieve successful recanalization after reperfusion therapy.
A proof-of-concept randomized controlled clinical trial evaluated the effect of prehospital remote ischemic perconditioning as an adjunctive therapy for patients with AIS who were candidates for intravenous thrombolysis (Hougaard et al., 2014). Four-cycles of perconditioning stimulus were performed by the ambulance staff during transportation to the hospital and the procedure was discontinued upon arrival at the stroke unit if it was not completed. The final infarction lesions were measured on 1 month T2 fluid attenuated inversion recovery scans, and the volume of the penumbral salvaged brain tissue was quantified by identifying the tissue voxels in the difference between diffusion-weighted imaging and perfusion imaging at the baseline. The trial randomized 443 patients in the ambulance: 247 were treated with perconditioning, whereas 196 were not. Of the 443 patients, 171 were confirmed to have AIS and were treated with intravenous thrombolysis 91 received perconditioning, and 80 received no treatment. The salvaged tissue in the penumbra, infarct growth over baseline, final infarct size, and clinical outcomes at 3 months were not significantly different between the perconditioning and control groups. The subgroup analysis results showed that the risk of infarction in brain tissue was significantly reduced after perconditioning.
In another proof-of-concept randomized trial, the effects of perconditioning on brain infarction volume growth were evaluated (Pico et al., 2020). A total of 188 patients who were confirmed to have carotid ischemic stroke by magnetic resonance imaging within 6 h of symptom onset were recruited. Of these, 93 were treated with lower-limb remote ischemic perconditioning in addition to standard care, while 95 received standard care alone. The growth in brain infarction volume between the baseline and 24 h was measured by a diffusion-weighted sequence of magnetic resonance imaging scans of the brain. Among all of the recruited patients, 164 (87.2%) received intravenous thrombolysis and 64 (34.0%) were treated with endovascular thrombectomy. The growth in brain infarction volume between the baseline and 24 h was 0.30 mL in the perconditioning group and 0.37 mL in the control group, which were not significantly different. No significant differences in 90-d functional outcomes or mortality were observed.
Based on previous preclinical studies, RIC seems to be a promising strategy for AIS patients, especially for those treated with reperfusion therapy. However, the overall results of these studies investigating RIC in patients treated with intravenous thrombolysis or endovascular therapy were neutral. Although the aforementioned two trials were well designed and conducted, the recruited AIS participants were those treated with intravenous thrombolysis, and only a small number of participants underwent endovascular thrombectomy. For patients treated with intravenous thrombolysis, the rate of recanalization ranges from 20% to 30%, which is much lower in patients with large artery occlusion, tandem occlusion, and cardiac stroke (Fisher and Saver, 2015). Even though remote ischemic perconditioning may be able to prevent salvageable cerebral tissue from evolving into the infarction core, the salvageable cerebral tissue will eventually infarct if the occluded arteries are not recanalized. As endovascular therapy can recanalize the occluded artery at a much higher rate, which ranges from 60% to 90%, AIS patients receiving endovascular therapy appear to be the optimal candidates in which to investigate the neuroprotective effects of remote ischemic perconditioning. In a study by Pico et al (2020), although approximately 90% of their participants received intravenous thrombolysis and over 30% received endovascular thrombectomy, only 40% actually achieved successful recanalization at 24 h and 27% had a diffusion-perfusion mismatch before reperfusion therapies. Among the participants who did not achieve successful recanalization and who had fully infarcted tissue before reperfusion therapy, remote ischemic perconditioning may have been ineffective in preventing infarction growth.
Currently, the optimal investigational candidates for remote ischemic perconditioning for AIS might be those who have a diffusion-perfusion mismatch upon imaging before reperfusion therapy and who subsequently receive endovascular therapy. The safety and feasibility of RIC in patients with AIS who were treated with endovascular thrombectomy was investigated. A pilot study was conducted with AIS patients suspected of having a large-vessel occlusion in the anterior circulation and who were scheduled for reperfusion therapy within 6 h of ictus. Remote ischemic perconditioning was performed before reperfusion therapy, and postconditioning was performed immediately following recanalization and once daily for seven subsequent days (Zhao et al., 2018). Results showed that RIC was well-tolerated and did not have adverse effects on intracranial pressure, cranial perfusion pressure, and cerebral hemodynamics. The greatest contribution of this pilot study may be that it demonstrated that RIC is safe and feasible for patients with AIS secondary to large-vessel occlusion and who are generally gravely ill and treated with endovascular thrombectomy. Further studies are warranted to investigate whether RIC could benefit this patient population.
We searched for ongoing studies on August 13, 2021, at ClinicalTrials.gov and found several ongoing clinical trials investigating RIC in patients treated with reperfusion therapy; these are summarized in Table 1. The largest trial was the REmote Ischemic conditioning In patients with acute STroke (RESIST) trial, which recruited patients with a suspected stroke identified prior to their hospital visit within 4 h of symptom onset. For that trial, RIC was applied in the prehospital setting and continued in-hospital for 7 days. Patients with AIS and ICH were both enrolled and analyzed separately. In addition, four trials investigated RIC in patients with AIS who were treated with endovascular thrombectomy, and two of these trials evaluated infarction volume, whereas one trial evaluated the clinical outcomes. These ongoing trials, especially those investigating patients undergoing thrombectomy, will determine the protective effects of perconditioning and postconditioning.
Table 1: Representative Clinical Trials of Remote Ischemic Condoning for Stroke in ClinicalTrials.gov
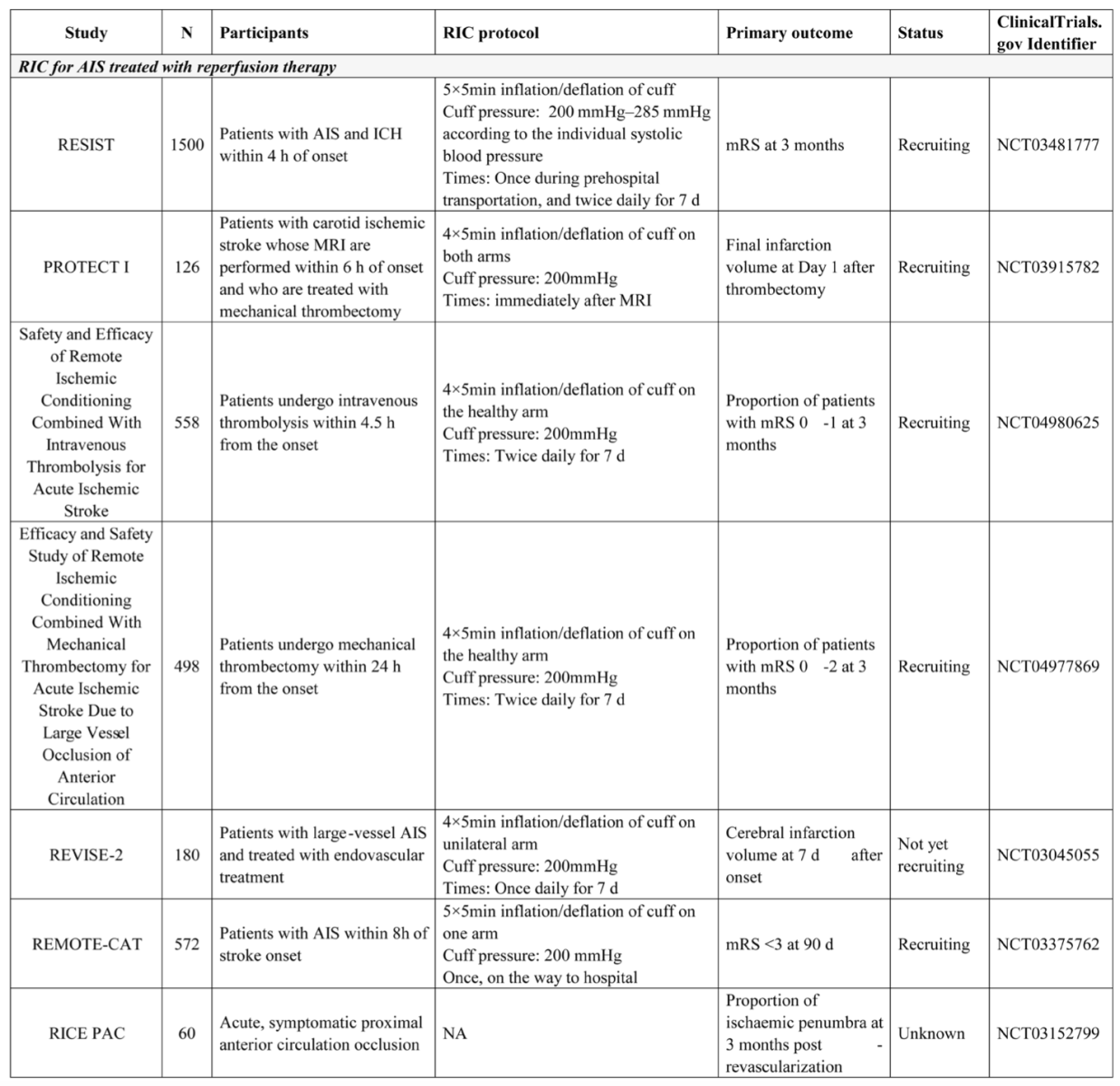
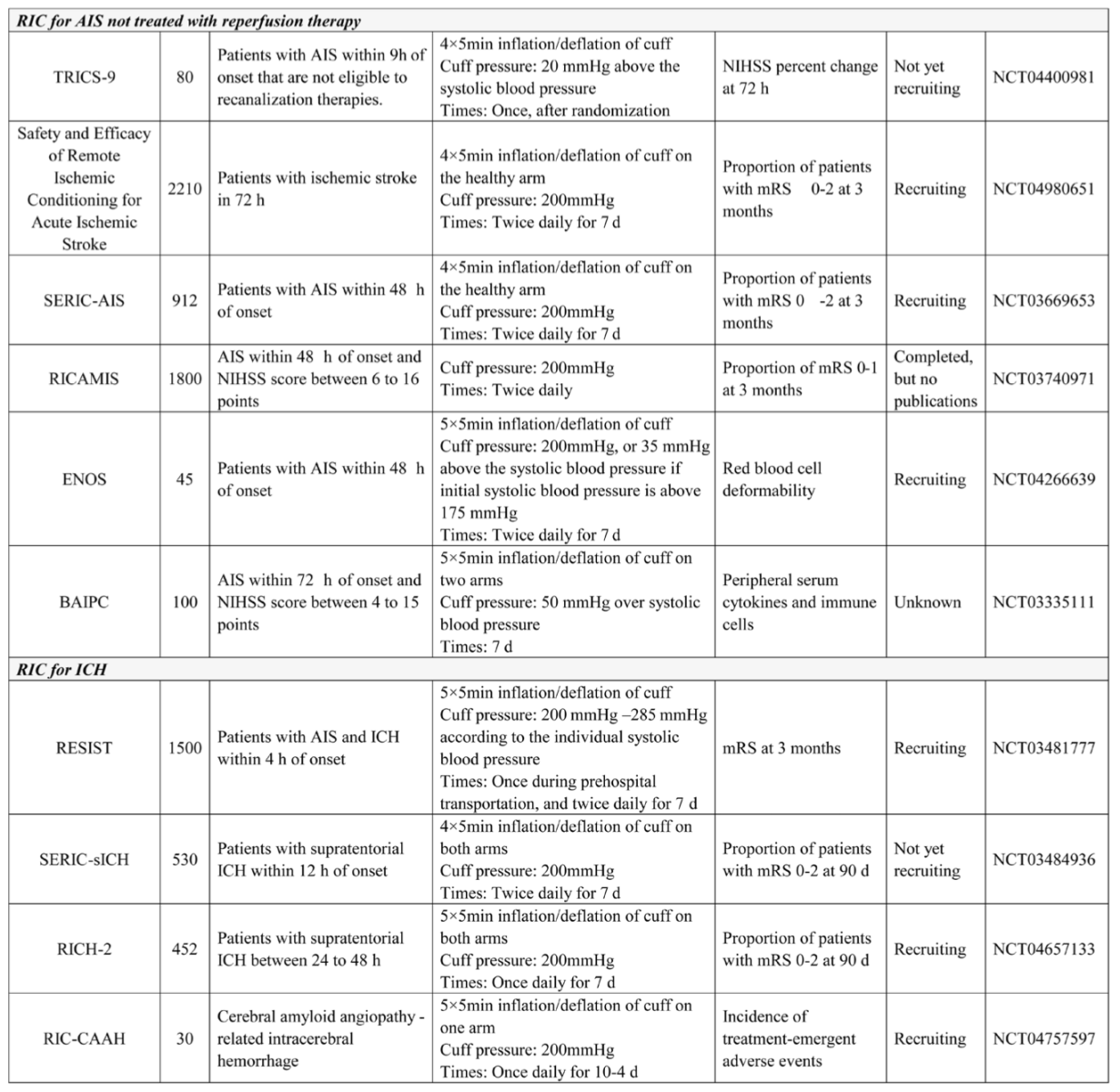
Studies of AIS not treated with reperfusion therapy
In addition to studies investigating RIC in AIS patients treated with reperfusion therapy, several studies also investigated RIC in patients not receiving intravenous thrombolysis or endovascular thrombectomy, which is the largest portion of the AIS population. In a pilot blinded placebo-controlled trial (REmote ischemic Conditioning After STroke trial [RECAST-1]) in patients with AIS within 24 h of ictus that did not receive intravenous thrombolysis, 26 were recruited and allocated to receive four cycles of RIC or sham RIC in the nonparetic arm (England et al., 2017). Results showed that RIC was safe and feasible in this patient population, that no severe adverse events were attributed to RIC treatment, and that RIC might improve the 90 day National Institutes of Health Stroke Scale scores. Based on this study, a phase IIb randomized controlled trial investigating RIC in hyperacute stroke (RECAST-2) was conducted to further investigate the feasibility, safety, and tolerability of RIC for patients with AIS within 6 h of ictus. A total of 60 patients were randomized to receive RIC or sham RIC in three blocks of increasing doses: participants 1-20 received four cycles of cuff inflation and deflation of RIC/sham, participants 21-40 received a second dose 1 h after the first dose, and participants 41-60 also received twice daily dosing starting the following morning up to and including day 4 (total of eight doses) (England et al., 2019). Results showed that RIC was well-tolerated, with adherence not differing between RIC and sham RIC, and no significant between-group differences in terms of serious adverse events, deaths, or modified Rankin Scale scores were detected. In another study, patients with AIS within 72 h were recruited if they did not receive intravenous thrombolysis, and RIC was performed from the time of enrollment to day 14 (Li et al., 2018). In total, 60 patients were recruited, and the results showed that functional outcomes and cerebral blood perfusion evaluated by perfusion weighted imaging were significantly improved by RIC.
In patients with AIS who did not receive reperfusion therapy, several small pilot trials discovered the potential benefits of RIC, but much larger studies are needed to confirm these results. Clinical trials that included patients with ischemic cerebrovascular events secondary to intracranial artery stenosis also demonstrated that repeated RIC for several months could promote the recovery of neurological function, improve cerebral perfusion evaluated by single photon emission computed tomography, and reduce the recurrence of stroke or transient ischemic attack (Meng et al., 2012; Meng et al., 2015). Currently, there are several trials investigating RIC in patients not treated with reperfusion therapy, as summarized in table 1. In this patient population with AIS, compared with RIC once or RIC for several days, repeated RIC for several months appears to be more effective and should be investigated in the future.
Studies for ICH
For ICH, hematoma exuding from vessels not only causes direct compressive injury to the cerebral parenchyma and disrupts the cellular architecture (Keep et al., 2012), but it also produces a mass effect that increases intracranial pressure and reduces cerebral perfusion, which then causes ischemic injury (Xu et al., 1993). In addition, further injuries can be caused by physiological responses to the hematoma and by the direct cellular toxicity arising from both the deposited hematoma and its subsequent degradation byproducts (Keep et al., 2012; Urday et al., 2015). RIC was found to have neuroprotective effects through multiple pathways, and it has also been investigated in ICH. Given the protection of RIC for AIS, its protective effects were initially investigated in an ICH model. In a previous study, ICH was induced by collagenase, and RIC was performed 1 h after ICH development; the results showed that although there were no significant differences in cerebral blood volume, brain water content, Evans blue extravasations, aquaporin-4 and matrix metallopeptidase-9 expressions, and 12-point neurological deficit score between the RIC and control groups, RIC did not exacerbate hematoma volume (Geng et al., 2012). Another study further investigated RIC in the ICH model. RIC was initiated 2 h after ICH and continued daily thereafter, and the results showed that RIC reduced the hematoma volume by 43% and improved cerebral blood flow by 24% compared with that noted in the control group on day 5 after ICH (Vaibhav et al., 2018).
To translate these preclinical data, the Remote Ischemic Conditioning for Intracerebral Hemorrhage (RICH-1) pilot clinical trial was conducted to evaluate the safety and preliminary efficacy of RIC in patients with ICH (Zhao et al., 2020c; Zhao et al., 2021). A total of 40 patients with supratentorial ICH presenting within 24-48 h of symptom onset were randomly assigned to receive RIC for seven consecutive days or medical therapy alone. The results showed that after seven days of treatment, the hematoma resolution rate in the RIC group was significantly higher than that in the control group (49.25 ± 9.17% vs. 41.92 ± 9.14%) and that relative perihematomal edema was significantly reduced by the RIC. There was no significant between-group difference in the proportion of patients who achieved favorable functional outcomes at the 90-d follow-up. As the first clinical trial of RIC for ICH, the RICH-1 study demonstrated the safety and feasibility of RIC in patients with ICH, and showed that RIC may also promote hematoma resolution and reduce perihematomal edema. Therefore, further studies are needed to confirm these results and larger studies that investigate the effects of RIC on clinical outcomes are urgently needed.
There are currently four ongoing trials investigating RIC for ICH. Interestingly, in contrast to previous studies of RIC for AIS, all four studies applied RIC for at least seven days. The RICH-2 study is a continuation of the RICH-1 study, which hypothesized that RIC could promote hematoma resolution and attenuate perihematomal edema, thus producing clinical benefits in patients with ICH. Patients within 24-48 h of symptom onset will be recruited, and those with a low risk of hematoma extension might be a better option as subjects to investigate the effects of RIC on hematoma clearance and cerebral edema. A pilot study specifically investigating RIC in patients with cerebral amyloid angiopathy-related ICH may provide insights in to previously unknown mechanisms of the protection of RIC for ICH.
In addition, hematoma volume is an independent determinant of the prognosis of ICH, but clinical trials have found that invasive and minimally invasive evacuation approaches could significantly reduce the hematoma volume effectively and that clinical outcomes were not significantly improved (Mendelow et al., 2005; Mendelow et al., 2013; Hanley et al., 2019). The secondary injury caused by the residual hematoma may continue to develop to the detriment of clinical outcomes; therefore, adjunctive neuroprotection followed by the minimally invasive evacuation of the hematoma might be effective in improving the therapeutic effects of hematoma evacuation, namely “hematoma evacuation-based neuroprotection” for ICH (Zhao et al., 2020a). Consequently, proof-of-concept clinical studies are urgently needed to determine the effects of RIC in patients with ICH who were treated with hematoma evacuation.
Lessons learned from cardiovascular diseases
Compared to studies on stroke, more large clinical trials on RIC for cardiovascular diseases (mainly patients undergoing coronary artery bypass graft and emergent primary percutaneous coronary intervention) have been completed, but all of them failed to demonstrate the clinical benefits of RIC. This may provide references for future trials on RIC for stroke. Trials that investigate RIC in patients undergoing coronary artery bypass grafting found that RIC during the operation did not significantly reduce the major adverse cardiovascular and cerebrovascular events at the time of hospital discharge or 12 months after randomization (Hausenloy et al., 2015; Meybohm et al., 2015). In addition, trials that investigated RIC in patients with ST-elevation myocardial infarction who were undergoing primary percutaneous coronary intervention found that RIC did not reduce cardiac death or hospitalization for heart failure at 12 months (Hausenloy et al., 2019). Although the precursor studies of these large trials have demonstrated the protective effects of RIC in reducing serum biomarkers of myocardial injury or infarction volume (Hausenloy et al., 2007; Botker et al., 2010; Candilio et al., 2015), these effects did not translate to clinical events. Many factors may contribute to the neutral results of these large trials, but the RIC protocol might be an important one.
The protection of RIC lasts for approximately 96 h, with an interval of 12–24 h. Therefore, protection induced by one RIC session may last for 2 or 3 days (Yellon and Downey, 2003), which may not provide consistent protection to patients or positively affect the clinical events. Conversely, repeated RIC for weeks to months may benefit patients by affecting clinical events, which has been proven by small trials of RIC in patients with cerebrovascular disease (Meng et al., 2012; Meng et al., 2015; Wang et al., 2017; Zhao et al., 2017), which indicates that chronic RIC is a choice in further studies. Studies of RIC in patients with AIS treated with endovascular thrombectomy are similar to those of RIC in acute myocardial infarction treated with percutaneous coronary intervention. Given the lessons from cardiovascular disease, investigating the protective effects of RIC once in this patient population may not be optimal. Therefore, when investigating RIC in patients with AIS treated with endovascular thrombectomy, RIC should be repeatedly used for several days, weeks, or even several months to ensure its clinical benefits.
Summary
RIC has been investigated in patients with AIS and ICH, but most studies involve a single center and enrolled a small number of patients, indicating the lack of multiple center randomized control trials with a large number of participants that evaluated its effects on clinical outcomes. Many specific studies are needed to determine the effects of perconditioning or postconditioning in patients with AIS treated with endovascular reperfusion, and repeated RIC for several weeks or months might be a better choice as the recovery of neurological function requires a period of several months. Compared with investigations of RIC in AIS, the mechanisms and effects of RIC for ICH are still unknown, and more research is urgently needed. However, based on the mechanisms of injuries after ICH and its overlap with that of AIS, RIC appears to be promising therapy for ICH. Therefore, research on RIC for stroke is still in its early stages, and more work is needed.
Conflicts of Interests
None
Acknowledgements
This work was supported by Beijing Nova Program (No. Z201100006820143), General Project of Science and Technology from Beijing Municipal Education Commission (No. KM202110025018), the National Natural Science Foundation of China (No. 82001257), and grant 2019A36 from Beijing Hundred Thousand and Ten Thousand Talent Project.
References
Wenbo Zhao1,2,3
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China. 2Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China. 3Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China.
Wantong Yu1
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Sijie Li3
3Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China.
Changhong Ren2,3
2Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China. 3Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China.
Xunming Ji2,4
2Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China. 4Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Corresponding author:
Xunming Ji MD, PhD
Email: jixm@ccmu.edu.cn
Table 1: Representative Clinical Trials of Remote Ischemic Condoning for Stroke in ClinicalTrials.gov


Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6112 | 16 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA