Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Left ventricular thrombus – management and review of evidence: Vitamin K Antagonists or Direct Oral Anticoagulants?
Time:2021-11-15
Number:8120
Debbie Falconer1, Ali Kirresh1, Luciano Candilio1
Author Affiliations


- 1Cardiology Department, CardioVascular Division, Royal Free Hospital, Pond Street, London, NW3 2QG, UK.
Conditioning Medicine 2021. 4(4): 161-167.
Abstract
Left ventricular thrombus (LVT) is most commonly detected in patients with a severely reduced left ventricular ejection fraction, particularly in the context of ischemic cardiomyopathy secondary to acute myocardial infarction. Stroke and acute limb ischemia are well known complications arising from systemic embolization of LVT, and are both associated with significant morbidity and mortality. For this reason, oral anticoagulation should be started promptly after diagnosis. Vitamin K antagonists (VKAs) have traditionally been the first-line anticoagulants of choice, however, direct oral anticoagulants (DOACs) are increasingly being used off-label. Case series and observational studies have been published demonstrating that DOACs are safe and effective in treating LVT, but no randomized controlled trial directly comparing the safety and efficacy of DOACs versus VKAs has so far been conducted. Additionally, the optimal duration of treatment and follow up of these patients remains unclear. We review current evidence on diagnosis, management, and follow-up of patients with LVT, comparing data on DOACs versus VKAs.
Keywords: Left ventricular thrombus, Vitamin K antagonist, Direct oral anticoagulant
Abstract
Left ventricular thrombus (LVT) is most commonly detected in patients with a severely reduced left ventricular ejection fraction, particularly in the context of ischemic cardiomyopathy secondary to acute myocardial infarction. Stroke and acute limb ischemia are well known complications arising from systemic embolization of LVT, and are both associated with significant morbidity and mortality. For this reason, oral anticoagulation should be started promptly after diagnosis. Vitamin K antagonists (VKAs) have traditionally been the first-line anticoagulants of choice, however, direct oral anticoagulants (DOACs) are increasingly being used off-label. Case series and observational studies have been published demonstrating that DOACs are safe and effective in treating LVT, but no randomized controlled trial directly comparing the safety and efficacy of DOACs versus VKAs has so far been conducted. Additionally, the optimal duration of treatment and follow up of these patients remains unclear. We review current evidence on diagnosis, management, and follow-up of patients with LVT, comparing data on DOACs versus VKAs.
Keywords: Left ventricular thrombus, Vitamin K antagonist, Direct oral anticoagulant
Introduction
Left ventricular thrombus (LVT) is a well recognized complication of both ischemic and non-ischemic cardiomyopathies. The vast majority of LVT occur following an acute myocardial infarction (AMI) involving the left anterior descending (LAD) coronary artery or in the context of congestive cardiac failure with a severely impaired left ventricular ejection fraction (LVEF, Table1) (Talle et al., 2014). Virchow’s triad of blood stasis, endothelial injury, and hypercoagulability refers to the conditions required for in vivo clot formation. Following an AMI, the left ventricle (LV) becomes an ideal milieu for clot formation with regional wall akinesia or dyskinesia leading to blood stagnation, and prolonged ischemia resulting in endocardial injury and pro-inflammatory, hypercoagulable changes (Delewi et al., 2012). Independent risk factors for LVT formation are anterior localization of AMI, larger infarct size, and reduced LVEF.
Table 1: Incidence of left ventricular thrombus by aetiology (Adapted from Talle et al., 2014)
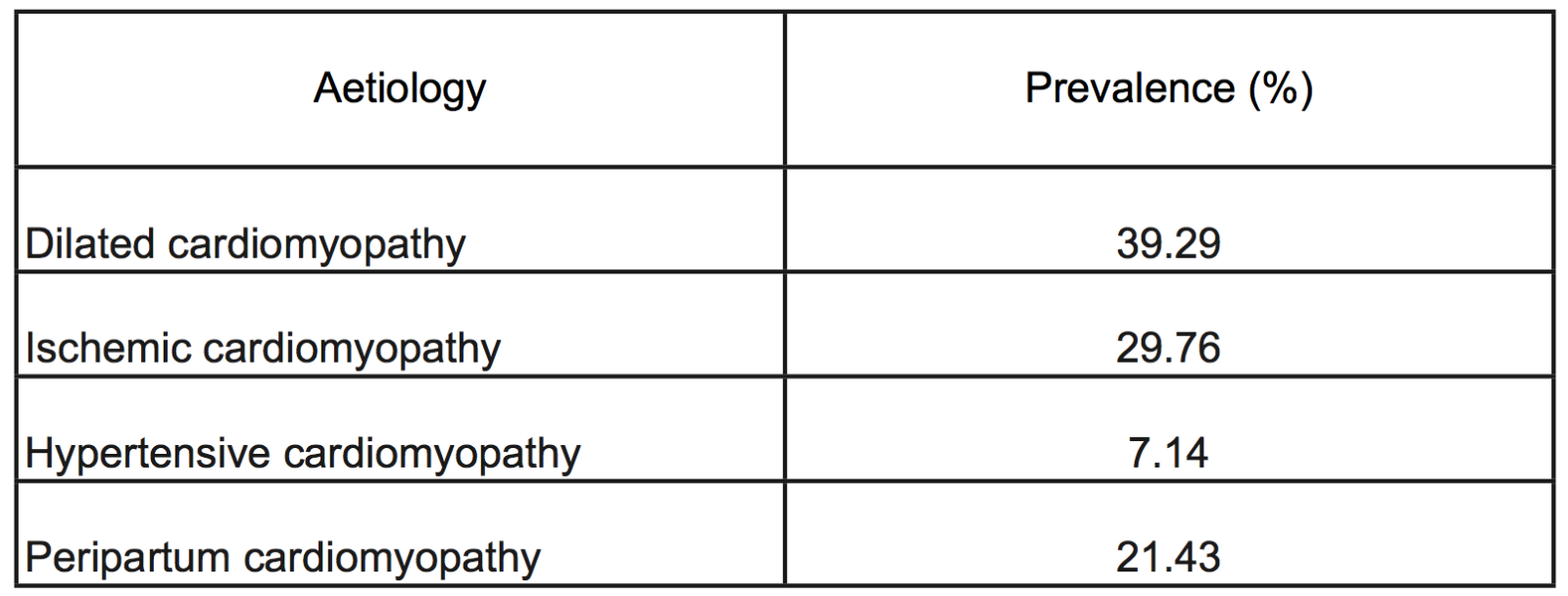
The advent of primary percutaneous coronary intervention (PPCI) and improved post-AMI care has led to a reduction in the incidence of LVT. Estimates of LVT incidence from the pre-PPCI era range between 17% and 21% of all AMI patients, and of up to 46% of those presenting with an anterior AMI (Asinger et al., 1981; Visser et al., 1983). However, more recent estimates from the PPCI-era are much lower, ranging between 4% and 12% of all AMI patients (Phan et al., 2019; Jones et al., 2020). This is postulated to be due to increased use of anticoagulants in the initial treatment phase, swift reperfusion resulting in smaller infarct sizes, and improved LV remodeling with evidence-based pharmacotherapy post-infarct (Habash and Vallurupalli, 2017). However, despite a reduction in incidence due to modern-day reperfusion strategies, LVT remains an important complication of AMI, with significant consequences. Untreated, LVT can result in acute embolic complications including stroke and acute limb ischemia, both of which are associated with significant morbidity and mortality (Stratton and Resnick, 1987; Clason et al., 1989). Following the diagnosis of LVT, anticoagulation should be started promptly to reduce the risk of such sequelae (Vaitkus and Barnathan, 1993). Management of LVT has been historically based on initial combination of unfractionated heparin/low molecular weight heparin with vitamin K antagonists (VKAs), with often discordant consensus on the optimal duration of subsequent VKAs monotherapy. However, following the introduction of direct oral anticoagulants (DOACs), new albeit controversial evidence has risen on their use in the context of LVT as a more “pragmatic” alternative for VKAs, despite the lack of consensus on the optimal drug-choice, duration of treatment, and timing of follow-up. We present a review of current evidence on the diagnosis and management of LVT, with a specific focus on the conflicting data surrounding the efficacy and safety of DOACs as an alternative to VKAs.
Diagnosis and follow-up imaging
The majority of LVT are diagnosed on transthoracic echocardiography (TTE), which is low cost and readily available at the bedside (Delewi et al., 2012). However, one recognized disadvantage of TTE is the poor visualisation of the LV apex, which is the most common site of LVT formation due to akinesia/dyskinesia subsequent to an AMI in the LAD artery territory. This may lead to under-detection of LVT on TTE, with potentially serious clinical consequences (Thanigaraj et al., 1999). In order to overcome this limitation, ultrasound contrast agents or alternative imaging modalities such as cine-cardiac magnetic resonance (CMR) have been used. Contrast agents are composed of injected intravenously microbubbles smaller than red blood cells, and are established adjunctive tools to increase LV opacification and endocardial border definition, and thereby LVT detection too, with a demonstrated near doubling of LVT detection from 33% to 61% and increased specificity from 94% to 99% (Weinsaft et al., 2009). Contrast enhanced CMR (CE-CMR) is the gold standard diagnostic tool for LVT diagnosis with the highest sensitivity and specificity in a systematic review of 7 studies comparing CE-CMR, non-CE CMR, TTE, and contrast-enhanced TTE (Table 2) (Roifman et al., 2015). Therefore, for patients with a high suspicion of LVT but a negative TTE, alternative imaging in the form of contrast TTE or CE-CMR should be sought to further investigate the presence of an LVT.
Table 2: Sensitivity and specificity of each modality in detecting
left ventricular thrombi (Adapted from Roifman et al., 2015). Abbreviations; CMR, cardiac magnetic resonance;LE-CMR, late gadolinium-enhanced cardiac magnetic resonance; TTE, transthoracic echocardiography.

LVT can also be seen on computed tomography (CT) as areas of low attenuation (Pagan et al., 2015). It has historically been considered that CT has a similar sensitivity and specificity to TTE in detecting LVT (Delewi et al., 2012), but Bittencourt et al. (2012) found a threshold of 65 Hounsfield units yielded a sensitivity of 94% and specificity of 97% in differentiating the LVT from the myocardial wall. However, this is rarely selected as the diagnostic modality of choice owing to the required exposure to ionizing radiation and use of iodine-based contrast. LVT are therefore generally detected on CT when the scan is being performed for an alternative reason.
The timing of imaging following AMI is also important. Data from CMR sub-studies of two ST-segment elevation myocardial infarction (STEMI) cohorts (537 patients) were retrospectively analyzed, comparing detection rates when imaging was performed “early” (< 5 days from STEMI) or “late” (> 5 days from STEMI). A total of 34 LVTs were detected in 265 patients admitted with anterior STEMI, severely impaired LVEF (≥ 10% of LV necrosis), and undergoing valid CMR within 34 days from admission. Of these, LVT was found in 13 of 160 (8.1%) patients within five days of AMI compared with 21 of 105 (20%) patients more than 5 days after admission (p = 0.0047) (Gellen et al., 2017). The cohort was further subdivided into five groups depending on “AMI to CMR time.” The highest LVT detection rate was found in patients undergoing CMR 9-12 days following STEMI (25%) compared with only 3% when the CMR was performed 0-2 days (p = 0.017) (Gellen et al., 2017). Current European Society of Cardiology (ESC) guidelines on STEMI management recommend that low risk patients with complete revascularization can be discharged safely by day three post-percutaneous coronary intervention (PCI) (Ibanez et al., 2018). Therefore, although delaying imaging is not practical in many clinical settings, clinicians should be aware of the continued risk of LVT formation following discharge and consider follow up imaging in those at higher risk, although significant inconsistency exists between studies as to which imaging modality can be used, with some patients followed up with TTE and others with CMR, thereby leaving LVT potentially undetected in patients when TTE is used (Siddiqui et al., 2018).
Management of LVT
The 2012 ESC guidelines on management of STEMI recommend that patients diagnosed with LVT should be anticoagulated with a VKA for up to six months (Steg et al., 2012). The updated guidelines from 2017 are less specific, recommending oral anticoagulation for up to six months, guided by repeated imaging, while acknowledging that there is a lack of randomized data on the optimal regimen and duration of treatment (Ibanez et al., 2018). In clinical practice, warfarin generally remains the first-line treatment for LVT, but DOACs are increasingly being used off-label for this indication. Advantages of DOACs over warfarin are well recognized, including less monitoring and minimal interaction with other foods and drugs through the cytochrome p450 enzymes (Bauer, 2013). The minimal monitoring required is particularly pertinent during the ongoing coronavirus 2019 pandemic, when travel and hospital visits can be a source of significant anxiety for this already vulnerable group of patients (Ibrahim et al., 2020; Rees et al., 2020).
DOACs have proven non-inferior to VKAs for stroke prevention in non-valvular atrial fibrillation (AF) and in the management of venous thromboembolism (Granger et al., 2011; Patel et al., 2011; Fiorelli et al., 2018). A number of case reports and series have subsequently highlighted the successful and safe use of DOACs for the treatment of LVT (Abdelnaby et al., 2019; Tomasoni et al., 2020). More recently, the publication of several larger observational studies comparing DOACs and VKAs (Table 3) have provided the strongest indication that DOACs may be a safe and efficacious alternative to VKAs. Rate of LVT resolution on imaging, incidence of stroke or systemic embolism (SSE), and major bleeding events were the most commonly compared outcomes between cohorts.
Jones et al. (2020) analyzed data on 2,328 patients admitted with AMI to a tertiary center in the United Kingdom, diagnosing LVT in 101 (4.6%) patients. Sixty patients were prescribed warfarin, while 41 received a DOAC. They found a higher rate of thrombus resolution on TTE in the DOAC group compared to the warfarin group (82% vs. 64.4%, p = 0.0018), as well as a lower rate of major bleeding events in the DOAC-treated group (0% vs. 6.7%, p = 0.03) with no significant difference in rates of stroke after a median follow up of 2.2 years. In addition to this, LVT resolution was seen sooner in the DOAC group compared to the VKA cohort with a median time to first imaging of 151 and 175 days, respectively. At repeat imaging, 29 patients (70.7%) in the DOAC group and 29 patients (48.3%) in the VKA group had resolution of LVT (p = 0.04). Importantly, the presence of AF and prior history of thromboembolic events were not reported in the baseline characteristics for this study. As both are important risk factors for future strokes, a difference in baseline prevalence might have confounded the results when assessing rates of SSE. Also, this group of patients underwent imaging 12-24 hours after admission with AMI, thereby potentially leaving a proportion of LVTs undetected.
There was no significant difference in rates of LVT resolution between VKA and DOAC-treated groups in six other studies (Bass et al., 2019; Ali et al., 2020; Cochran et al., 2020; Daher et al., 2020; Guddeti et al., 2020; Iqbal et al., 2020). Iqbal et al. (2020) followed-up 84 patients for a median of three years and found no difference in rates of LVT resolution (76% vs. 65%, p = 0.33), all-cause re-hospitalization (50% vs. 45%, p=0.523), or all-cause mortality (10% vs. 14%, p = 0.61) in the two groups. The authors admitted the lack of a standardized approach in the timing or modality of follow-up, as repeat imaging was performed at the discretion of the treating cardiologist. The detection rate of LVT, and therefore the rate of resolution on imaging, may therefore have been influenced by differences in the follow-up protocol. Clearly, any future randomized controlled trial (RCT) on this topic would require a standardized follow-up protocol for both groups in order to minimize the likelihood of such detection bias.
Daher et al. (2020) demonstrated no significant difference in LVT resolution on TTE between patients receiving DOAC or VKA following a minimum of three months of anticoagulation (70.6% vs. 71.4%, p = 0.9). Furthermore, when DOACs failed to resolve LVT on imaging (5 patients), warfarin was used as a second line alternative with international normalized ratio (INR) target of 3-4, with complete LVT resolution on subsequent imaging. In clinical practice, the ability to increase INR targets as per response to treatment is a unique advantage of warfarin over DOACs for patients who do not respond to standard measures. Therefore, if DOACs are adopted as a first-line treatment for LVT, warfarin will likely remain as the treatment of choice for LVT, which do not resolve after 3-6 months.
A number of studies have also reported on the rates of SSEs in both DOAC and VKA treated groups (Table 3). Bass et al. (2019) followed up a cohort of 949 patients, of whom 769 (81%) were prescribed warfarin, while 180 (19%) received a DOAC. There was no difference in the rate of thromboembolic events between the DOAC and the VKA groups (7.8% vs. 11.7%, p = 0.524). It should be noted that this cohort was followed up for only 90 days after diagnosis, thus only half of the currently recommended treatment duration of six months. This implies that LVTs may not have resolved after 90 days and therefore further SSEs may have occurred outside of this window, which would be unaccounted for in this study. Additionally, there were also significant differences in the baseline characteristics between the two cohorts, with a specific regard to a higher serum creatinine in the VKA group (1.0mg/dL vs. 1.33mg/dL, p < 0.0001). DOACs should be used with caution in patients with an estimated glomerular filtration rate (eGFR) of 15-29 ml/min, and avoided in those with an eGFR less than 15 ml/min. Therefore, patients with renal dysfunction are more likely to be prescribed VKAs. Additionally, patients with chronic kidney disease are at increased risk of both ischemic stroke (Nayak-Rao and Shenoy, 2017) and clinically significant bleeding (Ocak et al., 2018) compared to those with normal renal function, and therefore results should always be interpreted with caution when comparing groups with a statistically significant difference in baseline renal function.
Table 3: Summary of studies comparing outcomes of warfarin and DOACs. (Abbreviations; AMI: acute myocardial infarction; CCF: congestive cardiac failure; DOAC: direct oral anticoagulant; Echo: echocardiography; LVT: left ventricular thrombus; N; number of patients in study; SSE: stroke or systemic embolism; VKA: vitamin K antagonist (warfarin unless otherwise specified))
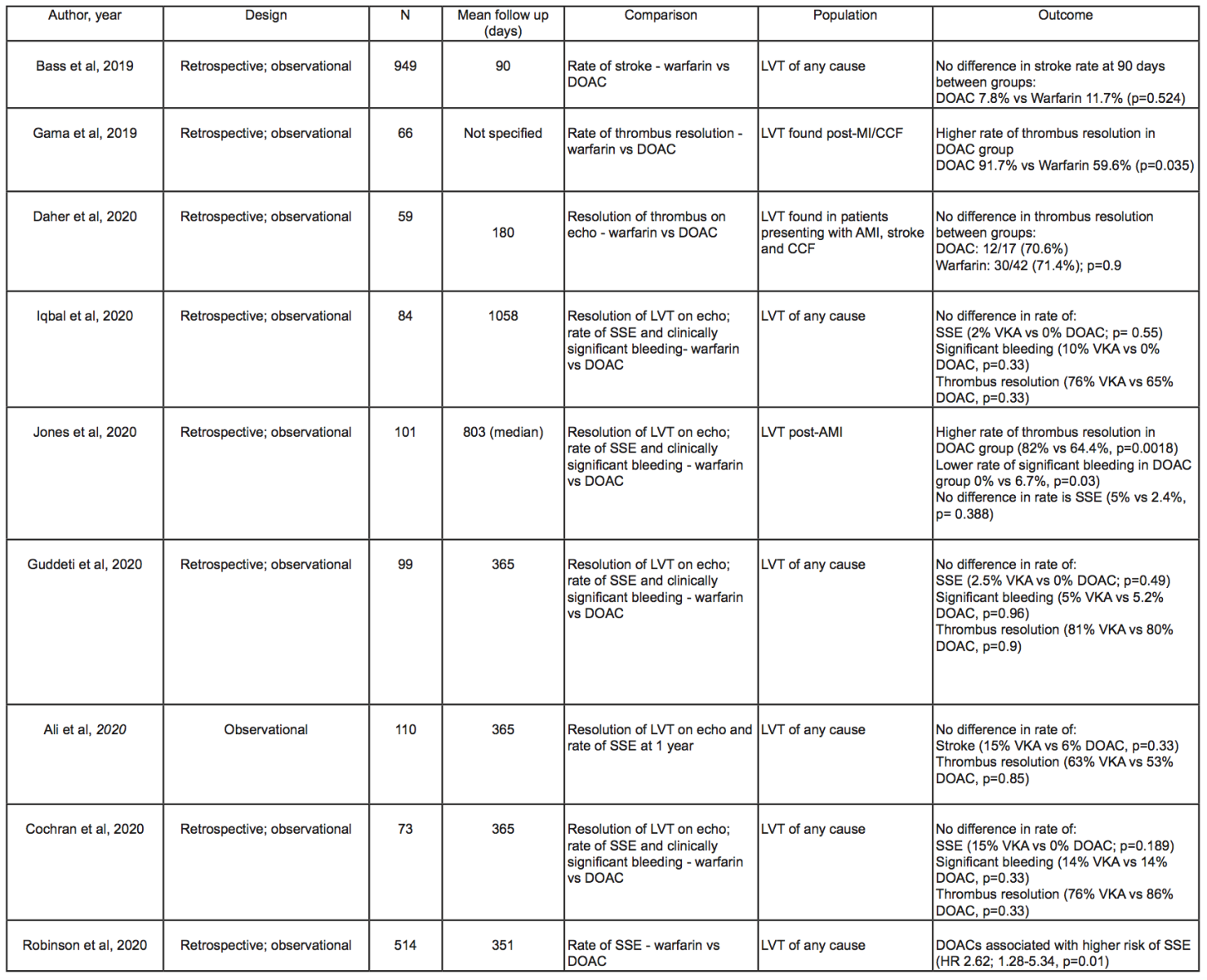
While most studies found no significant difference in the incidence of stroke between the DOAC and the VKA-treated groups (Bass et al., 2019; Ali et al., 2020; Cochran et al., 2020; Guddeti et al., 2020; Iqbal et al., 2020), one important multi-center study of 514 patients found a significantly higher incidence of SSEs in the DOAC group compared with VKA-treated patients after a mean follow up of 351 days (Robinson et al., 2020); out of the 54 SSE events detected, 17 occurred in the DOAC group and 14 in the warfarin group, with univariable Cox proportional hazards regression demonstrating a significant association between SSE and treatment with DOAC and prior SSE (respectively Hazard ratio [HR], 2.71; 95% confidence interval [CI], 1.31-5.57; p = 0.01, and HR, 2.13; 95% CI, 1.22-3.72; p = 0.01). This distinction persisted after a multi-variate analysis, which corrected for the differences in significant baseline characteristics, including AF and prior stroke (respectively HR, 2.64; 95% CI, 1.28-5.43; p = 0.01, and HR, 2.07; 95% CI, 1.17-3.66; p = 0.01). The authors proposed that the larger sample size, the multi-center design of the study, and the longer follow-up period were implicated as possible reasons for the significant differences with previous studies. Another interesting point arising from these data is the relatively late separation of event curves, with a landmark analysis demonstrating an association between DOAC and SSE in the interval from three months to the end of follow-up (HR, 2.88; 95% CI, 1.22-6.80; p = 0.2). The authors propose that some late embolic events are associated with phenomena outside the LV, including vascular atheroembolism or calcifications, leaflet thrombosis, or septic vegetations, which may be differentially treated with DOACs, and that LVT could in itself represent a marker of longer-term and persisting thrombotic risk despite anticoagulation therapy and LVT resolution.
There are several limitations to the studies to date. Most notably, all have been observational and therefore, despite efforts to correct for confounding factors, there are likely to be residual confounding variables that are unaccounted for. Furthermore, the majority of these studies are small (less than 100 patients over both groups) and single center, often with significant differences in critical baseline characteristics between groups. Additionally, as current practice still involves the use of VKA as first line treatment, the majority of patients in each study received warfarin, meaning the DOAC treated groups are smaller. For example, within the cohort presented by Guddeti et al. (2020), there were as few as 19 patients in the DOAC-treated group, and therefore it is difficult to draw wider conclusions. Furthermore, when assessing LVT resolution, most studies relied on TTEs reports to confirm the presence of LVT, which given the aforementioned limitations and in particular the poor sensitivity of non-contrast TTE and subsequent inconclusive reports, might have likely led to LVT under-detection in these studies. Additionally, none of the above analyses involved the use of CMR, the gold standard imaging modality for LVT diagnosis, thus firm conclusions on thrombus resolution might have been suboptimal (Jones et al., 2020). Lastly, some of the studies focus on patients presenting with LVT following an ischemic event, and therefore the results may not be generalizable to patients with other etiologies.
Thereby, although the initial results of these studies trend towards DOACs as a safe and effective VKA alternative in appropriately selected patients, data should be interpreted with caution and the conflicting outcomes among studies highlight the need for further investigation with larger groups and longer follow-ups to definitely recommend the routine use of DOACs in the management of LVT.
Bleeding risk: DOACs vs. VKAs
Although studies comparing VKAs and DOACs for the management of LVT are relatively small, larger trials have compared the safety and bleeding profiles of DOACs and warfarin in stroke prevention in AF (Vinogradova et al., 2018). A study of 196,061 patients in primary care in the United Kingdom compared the risk of significant bleeding (requiring hospitalization or resulting in death) in patients prescribed warfarin, dabigatran, apixaban, and rivaroxaban for any indication. Overall, apixaban was found to have a lower bleeding risk than warfarin (adjusted hazard ratio 0.66, p < 0.01), the biggest difference observed in the rates of gastrointestinal bleeds (hazard ratio 0.55, p < 0.01). There was no difference in the risk of major bleeds between warfarin and dabigatran, or rivaroxaban (Vinogradova et al., 2018). A lower bleeding risk with apixaban than warfarin has been observed in other large cohort studies, in which the majority of patients are anticoagulated for AF (Abraham et al., 2017; Amin et al., 2017). Where LVT is the indication for anticoagulation, Jones et al. (2020) found a lower rate of clinically significant bleeds in the DOAC cohort versus the VKA group (0% vs. 6.7%, p = 0.03). It is not reported if the significant bleeding events secondary to warfarin therapy occurred while the INR was super-therapeutic or in-range. Two other studies found no significant bleeding risk between groups (Guddeti et al., 2020; Iqbal et al., 2020).
A key consideration is the concomitant use of dual antiplatelet therapy (DAPT) in a triple therapy regimen, which confers a significantly increased bleeding risk. This is particularly pertinent given that significant proportions of patients with LVT have an ischemic etiology (Table 1) and are therefore likely to have undergone recent PCI, mandating the use of DAPT alongside anticoagulation (Valgimigli et al., 2018). Triple therapy regimens should comprise an oral anticoagulant and aspirin alongside clopidogrel, rather than a more potent P2Y12 inhibitor. Both ticagrelor (Gimbel et al., 2020) and prasugrel (Jia et al., 2015) confer greater bleeding risks than clopidogrel, and therefore their inclusion in a triple therapy regimen would likely augment the bleeding risk beyond that seen with aspirin and clopidogrel.
Triple antithrombotic therapy with warfarin has been shown to increase the risk of fatal and non-fatal bleeding compared with DAPT alone. A Danish cohort study demonstrated that aspirin and clopidogrel led to a bleeding risk of 7.5% per year, which increased to 15.4% per year with the addition of warfarin (Hansen et al., 2010). Notably, in the same cohort, the rate of intracranial bleeds increased from 0.2% to 1% per year when adding warfarin to DAPT (Hansen et al., 2010).
The evidence that anticoagulation reduces the risk of systemic embolization in LVT pre-dates the use of DAPT (Vaitkus and Barnathan, 1993), and therefore it is unknown if the reduction in the rate of SSE outweighs the risk of the increased bleeding. Up-to-date randomized trials are required to aid clinical decision-making in this patient group. A nationwide observational study in Denmark compared the bleeding risk of patients on VKA and DOAC-based triple therapy for AF (van Rein et al., 2019). When compared with warfarin monotherapy, the hazard ratio for major bleeding events on warfarin-based triple therapy was 3.13 (95% CI, 2.84–3.45), which was reduced to 2.28 (95% CI, 1.67–3.12) for patients on DOAC based triple therapy. These data suggest that DOAC-based regimens for LVT may confer a lower bleeding risk than VKA-based regimens, and therefore DOACS may be more suitable in higher-risk patients requiring triple therapy.
Follow-up duration of treatment and when to re-image
So far no trial has focused on outcomes of long-term anticoagulation in patient with a diagnosis of LVT after the recommended six month treatment period, either in the case of resolution or persistence of the thrombus. ESC 2017 STEMI guidelines recommend oral anticoagulation for three to six months, guided by repeat imaging and with careful consideration of bleeding risk and need for concurrent antiplatelet therapy (Ibanez et al., 2018). Therefore, a pragmatic approach may be to perform repeat imaging after 3-6 months and in patients where the LVT has resolved, with an improved LVEF, anticoagulation may be stopped. However, in instances where the LVT has resolved, but there is ongoing severe LV systolic dysfunction and a high risk of LVT recurrence, the optimal approach is less clear, and decisions regarding ongoing anticoagulation should be made on a case-by-case basis and with close balance of potential risks and benefits (Habash and Vallurupalli, 2017).
Prophylactic oral anticoagulation in high-risk patients without LVT
The American College of Cardiology/American Heart Association guidelines on STEMI management recommend prophylactic oral anticoagulation in patients with no LVT, but with high risk features for LVT formation on imaging such as anterior akinesis or dyskinesis (O'Gara et al., 2013). However, observational data of 460 STEMI patients with high-risk echocardiographic findings, found there was no benefit of adding warfarin to DAPT. The warfarin treated group had higher rates of death (5.4% vs. 1.5%, p = 0.04), stroke (3.1% vs. 0.3%, p = 0.02), and major bleeding (8.5% vs. 1.8%, p < 0.0001) (Le May et al., 2015). Shavadia et al. (2017) found no benefit to adding warfarin to DAPT in an observational study of 436 anterior STEMI patients with high-risk features on TTE in terms of thromboembolic events (2.1% vs. 1.2%, p = 0.343) and bleeding requiring hospitalization (2.5% vs. 1.2%, p = 0.361) at one year (Shavadia et al., 2017). Therefore, based on these recent data, prophylactic anticoagulation does not seem to confer significant benefit in patients with high-risk features for LVT thrombus formation on TTE after AMI.
Conclusion
We presented a general overview of the current evidence relating to the diagnosis, management, and follow-up of patients with LVT. Although LVT is an important complication of ischemic and non-ischemic cardiomyopathies, there are significant gaps in data relating to the optimal choice of oral anticoagulation and duration of treatment. Much of the data on the benefits of anticoagulation stems from the pre-PCI and DAPT era. VKAs such as warfarin are currently the first-line anticoagulant of choice, however there is an increasing body of data suggesting that DOACs may be a safe and effective alternative. The use of DOACs over VKAs may benefit patients by reducing the interactions with other medications, as well as the need for hospital visits and INR monitoring, although robust evidence on safety and efficacy advantages over warfarin is needed before they are brought into routine clinical practice. The current evidence stems from small, observational studies, and RCTs may be warranted to investigate this further.
Declaration of conflicting interests
The authors declare that they have no conflicts of interest
References
Debbie Falconer1
1Cardiology Department, CardioVascular Division, Royal Free Hospital, Pond Street, London, NW3 2QG, UK.
Ali Kirresh1
1Cardiology Department, CardioVascular Division, Royal Free Hospital, Pond Street, London, NW3 2QG, UK.
Luciano Candilio1
1Cardiology Department, CardioVascular Division, Royal Free Hospital, Pond Street, London, NW3 2QG, UK.
Debbie Falconer and Ali Kirresh contributed equally to the manuscript.
Corresponding author:
Dr. Debbie Falconer
Email: Debbie.falconer@nhs.net
Table 1: Incidence of left ventricular thrombus by aetiology (Adapted from Talle et al., 2014)

Table 2: Sensitivity and specificity of each modality in detecting
left ventricular thrombi (Adapted from Roifman et al, 2015). Abbreviations; CMR, cardiac magnetic resonance;LE-CMR, late gadolinium-enhanced cardiac magnetic resonance; TTE, transthoracic echocardiography.

Table 3: Summary of studies comparing outcomes of warfarin and DOACs. (Abbreviations; AMI: acute myocardial infarction; CCF: congestive cardiac failure; DOAC: direct oral anticoagulant; Echo: echocardiography; LVT: left ventricular thrombus; N; number of patients in study; SSE: stroke or systemic embolism; VKA: vitamin K antagonist (warfarin unless otherwise specified))

Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8120 | 6 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA