International bi-monthly journal of cell signaling, tissue protection, and translational research.
Remote Conditioning Modifies the Cardiorespiratory Responses During Exercise in Simulated Altitude
Silvia Gaspard1,2, Cecilia Thairi1, Guido Giardini2, Paola Berchialla1, Piergiorgio Montarolo3, Pasquale Pagliaro1
Author Affiliations


- 1Department of Clinical and Biological Sciences, University of Torino, Turin, Italy.
- 2Centro di Medicina di Montagna, Aosta, Italy.
- 3Department of Neurosciences, Rita Levi Montalcini, University of Torino, Turin, Italy
Abstract
The role of remote ischemic preconditioning (rIPC) in hypoxia is not yet fully understood. Since rIPC influences autonomic nervous system activity, we investigated the effects of rIPC on cardiopulmonary parameters during exercise in hypoxia.
Eighteen healthy male participants performed a first incremental-exercise (1st-Exercise) in normobaric hypoxia (12.5% O2). Five minutes after the 1st-Exercise, participants received either rIPC (3x5-min ischemia/5-min reperfusion cycles at 200 mmHg; n = 9) or sham-test (15 mmHg; n = 9), which served as control. After a 2-h wash-out in normoxia, participants performed a second incremental-exercise (2nd-Exercise) in hypoxia. During the 2-h wash-out, the heart rate (HR) recovered more in rIPC than sham group and the difference in HR recovery was by about 5 beats per minute (p < 0.001, sham vs rIPC), as revealed by the time-group and time difference analysis. Then, the achievement of 80% of predicted HR-max occurred, on average, 81 seconds earlier in the sham group during the 2nd-Exercise (p = 0.048, sham vs. rIPC). Carbon dioxide (CO2) production (VCO2) and oxygen consumption (VO2) were not different between the two groups when comparing the two exercises. However, during the 2nd-Exercise the difference in ventilation (ΔVE) between Sham and rIPC were statistically significant (-3.32 l/min, p < 0.001 sham vs. rIPC) at submaximal intensity (70-80% of the total exercise time). Nevertheless, ventilator efficiency (VE/VCO2) was not different among groups. Effort in hypoxia is capable of impairing cardiopulmonary performance during a subsequent exercise. Our data suggest that rIPC modifies HR recovery after the effort and this may mitigate the performance worsening during the 2nd-Exercise.
Keywords: Heart rate, Hypoxia, Ischemic conditioning, Preconditioning, Pulmonary ventilation
Abstract
The role of remote ischemic preconditioning (rIPC) in hypoxia is not yet fully understood. Since rIPC influences autonomic nervous system activity, we investigated the effects of rIPC on cardiopulmonary parameters during exercise in hypoxia.
Eighteen healthy male participants performed a first incremental-exercise (1st-Exercise) in normobaric hypoxia (12.5% O2). Five minutes after the 1st-Exercise, participants received either rIPC (3x5-min ischemia/5-min reperfusion cycles at 200 mmHg; n = 9) or sham-test (15 mmHg; n = 9), which served as control. After a 2-h wash-out in normoxia, participants performed a second incremental-exercise (2nd-Exercise) in hypoxia. During the 2-h wash-out, the heart rate (HR) recovered more in rIPC than sham group and the difference in HR recovery was by about 5 beats per minute (p < 0.001, sham vs rIPC), as revealed by the time-group and time difference analysis. Then, the achievement of 80% of predicted HR-max occurred, on average, 81 seconds earlier in the sham group during the 2nd-Exercise (p = 0.048, sham vs. rIPC). Carbon dioxide (CO2) production (VCO2) and oxygen consumption (VO2) were not different between the two groups when comparing the two exercises. However, during the 2nd-Exercise the difference in ventilation (ΔVE) between Sham and rIPC were statistically significant (-3.32 l/min, p < 0.001 sham vs. rIPC) at submaximal intensity (70-80% of the total exercise time). Nevertheless, ventilator efficiency (VE/VCO2) was not different among groups. Effort in hypoxia is capable of impairing cardiopulmonary performance during a subsequent exercise. Our data suggest that rIPC modifies HR recovery after the effort and this may mitigate the performance worsening during the 2nd-Exercise.
Keywords: Heart rate, Hypoxia, Ischemic conditioning, Preconditioning, Pulmonary ventilation
Introduction
In 1986, Murry et al. (1986) described ischemic preconditioning as endogenous cardioprotective mechanisms to limit ischemia/reperfusion injury (IRI) via repeated cycles of brief ischemia/reperfusion. Subsequently, in 1993, Przyklenk et al. (1993) introduced remote ischemic preconditioning (rIPC) as a strategy to limit IRI in an area far from that subject to the preconditioning protocol. The mechanisms underlying rIPC appear to be both neural and humoral in nature, with a preeminent role for the autonomic nervous system (Lim et al., 2010). It produces an early cardioprotective effect (first window of protection), which lasts 2-3 h, then, after 12-24 h, it is followed by a late cardioprotection period (second window of protection), which lasts 24-36 h (Kuzuya et al., 1993; Baxter et al., 1997). It was soon clear that rIPC can be obtained by the intermittent interruption of the blood flow of a limb (Kuzuya et al., 1993; Baxter et al., 1997; Bushell et al., 2002; de Groot et al., 2010; Foster et al., 2014; Horiuchi 2017). This is typically obtained by inflating a sphygmomanometer cuff to 200-220 mmHg and deflating it to 0 mmHg for several cycles with each inflation-deflation being 3-5 min in duration. Also intermittent hypoxic conditioning (I-Hypo-C), obtained with intermittent hypoxia and normoxia can trigger similar protective effects in vital organs (Sprick et al., 2019).
Many authors have studied the impact of rIPC and I-Hypo-C protocols on exercise performance (Kuzuya et al., 1993; Crisafulli et al. 2011; Foster et al., 2014; Hittinger et al. 2014; Tocco et al., 2015; Banks et al., 2016; Griffin et al., 2018; Paull 2018; Turnes et al., 2018; Slysz and Burr, 2019; Sprick et al., 2019). However, the effectiveness of these procedures in improving physical performance is not yet fully understood. In particular, in recent years, rIPC has also been tested on physical exercise under high altitude conditions. In most studies, the experimental setting is obtained in normobaric hypoxic conditions (Hittinger et al. 2014; Rieger et al., 2017; Kim et al., 2019; Wiggins et al., 2019). Only a few studies have used one similar to the real environment, namely hypobaric hypoxia (Kuzuya et al., 1993; Berger et al. 2017; Rieger et al., 2017). For example, Kim et al. (2019) noted that during exercise following rIPC, mean pulmonary arterial pressure (mPAP) and pulmonary arterial systolic pressure (PASP) are reduced compared to controls, and there is an increase in ventilation (VE) as well . However, Wiggins et al. (2019) have failed to demonstrate an increase in time trial (TT) in participants undergoing normobaric hypoxia. Also Hittinger et al. (2014) did not detect any difference compared to controls concerning parameters such as maximal power output (W max) and peripheral saturation of oxygen (SpO2). Despite a different experimental setting (hypobaric hypoxia), Foster et al. (2014) have experienced a decrease in mPAP, as well as an increased total exercise time (TET). However, in the same hypoxic conditions but at rest, Berger et al. (2017) did not notice any influence of rIPC on acute mountain sickness (AMS) development or on parameters such as SpO2, heart rate (HR), arterial pressure (PA), and PASP, a finding confirmed by Rieger et al. (2017).
Therefore, the effectiveness of rIPC in influencing cardiorespiratory parameters and physical performance is not entirely clear, especially under hypoxia or high altitude environments. With this study conducted on healthy, sedentary participants, we aimed to check if the early phase of rIPC (first window of protection) affects the recovery of cardiorespiratory parameters from a submaximal exercise and whether it influences them in a subsequent exercise performed in normobaric hypoxic conditions.
Methods and Materials
Eighteen healthy males participated in this study. All the participants were declared to not be affected by any respiratory or cardiovascular diseases. Exclusion criteria for the study are shown in Table 1. At the time of the trial, none of the participants took drugs daily. In addition, all were non-smokers and normotensives. Participants were asked to refrain from drugs and alcohol intake and physical exercise 24 hours before the test. On the morning of the scheduled cardiovascular assessment, participants arrived in a 1.5-2-h fasted condition. After an exhaustive explanation of methods and purposes of the study, they provided written informed consent. This trial was approved by the ethics committee of the "Parini" Hospital of the Aosta AUSL in accordance with Helsinki Declaration’s rules.
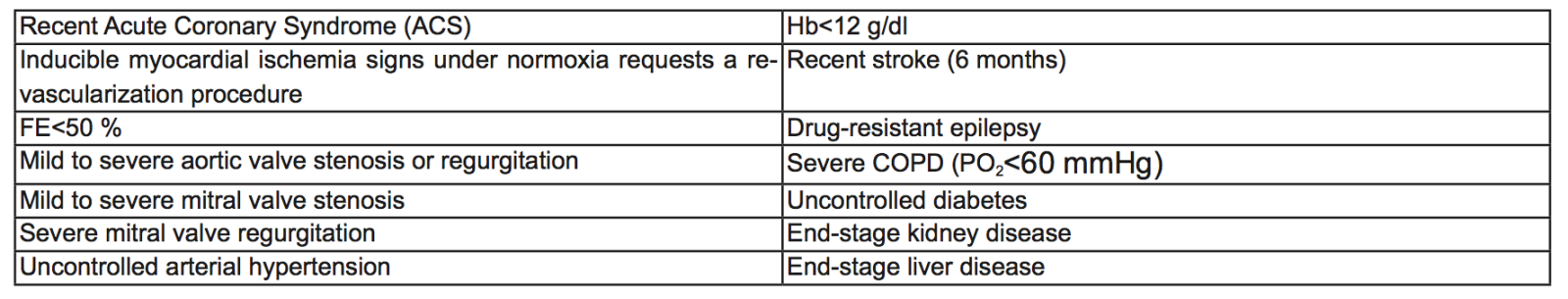
Table 1. Exclusion Criteria
Protocol overview
The experimental setting was carried out under fraction of expired O2 (FiO2) conditions of 12.6 ± 0.2%, equivalent to approximately 4100 m above sea level. Atmospheric pressure, room temperature, and humidity were 714.9 ± 5.6 mmHg, 24.9 ± 0.7 °C, and 25.3 ± 7.4 %, respectively.
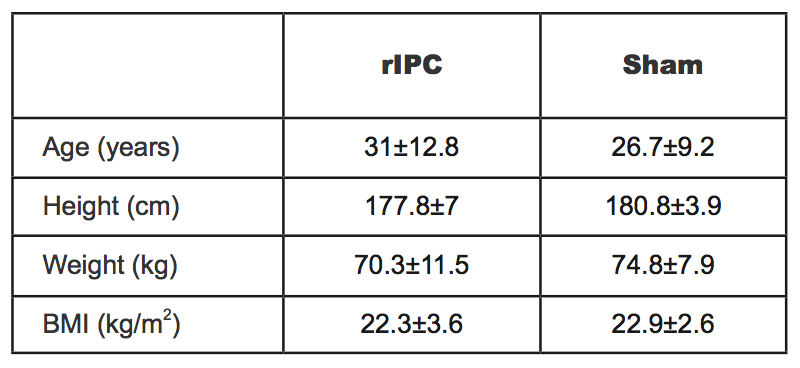
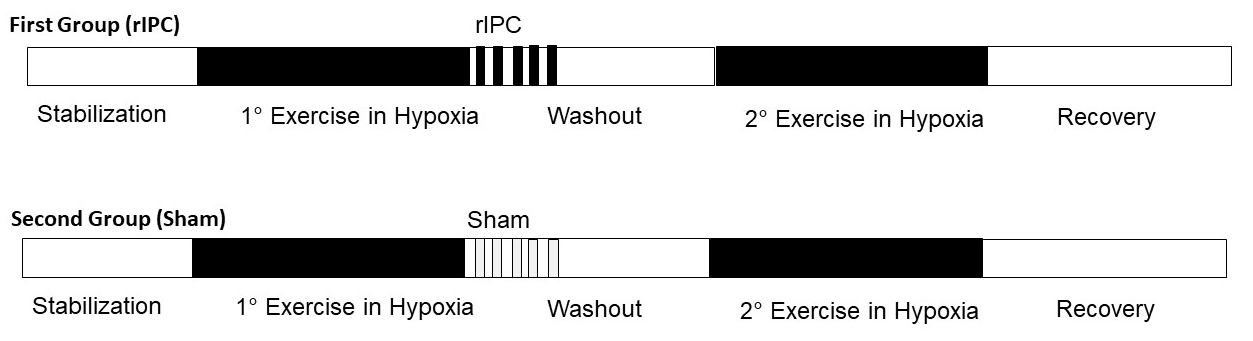
The participants were randomly assigned to two groups both containing nine participants. Their characteristics are shown in Table 2. After a first effort in normobaric hypoxia, while the participants of the first group were subjected to the rIPC maneuver, the participants of the second group underwent the sham test and served as control (see below). The experimental protocol was divided into four main phases; 1) first bout of physical exercise under normobaric hypoxic conditions 2) rIPC treatment/sham test during normoxia 3) wash-out during normoxia 4) second bout of physical exercise under normobaric hypoxic conditions (Figure 1). The normobaric hypoxic environment was reproduced thanks to Alti Trainer made by SMTEC. It delivered airflow with reduced inspired FiO2 through a respiratory mask. In this study, we administered a FiO2 equal to 12.6%, equivalent to an altitude of 4100 m above sea level. To perform the physical exercise, participants used a supine ergometer ergoselect 1200 made by ergoline. Data were collected by QUARK RMR with CPET made by COSMED, which allowed recording of all parameters every 3 to 10 s. During the first and fourth phases, participants underwent a 12 lead surface ECG-monitoring.
Table 2. Physical features of two groups expressed as mean ± SD (acronyms as in the text).
The protocol of the phases of physical exercise was designed on the basis of previous studies (Hittinger et al. 2014; Paradis-Deschênes et al., 2018; Kim et al., 2019; Wiggins et al., 2019). The protocol involved four stages: 1) rest, 2 min in normoxia followed by hypoxia; 2) physical exercise, incremental starting from 25 Watt (W) at 60-70 rpm, followed by an increase of 25 W every 3 min up to 80-90% of the predicted HR max (HRmax-pred) of each subject (Riebe et al., 2018); 3) recovery, 3 min at 20 W; 4) same as stage 1. After randomization, participants were subjected to rIPC treatment or sham test after step 3. The procedure used in this study involved the application of the inflated/deflated sphygmomanometer cuff to the upper left limb. The procedure duration was about 25 min, including 3 cycles. Each cycle consisted of 5 min of cuff inflation (ischemic stimulus) followed by 5 min of its deflation (reperfusion phase) (Kuzuya et al., 1993; Turnes et al., 2018). According to the literature, administered pressures were 200 mmHg during the rIPC treatment and 15 mmHg during the sham test (Kuzuya et al., 1993).
In order to not influence participants, they were told that we intended to study the maneuver effect on physical exercise, without mentioning the existence of two different procedures. After that, participants experienced a 2-h wash-out. During this period, they were asked to eat a light meal, to avoid alcohol or drug intake, as well as to rest. The procedures and their duration were chosen on the basis of previous literature (Kuzuya et al., 1993; Baxter et al., 1997; Bushell et al., 2002; de Groot et al., 2010; Lisbôa et al., 2017; Turnes et al., 2018). In particular, Lisbôa et al. (2017) reported how the effectiveness of preconditioning is evident only in exercise after 2 h and 8 h, but not after 1 h from rIPC administration. Since, our purpose was to study the first window of protection induced by rIPC, which lasts up to 2-3 h (Marber et al., 1993; Kuzuya et al., 1993; Baxter et al., 1997; Gross, 2005), all participants performed the same physical exercise 2 h after the end of the rIPC protocol.
Pulmonary gas exchange
Since oxygen uptake (VO2, ml/min/kg), carbon dioxide production (VCO2, ml/min), and VE (l/min) increase in parallel to exercise intensity, we decided to analyze these parameters to have indices of exercise respiratory performance. Moreover, we evaluated the ventilator efficiency (VE/VCO2).
Cardiovascular parameters
Heart rate is considered a linear function of exercise intensity. In young and healthy participants HR max can reach 3-4 times basal values, namely the theoretical HRmax-pred, which in our participants differed by 2-4 beats per minute (bpm) whether calculated as = 220 – age or 208 – 0.7*age. Therefore, we evaluated HR at rest and during exercise and stopped the 1st exercise when the participant reached 80-90% of HRmax-pred. Blood pressure was also measured before exercise.
Statistical analysis
To have comparable parameters between the experimental groups, statistical analysis, as well as intra- and inter-group comparisons, were conducted over defined time intervals. These were chosen to be 60-70% and 70-80% of the total exercise time (TET) and the time period at which the 75-80% of HRmax-pred was reached. In these time intervals, the exercise intensity is considered submaximal.
The Linear mixed model approach was used to analyze the variation of cardiovascular parameters and pulmonary gas exchange between experimental groups (rIPC and sham). First exercise and second exercise are to be referred to the exercise before the rIPC/sham test and to the one after, respectively. Interaction between group and time measurement (1st and 2nd exercise) was included.
The linear mixed model was fit using restricted maximum likelihood criterion. P-value adjustment for the post-hoc test was applied. Statistical significance was set at 0.05. All analyses were carried out using R version 4.0.2 (R Core Team, 2020).
Results
All the participants completed the protocol. However, two participants were excluded: a subject belonging to rIPC group was excluded because the participant did not fully record his physical performance before rIPC protocol, whereas a subject belonging to the sham group was excluded because, in the first exercise, he only reached 70% of his theoretical HRmax-pred due to insufficient hemoglobin (Hb) saturation (SpO2˂60 %).
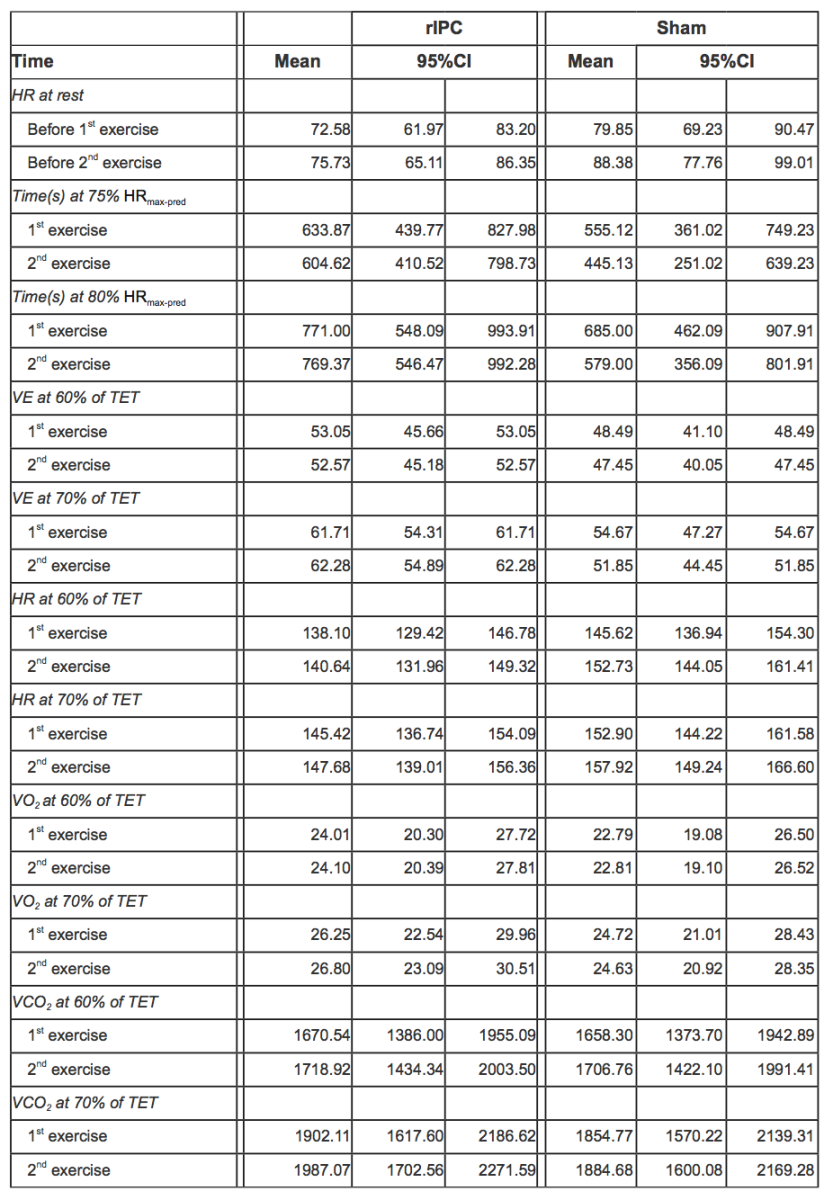
In Table 3, mean values of pulmonary gas exchange and cardiovascular parameters adjusted for individual variability along with 95% CI are reported.
Table 3. HR at rest before exercises and during 1st and 2nd exercise for both rIPC and sham group, mean values adjusted for individual variability with 95%CI (acronyms as in the text).
Heart Rate
Before exercise, HR was not significantly different between groups: mean HR was 72.58 (95% CI 61.97-83.20) in the rIPC group vs. 79.85 (95% CI 69.23-90.47) in the sham group, with a mean difference of 7.27 bpm with a p value not statistically significant (Table 4). After the first exercise and before the second exercise, HR was on average 3.15 bpm higher (p < 0.001). Participants in the sham group showed an additional statistically significant increase of 5.38 bpm (p < 0.001).
Table 4. Mixed effect models for HR (bpm) at rest and HR at 60-70% of TET. In square brackets is reported the reference category (acronyms as in the text).
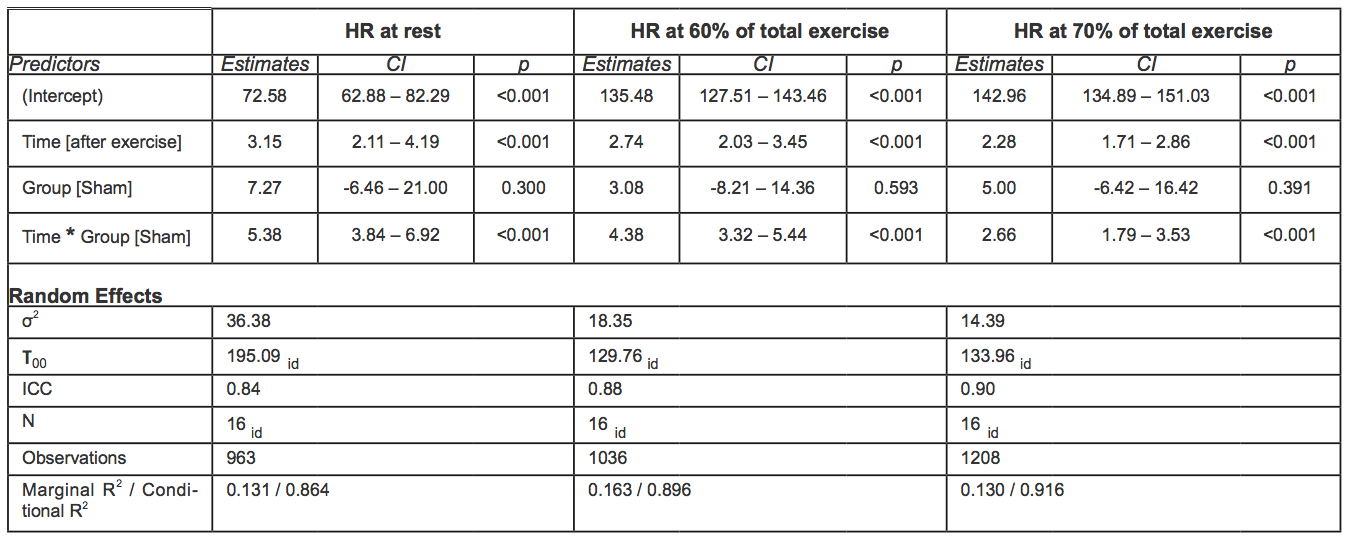
During the second exercise, at 60-70% of the total exercise time, HR was on average 4.57 bpm higher in the sham group than in rIPC group (p < 0.001). At 70-80% time of the total exercise, the difference between groups was smaller (2.66 bpm) but still significant (p < 0.001).
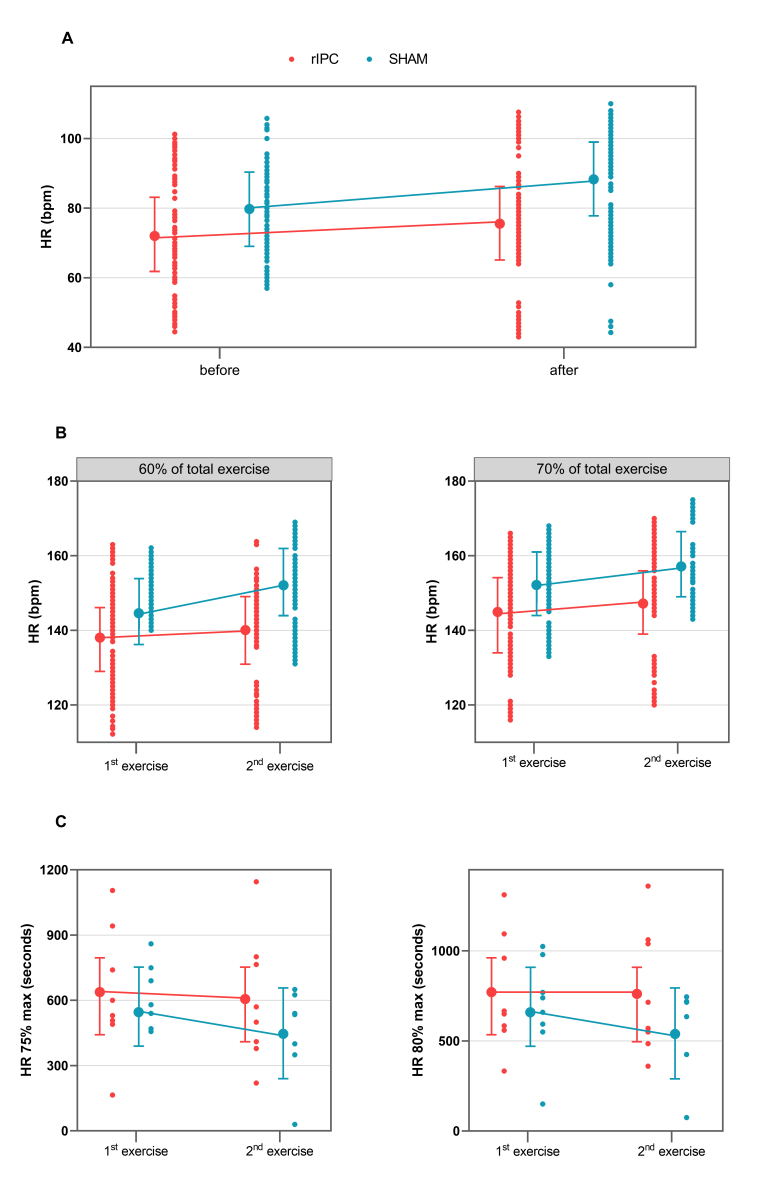
Raw data and mean adjusted values estimated by the models are reported in figures 2A and B and in Table 5.
Table 5. Mixed effect models for time (s) at HR 75 and 80% of HRmax. In square brackets is reported the reference category (acronyms as in the text).
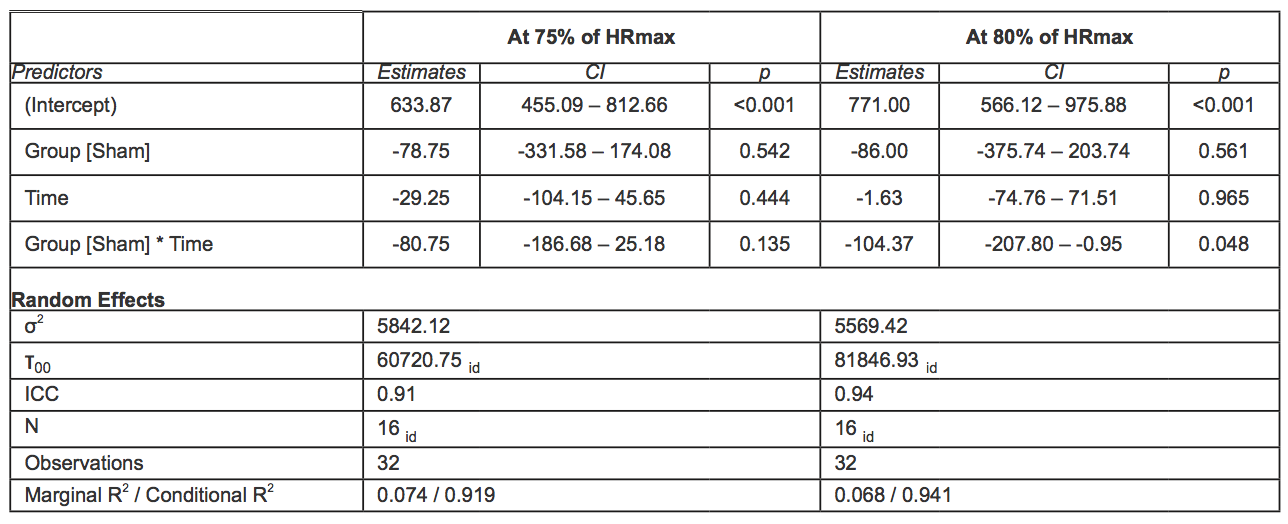
Time
During the first exercise, the time at which the 75% of HRmax-pred was reached was 633.87s (95% CI 439.77 – 827.98) and 555.12s (95% CI 361.02 – 749.23) in the rIPC group and the sham group, respectively, with a mean difference equal to 78.75sn, which was not statistically significant. No significant difference was observed in the time at which the 80% of HRmax-pred was reached (771s, 95% CI 548.09 – 993.91 in the rIPC group; 685s 95% CI 462.09 – 907.91 in the sham group).
During the second exercise, the time at which the 80% of HRmax-pred was achieved decreased significantly in the sham group (-104.37s, 95% CI -207.8; -0.95, p = 0.048), whereas in the rIPC group a significant reduction was not observed. The differences were not significant for the time at which the 75% of HRmax-pred was achieved either in the sham group or the rIPC group. Raw data and mean adjusted values estimated by the models are reported in figure 2C.
Ventilation
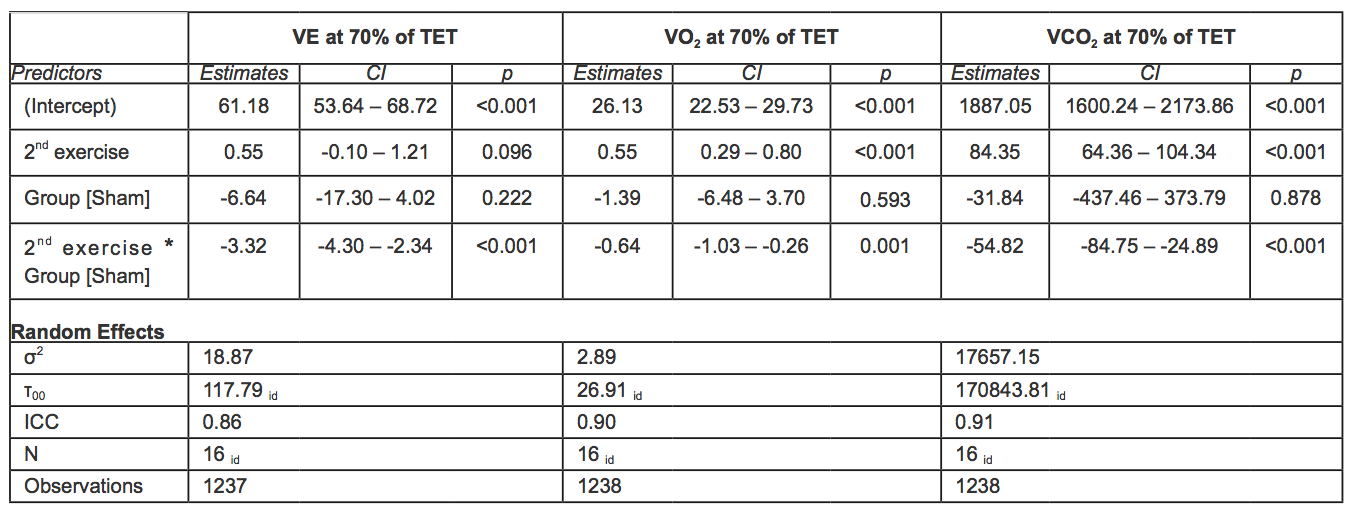
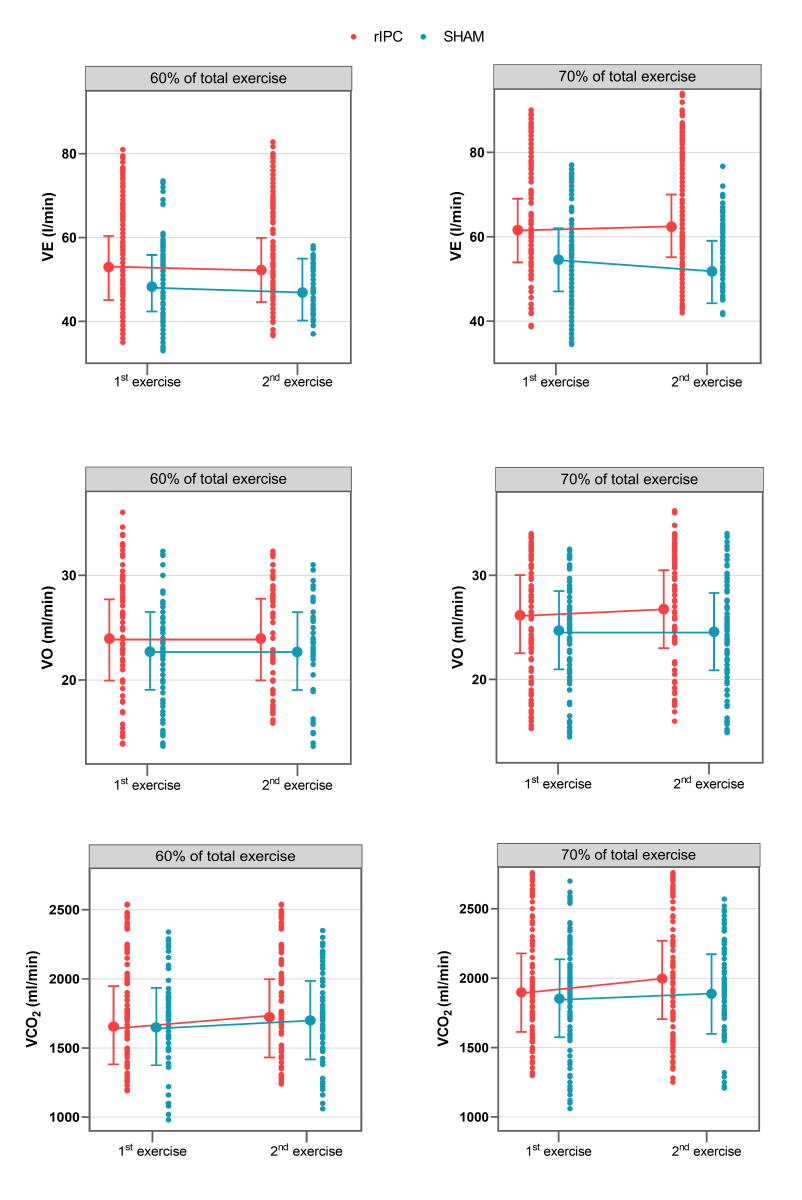
At 60-70% of the total exercise time, no statistically significant differences in VE were observed between groups when comparing the first and second exercise. At 70-80% of the total exercise time, VE was significantly lower during the second exercise among individuals in the sham group (-3.32 l/min, 95% CI -4.30; -2.34, p < 0.001). Raw data and mean adjusted values estimated by the models are reported in figure 3 and Tables 6-7.
Oxygen consumption (VO2)
At 60-70% of the total exercise time, no statistically significant differences in VO2 were observed between groups when comparing the first and second exercise,.
At 70-80% of the total exercise, the VO2 increase was significantly lower in the sham group. Indeed, the increase over time of VO2 was 0.64ml/min/kg higher in the rIPC group (p = 0.01).
Raw data and mean adjusted values estimated by the models are reported in figure 3 and Tables 6-7.
CO2 production (VCO2)
Comparing the first and second exercise, we observed that at 60-70% of the total exercise time, VCO2 increased significantly in both groups (+56.38, 95%CI: 35.30 – 77.31, p < 0.001).At 70-80% of the total exercise (submaximal exercise), the increase was significantly lower in the sham group. Indeed, the increase over time of the increment of VCO2 was 54.82 ml/min higher in the rIPC group (p < 0.001). At submaximal exercise, ventilator efficiency (VE/VCO2) was similar in all conditions.
Raw data and mean adjusted values estimated by the models are reported in figure 3 and Tables 6-7.
Table 6. Mixed effect models for VE, VO2 and VCO2 at 60% of TET. In square brackets is reported the reference category (acronyms as in the text).
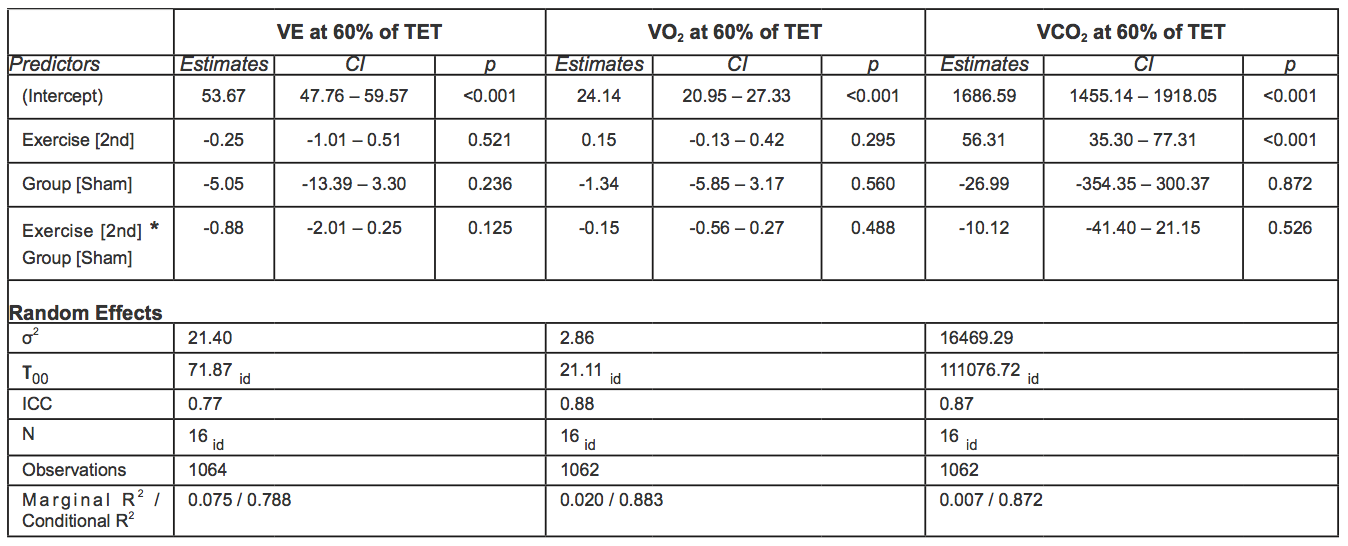
Table 7. Mixed effect models for VE, VO2 and VCO2 at 70% of TET. In square brackets is reported the reference category (acronyms as in the text).
Discussion
Here we demonstrate that rIPC modifies recovery from submaximal exercise performed under normobaric hypoxic conditions, as revealed by faster HR recovery in two hours in preconditioned participants compared to the sham group. Furthermore, in a subsequent physical exercise, carried out during the first window of protection under hypoxic conditions, the preconditioned participants performed the exercise longer before reaching 75-80% of HRmax-pred. Indeed, during the 2nd exercise in the sham group the value of HR was greater under the same power output, but this alteration was mitigated by rIPC maneuver.
Preconditioning probably represents one of the best strategies for efficient prevention against acute cardiovascular diseases (CVDs) (Keith et al., 1990; Crisafulli et al., 2015; Pagliaro and Penna, 2015). As said, preconditioning is composed of an immediate/early (hours) and a delayed (days) phase. While the first is caused by rapid posttranslational modifications of existing proteins, the latter provides sustained protection as a result of the de novo synthesis of additional proteins (Qian et al., 1999; Bolli, 2000). Also I-Hypo-C, is under scrutiny for CVD prevention (Sprick et al., 2019). In principle, I-Hypo-C is thought to drive responses similar to those induced by rIPC. For example, I-Hypo-C was shown to increase exercise tolerance in elderly men with and without coronary artery disease (Burtscher et al., 2004) and to improve several metabolic and cardiovascular parameters in patients (del Pilar Valle et al., 2006; Serebrovska et al., 2016). Moreover, I-Hypo-C benefits not only the cardiovascular, but the nervous, digestive, and pulmonary systems as well (Burtscher et al., 2009; Milano et al., 2011; Faulhaber et al., 2015; Estrada et al., 2016; Serebrovska et al., 2016; Glazachev et al., 2017). Most likely, the reoxygenation of hypoxic tissues plays a crucial role in developing protection during I-Hypo-C, but the underlying mechanisms are not yet fully understood.
Here we decided to perform a rIPC protocol after a first hypoxic exercise to add the effects of short intermittent ischemia, namely rIPC, to those of alternating hypoxia/normoxia and to analyze the effects of these protocols in a time window (the first window of protection) in which it is still likely that there will be the effects of the conditioning induced by the rIPC. Intermittent hypoxia was chosen, alternating hypoxia and normoxic washout, since it is at the time of reintroduction of oxygen that reactive oxygen species-dependent and other protective mechanisms are likely to be evoked (Burtscher et al., 2009; Milano et al., 2011; Faulhaber et al., 2015; Estrada et al., 2016; Serebrovska et al., 2016; Glazachev et al., 2017). We therefore chose to superimpose the mechanisms induced by rIPC to those induced by intermittent hypoxia.
Our data suggest that performing exercise in hypoxia and recovery in normoxia does not induce per se an improvement of cardiovascular parameters during the time window of the early protection. Actually, it delays the complete recovery of cardiovascular parameters. Consequently, the second exercise has an impaired cardiovascular response. This is in agreement with the observed alterations in repeated-sprint ability (i.e. with earlier and larger performance decrements) occurring under hypoxic conditions (Girard et al., 2017). The novelty of our study is in the addition of a rIPC protocol during the initial part of the post-exercise recovery, which accelerates the recovery of cardiovascular parameters and allows a better cardiorespiratory performance during the second exercise under hypoxic conditions. Actually the main differences are in terms of HR, as VCO2 and VO2 are similar among groups and during the two exercises. Yet, VE parameters are slightly improved in the second exercise of the rIPC group, whose physiological relevance may be negligible. Indeed, breathing efficiency (VE/VCO2) was not different between groups.
We do not know the mechanisms responsible for cardiovascular parameters worsening after switching from hypoxic exercise to normoxic recovery and hypoxic exercise again, and we do not know the mechanism of limitation in impairment by rIPC. We can speculate that the mechanisms may reside in different acid-base responses in the sham and rIPC group. It is well known that acidosis is necessary for inducing cardioprotection by conditioning protocols (Cohen and Downey, 2011; Inserte et al., 2011; Pagliaro and Penna, 2015). Exercise at high altitude (or in hypoxia) is characterized by tissue hypoxemia, which may therefore represent a sort of ischemia-reperfusion stress. However, hypoxia-drives the hyperventilation response mediated by the carotid bodies and aimed at restoring normal oxygenation. This necessarily implies excessive washout of carbon dioxide, which shifts the acid-base balance toward alkalosis (Samaja et al., 1997). Therefore, this balance may limit the potential to trigger protective mechanisms. The addition of rIPC might re-establish tissue acidosis, which may help to trigger a stronger protection response and to improve subsequent cardiorespiratory performance. Indeed, recovery from hypoxic exercise is modifiable by rIPC, which has the potential to improve exercise performance via both local and remote mechanisms (Kuzuya et al., 1993; de Groot et al., 2010). For instance, it has been reported that rIPC improves maximal performance in highly trained swimmers (Jean-St-Michel et al., 2011). Recently, it has been shown that rIPC induces a sort of "vascular conditioning," which is likely to be attributed to the upregulation of some cardio-protective microRNAs (such as miR-150, -21, -208), to the reduction of oxidative stress, and to the production of oxides of nitrogen (Ikonomidis et al., 2021). It has also been suggested that preconditioning may provide an immediate and effective strategy to enhance high-intensity endurance performance at moderate altitude (Paradis-Deschênes et al., 2018). Here we suggest that rIPC may represent a way for a better recovery of cardiorespiratory parameters from an effort in hypoxia.
Methodological considerations and limitations of the study
Our study data was acquired in laboratory settings of supine exercise and in normobaric hypoxia that do not represent the high altitude environment. However, our setting allows us to easily shift from hypoxia (during exercise) to normoxia (during recovery from exercise). Nevertheless, the wash-out time - dictated by the duration of the first window of protection - was insufficient for a full HR recovery, especially for sham group participants. Moreover, we had a low sample size, due to the Covid-19 lockdown, which could have compromised the statistical significance of some of the analyzed parameters. Yet, we cannot rule out that a stronger ischemic preconditioning stimulus (e.g. intermittent ischemia of two limbs) may achieve different results, as dose response studies achieved contradictory results in terms of cardioprotection (Whittaker and Przyklenk, 2014; Gaspar et al., 2018) and the concept of ‘hyperconditioning’ was introduced (Whittaker and Przyklenk, 2014). Finally, to have mechanistic assumptions on how rIPC can affect the cardiorespiratory system, in future studies, autonomic nervous system factors, such as acetylcholine, epinephrine, or norepinephrine could be analyzed, at the different time points.
Clinical implications
In various pathological and clinical conditions, including CVDs, intermittent hypoxic interventions have been shown to be effective as therapeutic approaches (Burtscher et al., 2004; Millet et al., 2016; Serebrovskaya et al., 2008; Fernandez et al., 2018; Kayser et Verges, 2013). In particular, for respiratory diseases, evidence is accumulating that intermittent hypoxia may be effective as a treatment in chronic obstructive pulmonary disease, improving both exercise tolerance and decreasing adrenergic reactivity, thereby improving respiratory control (Haider et al., 2009; Burtscher et al., 2009; Burtscher et al., 2010). With due caution, it could also be useful in some patients who have suffered from COVID-19 (Millet et al., 2021). Nevertheless, we are aware that there is an extraordinary need to better understand conditioning mechanisms and to obtain their benefits by other means, for example pharmacologically and through an integrated and multitargeted approach in some multifactorial conditions (Davidson et al., 2019).
In conclusion, physical performance and cardiorespiratory parameters in normobaric hypoxia are appreciably compromised in cases of incomplete recovery from a previous submaximal exercise. Remote ischemic preconditioning, performed under normoxic conditions, is able to accelerate recovery from an exercise performed under hypoxic conditions, allowing faster recovery of HR and, possibly, better cardiorespiratory performance during a second effort (Kim et al., 2019). Thus rIPC may allow better execution of an effort performed after an alternation of hypoxia/normoxia. Therefore, we suggest that rIPC could mitigate the impairment of physical performance due to incomplete recovery from a previous effort in normobaric hypoxia.
An interesting and lively debate, which goes beyond the scope of the present work, has long been underway among researchers on whether or not hypobaric hypoxia induces responses other than normobaric hypoxia, especially in the context of exercise physiology. For a more in-depth analysis on this point, the reader is redirected to the review by Viscor et al. (2018).
Acknowledgments
This study was funded by the University of Turin, Ricerca Locale Ex-60% (Grants: PAGP_RILO_16_01) and by MIUR (PAGP_FFABR_17_01). The authors thank the participants for their voluntary involvement in the study.
Declaration of conflicting interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be perceived as a potential conflict of interest.
References
Silvia Gaspard1,2
1Department of Clinical and Biological Sciences, University of Torino, Turin, Italy. 2Centro di Medicina di Montagna, Aosta, Italy.
Cecilia Thairi1
1Department of Clinical and Biological Sciences, University of Torino, Turin, Italy.
Guido Giardini2
2Centro di Medicina di Montagna, Aosta, Italy.
Paola Berchialla1
1Department of Clinical and Biological Sciences, University of Torino, Turin, Italy.
Piergiorgio Montarolo3
3Department of Neurosciences, Rita Levi Montalcini, University of Torino, Turin, Italy.
Pasquale Pagliaro1
1Department of Clinical and Biological Sciences, University of Torino, Turin, Italy.
Corresponding author:
Pasquale Pagliaro, M.D., Ph.D.
Email: pasquale.pagliaro@unito.it
In a new window | Download PPT
Figure 1: Study protocol. The experimental protocol was divided into four main phases; 1) first physical exercise in normobaric hypoxic conditions, 2) remote ischemic conditioning (rIPC) treatment or sham test during normoxia, 3) wash-out during normoxia, and 4) second physical exercise in normobaric hypoxic conditions.
In a new window | Download PPT
Figure 2: A: Values of HR (bpm) before and 2-h after the 1st exercise. B: Values of HR (bpm) during the 1st and 2nd exercises at 60% and 70% of TET. C: Values of time (seconds) at 75-80% of HRmax-pred during the 1st and 2nd exercises. Points connected by lines are the mean adjusted values estimated by the models (acronyms as in the text).
In a new window | Download PPT
Figure 3: Values of VE (l/min), VO2 (ml/min/kg), and VCO2 (ml/min) during the 1st and 2nd exercises. Points connected by lines are the mean adjusted values estimated by the models (acronyms as in the text).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7118 | 17 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA