Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The Effect of Remote Ischemic Conditioning on Blood Pressure control in patients with Chronic Kidney Disease: the ERIC-BP-CKD Pilot Study
Time:2021-12-28
Number:4854
Jason Chon Jun Choo1, Jia Liang Kwek1, Cynthia Ciwei Lim1, Irene Yanjia Mok1, Horng Ruey Chua2,10, Yilin Jiang3, Fei Gao3,4, Kearney Jun Yao Tan5, Gustavo Crespo-Avilan3,6,7, Tazeen Hasan Jafar1,8,9, Derek J Hausenloy3,6,10,11,12
Author Affiliations


- 1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore.
- 2Division of Nephrology, Department of Medicine, National University Hospital, Singapore.
- 3National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 4Programme in Health Services & Systems Research, Duke-NUS Medical School, Singapore.5Clinical Trials and Research Centre, Singapore General Hospital.
- 5Clinical Trials and Research Centre, Singapore General Hospital.
- 6Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.
- 7Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
- 8Programme in Health Services & Systems Research, Duke-NUS Medical School, Singapore.
- 9Duke Global Health Institute, Duke University, Durham, NC, USA.
- 10Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 11The Hatter Cardiovascular Institute, University College London, London, United Kingdom.
- 12Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Conditioning Medicine 2021. 4(5): 251-254.
Abstract
Inadequately controlled hypertension in patients with chronic kidney disease accelerates progression of kidney dysfunction. Remote ischemic conditioning (RIC) using transient limb ischemia has been shown to protect the kidney and microvasculature in experimental and clinical studies. The ability to lower blood pressure in patients with CKD through application of daily chronic RIC (CRIC) is unknown. This study aimed to assess the feasibility and efficacy of lowering systolic blood pressure (SBP) in hypertensive CKD patients after administering 28 days of CRIC compared to sham. The study included stages one to four CKD patients with automated office blood pressure (AOBP) SBP >140mmHg on stable dose antihypertensives. Six patients were randomized to the CRIC treatment group and four to the control group, and treatments were performed daily for 28 days. AOBP and central aortic systolic blood pressure (CASP) readings were measured at baseline, day 28, and day 42. There were no differences in AOBP SBP or CASP between CRIC and control on either day 28 or 42. However, in the CRIC group, AOBP diastolic blood pressure (DBP) and mean arterial pressure (MAP) were significantly decreased on day 28 when compared to control, and AOBP MAP remained significantly lower on day 42. Treatment adherence was excellent with completion of CRIC or control in 96% and 93% of participants, respectively. There were no adverse events reported. The application of 28 days of CRIC improved DBP and MAP but not SBP in this small proof of concept study. A larger study is necessary to confirm these findings.
Keywords: Remote ischemic conditioning, chronic kidney disease, hypertension
Abstract
Inadequately controlled hypertension in patients with chronic kidney disease accelerates progression of kidney dysfunction. Remote ischemic conditioning (RIC) using transient limb ischemia has been shown to protect the kidney and microvasculature in experimental and clinical studies. The ability to lower blood pressure in patients with CKD through application of daily chronic RIC (CRIC) is unknown. This study aimed to assess the feasibility and efficacy of lowering systolic blood pressure (SBP) in hypertensive CKD patients after administering 28 days of CRIC compared to sham. The study included stages one to four CKD patients with automated office blood pressure (AOBP) SBP >140mmHg on stable dose antihypertensives. Six patients were randomized to the CRIC treatment group and four to the control group, and treatments were performed daily for 28 days. AOBP and central aortic systolic blood pressure (CASP) readings were measured at baseline, day 28, and day 42. There were no differences in AOBP SBP or CASP between CRIC and control on either day 28 or 42. However, in the CRIC group, AOBP diastolic blood pressure (DBP) and mean arterial pressure (MAP) were significantly decreased on day 28 when compared to control, and AOBP MAP remained significantly lower on day 42. Treatment adherence was excellent with completion of CRIC or control in 96% and 93% of participants, respectively. There were no adverse events reported. The application of 28 days of CRIC improved DBP and MAP but not SBP in this small proof of concept study. A larger study is necessary to confirm these findings.
Keywords: Remote ischemic conditioning, chronic kidney disease, hypertension
Introduction
Patients with chronic kidney disease (CKD) often have inadequately controlled hypertension (Lee et al., 2017), the presence of which is associated with more rapid progression of CKD and cardiovascular complications (Wan et al., 2019; Kim et al., 2020). As such, novel treatments are required to better control blood pressure and improve health outcomes in CKD. Remote ischemic conditioning (RIC) using transient limb ischemia/reperfusion has been shown to protect the kidney and microvasculature in experimental and clinical studies. Daily episodes of RIC (termed chronic RIC [CRIC]) applied for 1 to 12 months have been shown to lower systemic blood pressure (SBP), prevent stroke, and reduce post-myocardial infarction left ventricular remodeling in experimental and clinical studies (Wei et al., 2011; Meng et al., 2012; Jones et al., 2014; Chong et al., 2019). In addition, CRIC applied daily for 28 days has been shown to reduce SBP in patients with chronic ischemic heart failure (Pryds et al., 2017). Whether CRIC can reduce SBP in hypertensive patients with CKD is not known. The primary objective of the ERIC-BP-CKD study was to assess the feasibility and efficacy of CRIC administered for 28 days on lowering SBP in patients with CKD and hypertension when compared to sham. The secondary objectives were to measure central aortic systolic pressures, proteinuria, and kidney function.
Materials and methods
This was a proof-of-concept, single-center, randomized, controlled, double-blinded trial (NCT03236350). Ethics approval was granted by the SingHealth Centralized Institutional Review Board (Reference 2016-2966). Study inclusion criteria included: participants aged ≥ 21 years with CKD stages 1 to 4; stable treatment for hypertension; and automated office blood pressure (Agarwal, 2017) (AOBP) systolic blood pressure (SBP) >140mmHg. Average AOBP readings were measured after patients had rested alone for 5 minutes, followed by a fully automated sequence of two readings over 5-minutes. Exclusion criteria included: polycystic kidney disease; atrial fibrillation; peripheral arterial disease of the arms, long-acting sulphonylurea, nicorandil, or anticoagulation treatment. Eligible participants were randomized to receive either CRIC treatment (comprising four 5-minute inflations/deflation of CellAegis autoRIC® device on the upper arm) or control (comprising four 5-minute sham inflations and deflations of CellAegis autoRIC® device on the upper arm) performed daily for 28 days. The control sham device provides the same sound and vibration as the active CellAegis autoRIC® device but with no inflation of the cuff. Adherence to CRIC based on completed days and treatment cycles were automatically captured by the autoRIC® device. Demographics and clinical data were collected at baseline, whereas serum creatinine (including calculated eGFR CKD-EPI), spot urine protein-creatinine ratio (UPCR), and serum inflammatory biomarkers were measured at baseline and on day 28. AOBP readings (Omron® HEM-907), central aortic systolic pressures (CASP) BPro® Intro(Williams et al., 2011) were measured at baseline, day 28 and day 42 (2 weeks after stopping CRIC or sham). Anti-hypertensives were not adjusted throughout the study period and no specific lifestyle modification advice was given.
Statistical analysis was performed using Stata version 16 (StataCorp, Texas). Categorical variables were presented as proportions and continuous variables summarized as medians with interquartile ranges (IQR). Baseline characteristics were compared using Pearson chi-square test or Fisher’s exact test for categorical variables and Mann-Whitney U test for non-normally distributed continuous variables. Our primary analysis compared changes in SBP between the CRIC and control groups using the intention to treat approach for all randomly assigned participants. Wilcoxon signed rank test was used to compare between two time-points and Wilcoxon rank-sum test was used to compare between control and treatment groups. Generalized Estimation Equations (GEE) were used to compare control and treatment groups over time. All analyses were two-tailed and p < 0.05 was considered statistically significant.
Results
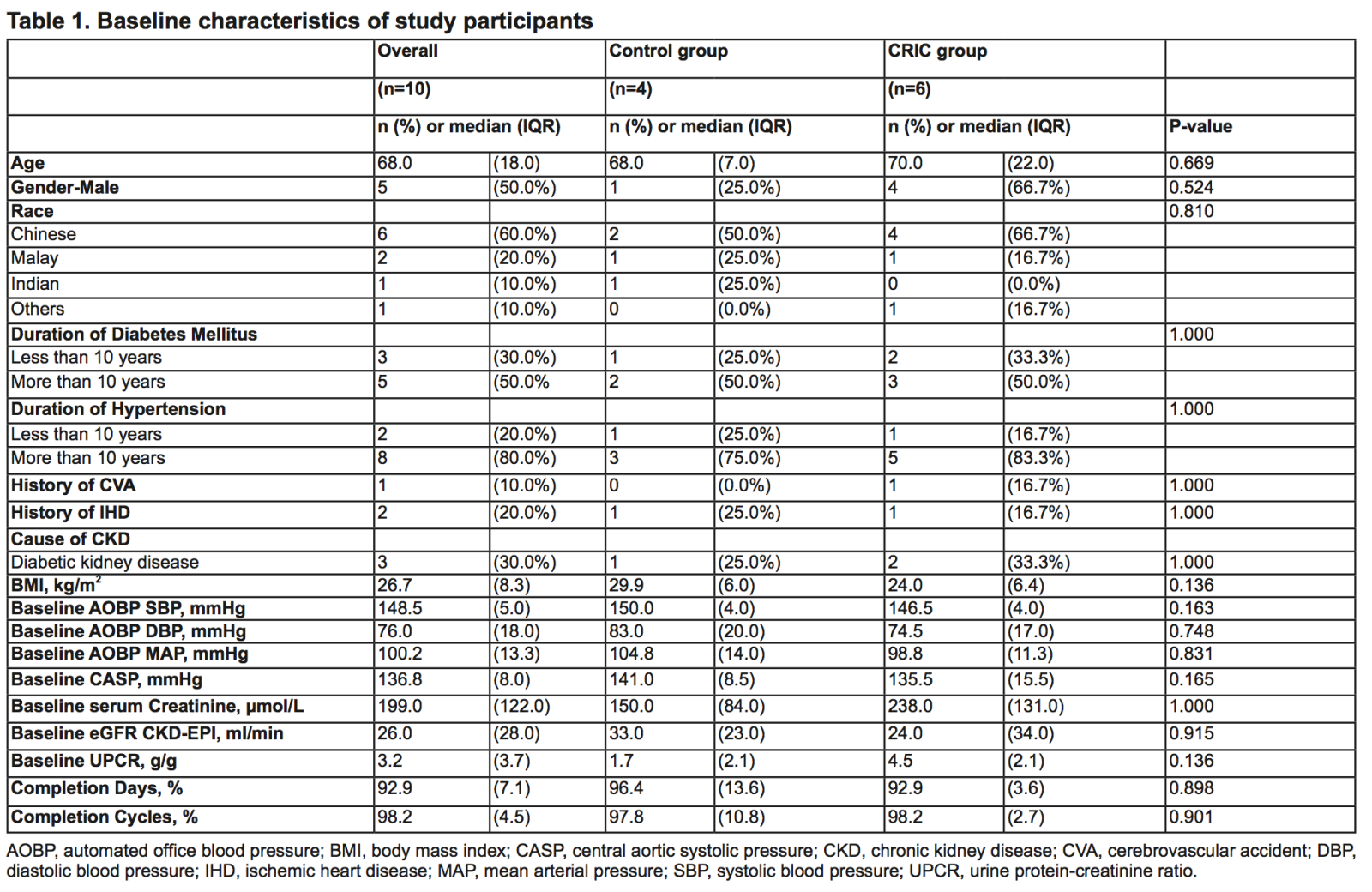
Sixteen patients were screened with 6 screen failures due to AOBP criteria. Ten patients were recruited with 6 patients randomised to CRIC and four to control. One participant in the CRIC group withdrew consent after randomization. Baseline demographic and clinical characteristics are shown in Table 1. Adherence to treatment was excellent with completion of CRIC or sham control in 96% and 93% of participants, respectively.
Table 1. Baseline characteristics of study participants
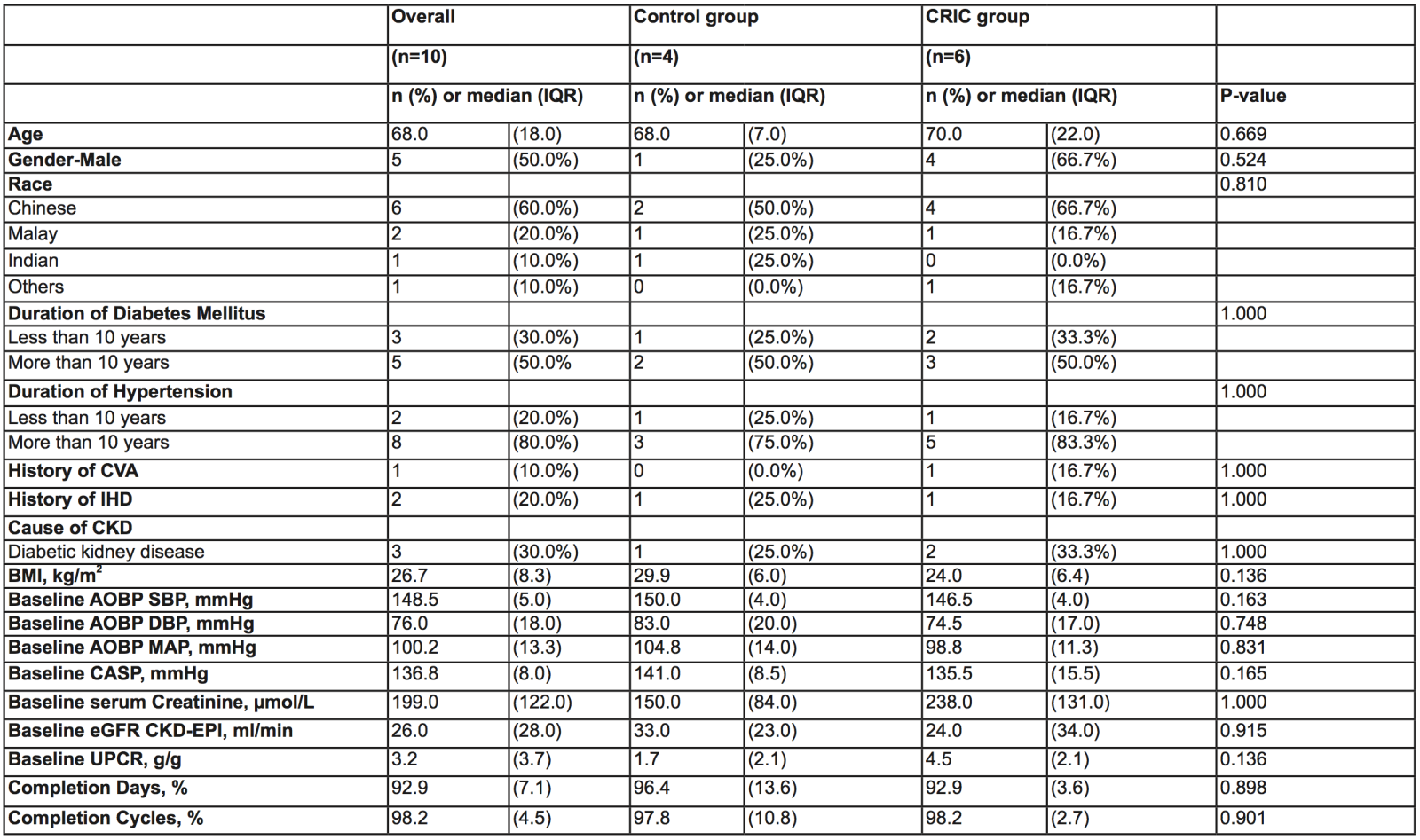
AOBP, automated office blood pressure; BMI, body mass index; CASP, central aortic systolic pressure; CKD, chronic kidney disease; CVA, cerebrovascular accident; DBP, diastolic blood pressure; IHD, ischemic heart disease; MAP, mean arterial pressure; SBP, systolic blood pressure; UPCR, urine protein-creatinine ratio.
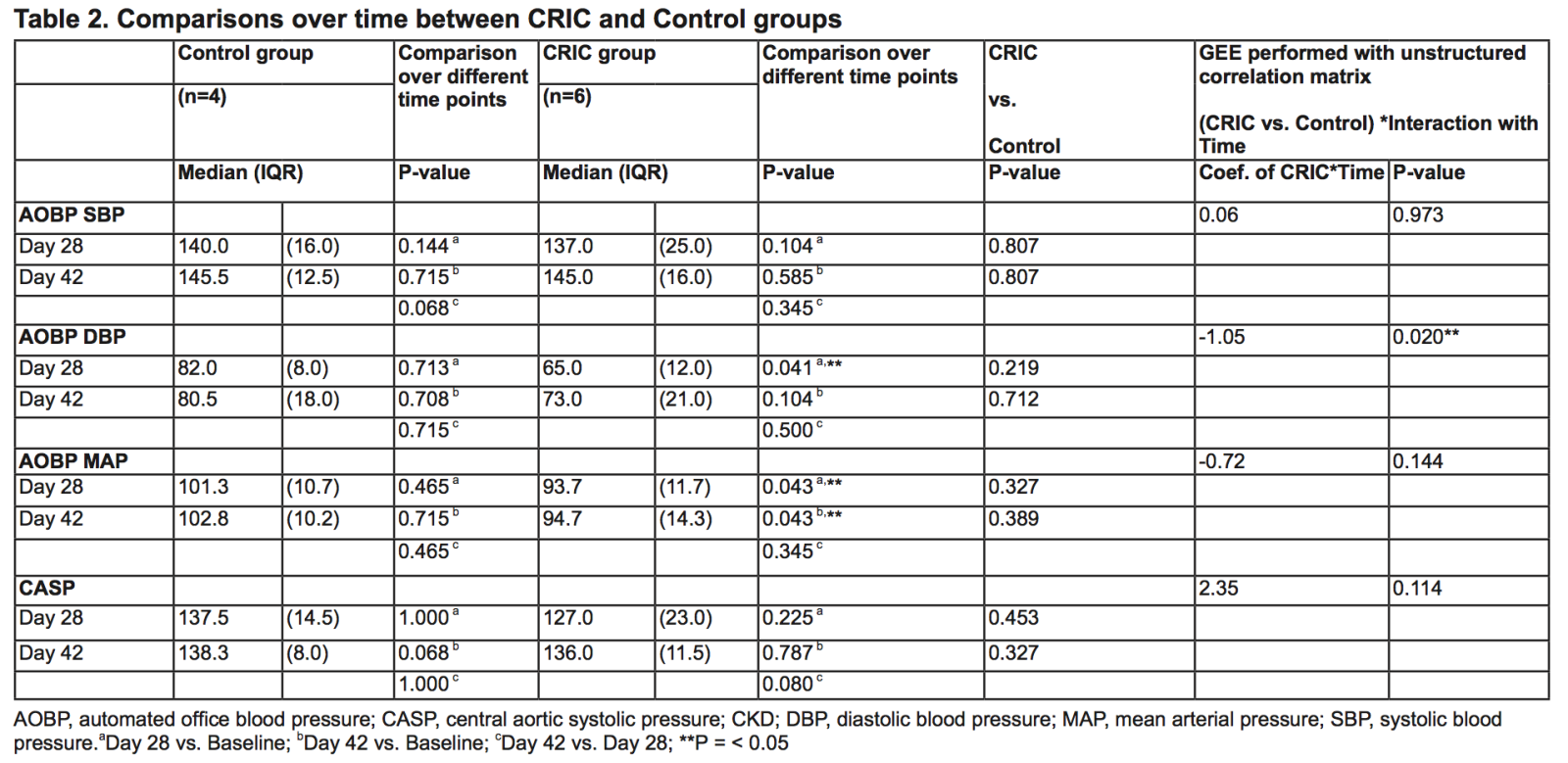
There were no differences in AOBP SBP or CASP between CRIC and control after either 28 or 42 days (Table 2). However, in the CRIC group, AOBP diastolic blood pressure (DBP) and mean arterial pressure (MAP) were significantly decreased after 28 days when compared to control, and AOBP MAP remained significantly lower after 42 days. In addition, GEE analysis of AOBP DBP comparing CRIC and control groups showed significant differences over time. Differences over time were not observed for GEE analyses of AOBP SBP and MAP. There were also no differences in spot UPCR, serum creatinine, eGFR CKD-EPI or inflammatory biomarkers (interleukin-1α, interleukin-6, monocyte chemoattractant protein-1, and tumor necrosis factor- α) between CRIC and control groups at 28 days. No adverse events were detected in this study.
Table 2. Comparisons over time between CRIC and Control groups
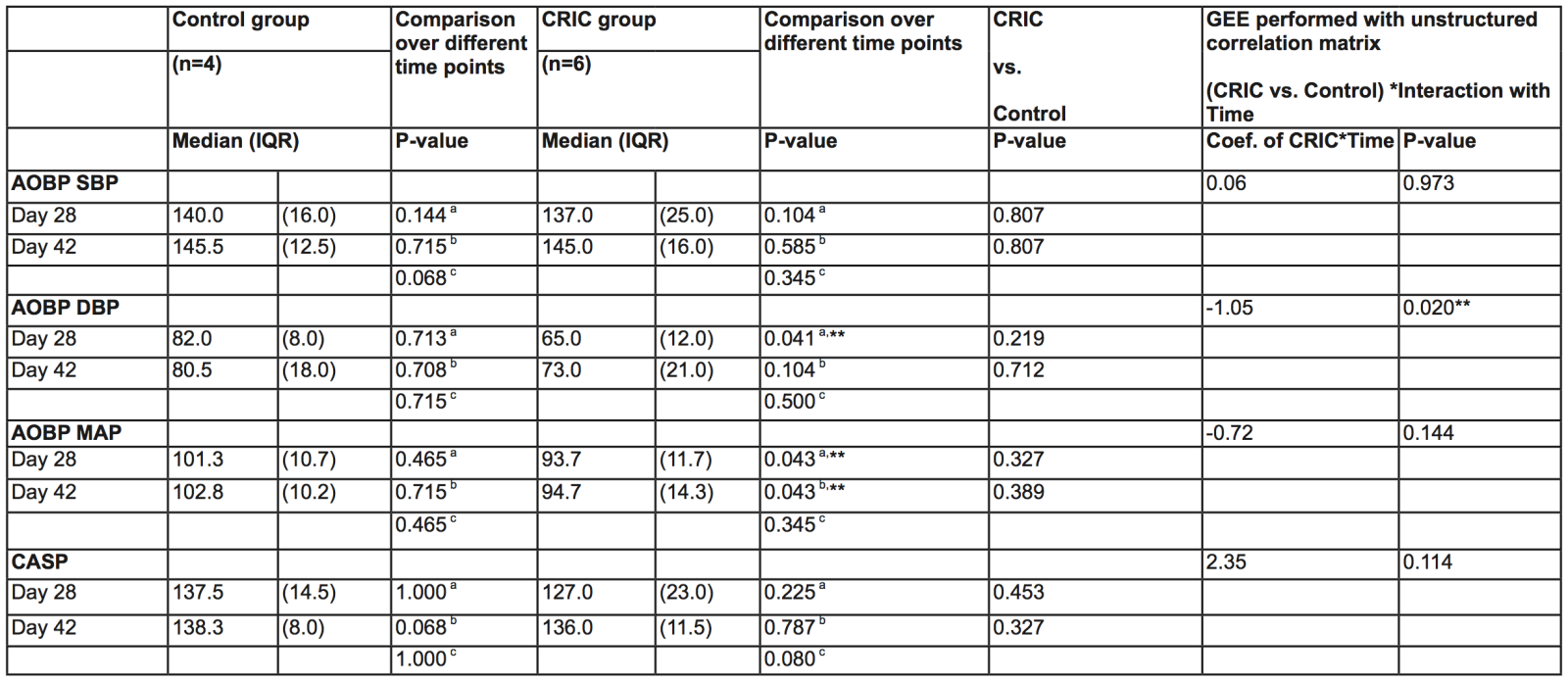
AOBP, automated office blood pressure; CASP, central aortic systolic pressure; CKD; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.aDay 28 vs. Baseline; bDay 42 vs. Baseline; cDay 42 vs. Day 28; **P = < 0.05
Discussion
In this proof-of-concept study, CRIC did not improve SBP control when compared to control treatment after 28 days, although there were significant reductions in DBP and MAP as measured via AOBP. The significance of this is uncertain in view of the small sample size. The lowest DBP was 60.0mmHg in the CRIC group (median baseline 74.5mmHg) after 28 days, which should not pose a safety issue considering that coronary artery filling is only affected at DBP levels <55mmHg. The overall reduction in MAP and DBP with CRIC warrants further investigation to understand if blood vessel compliance or cardiac output was temporarily altered. Interestingly, at day 42 (14 days after completion of CRIC), the MAP remained low signifying a potential legacy effect of CRIC. In addition, although RIC has anti-inflammatory benefits in vivo in animal models (Pearce et al., 2021), serum inflammatory biomarkers were not significantly different in our small cohort of patients. A larger study population would be necessary to validate these findings and confirm safety issues in terms of the observed reduction in DBP with CRIC.
Sources of Funding
This study was funded by the SingHealth Foundation research fund (SHF/CTG059/2016). DJH is supported by the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017), Centre Grant (CGAug16M006), and Collaborative Centre Grant scheme (NMRC/CGAug16C006). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
Disclosure
All authors declare no potential conflict of interest that might be relevant to the contents of this article.
References
Jason Chon Jun Choo1
1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore.
Jia Liang Kwek1
1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore.
Cynthia Ciwei Lim1
1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore.
Irene Yanjia Mok1
1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore.
Horng Ruey Chua2,10
2Division of Nephrology, Department of Medicine, National University Hospital, Singapore. 10Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Yilin Jiang3
3National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
Fei Gao3,4
3National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 4Programme in Health Services & Systems Research, Duke-NUS Medical School, Singapore.
Kearney Jun Yao Tan5
5Clinical Trials and Research Centre, Singapore General Hospital.
Gustavo Crespo-Avilan3,6,7
3National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 6Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore.7Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
Tazeen Hasan Jafar1,8,9
1Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore. 8Programme in Health Services & Systems Research, Duke-NUS Medical School, Singapore. 9Duke Global Health Institute, Duke University, Durham, NC, USA.
Derek J Hausenloy3,6,10,11,12
3National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 6Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 10Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 11The Hatter Cardiovascular Institute, University College London, London, United Kingdom.12Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Corresponding author:
Professor Derek J Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4854 | 14 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA