Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Animal models of peripheral arterial disease
Time:2021-12-28
Number:6620
Jasper Chua1,2, Gustavo E. Crespo-Avilan1,2,3, Elisa Liehn4,5,6, Sauri Hernandez-Resendiz1,2, Derek J. Hausenloy1,2,7,8,9
Author Affiliations


- 1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 2Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
- 3Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
- 4Department of Intensive Care and Intermediate Care, University Hospital, RWTH Aachen University, Aachen, Germany.
- 5Department of Cardiology, Angiology and Intensive Medicine, University Hospital Aachen, Aachen, Germany.
- 6Human Genetic Laboratory, University for Medicine and Pharmacy, Craiova, Romania.
- 7Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 8The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, United Kingdom.
- 9Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Conditioning Medicine 2021. 4(5): 209-215.
Abstract
Peripheral arterial disease (PAD) affects >200 million people worldwide and is associated with significant mortality, morbidity, and healthcare costs. It results from atheroma plaque build-up in the lower limb arteries restricting blood flow to the leg and foot muscles, leading to intermittent claudication, and complications such as wound ulcers/infections and chronic limb-threatening ischemia, which can progress to gangrene and lower extremity amputation. As such, new treatments are needed to prevent the onset and progression of PAD in order to improve health outcomes in patients with PAD. In this regard, animal models of PAD are essential for studying the complex pathophysiological mechanisms underlying this condition and for identifying and validating novel treatment targets for PAD. In this short review, we describe two animal models based on endothelial denudation in the peripheral vasculature, which recapitulate the vascular pathophysiology observed in PAD patients. We discuss their benefits and shortcomings and how these models may be improved to be more clinically relevant, for example including vascular risk factors such as diabetes in animal PAD models. The use of more clinically relevant animal models of PAD will facilitate the identification and the validation of novel treatment targets for preventing the onset and progression of PAD and will result in improved outcomes in patients with PAD.
Keywords: Atherosclerosis, Peripheral arterial disease, Wire-injury model, Balloon-injury model, Diabetes.
Abstract
Peripheral arterial disease (PAD) affects >200 million people worldwide and is associated with significant mortality, morbidity, and healthcare costs. It results from atheroma plaque build-up in the lower limb arteries restricting blood flow to the leg and foot muscles, leading to intermittent claudication, and complications such as wound ulcers/infections and chronic limb-threatening ischemia, which can progress to gangrene and lower extremity amputation. As such, new treatments are needed to prevent the onset and progression of PAD in order to improve health outcomes in patients with PAD. In this regard, animal models of PAD are essential for studying the complex pathophysiological mechanisms underlying this condition and for identifying and validating novel treatment targets for PAD. In this short review, we describe two animal models based on endothelial denudation in the peripheral vasculature, which recapitulate the vascular pathophysiology observed in PAD patients. We discuss their benefits and shortcomings and how these models may be improved to be more clinically relevant, for example including vascular risk factors such as diabetes in animal PAD models. The use of more clinically relevant animal models of PAD will facilitate the identification and the validation of novel treatment targets for preventing the onset and progression of PAD and will result in improved outcomes in patients with PAD.
Keywords: Atherosclerosis, Peripheral arterial disease, Wire-injury model, Balloon-injury model, Diabetes.
Introduction
Peripheral arterial disease (PAD) affects >200 million people worldwide and is associated with significant mortality, morbidity, and healthcare costs (Polonsky and McDermott, 2021). It results from atheroma plaque build-up in the lower limb arteries restricting blood flow to the leg and foot muscles, where it results in intermittent claudication, and complications such as wound ulcers/infections and chronic limb-threatening ischaemia, which can progress to gangrene and lower extremity amputation (Shu and Santulli, 2018; Polonsky and McDermott, 2021). As such new treatments are needed to prevent the onset and progression of PAD in order to improve health outcomes in patients with PAD.
The use of animal models has paved the way for many medical advances, often providing critical insights into disease mechanisms and management. For instance, in the case of PAD, post-angioplasty restenosis remains a major stumbling block. Proof-of-concept studies in small and large animal models have contributed tremendously to the understanding of the vascular response to injury, such as atherosclerosis, restenosis (Isoda et al., 2003; Morton et al., 2005), and neointimal formation (Chamberlain et al., 2010). Additionally, pre-clinical models have contributed to the discovery of new interventions such as drug-coated balloons (Scheller et al., 2003), which eventually led to clinical trials (Scheinert et al., 2014; Tepe et al., 2015; El Sayed et al., 2016) and subsequent FDA approval. This mini-review summarizes two animal models that recapitulate the pathophysiology of human PAD through endothelial denudation and stretch in the femoral artery (FA). The inclusion of vascular risk factors such as diabetes mellitus (DM) (Fowkes et al., 2013; Eraso et al., 2014) in animal PAD models will improve the clinical relevance of these models and facilitate the identification and validation of novel treatment targets for preventing the onset and progression of PAD, and result in improved outcomes in patients with PAD.
Limb revascularization using techniques such as angioplasty significantly lower the risk of acute ischemic complications of PAD but are often complicated by post-angioplasty restenosis due to neointimal hyperplasia – the prototypic response to vascular injury (Vartanian and Conte, 2015). An inflammatory response ensues, and is followed by the activation of vascular smooth muscle cells (VSMCs), resulting in a proliferative lesion with subsequent elaboration of extracellular matrix and fibrosis (Vartanian and Conte, 2015). The resultant neointimal hyperplasia often leads to restenosis, where 30 to 40% of the patients experience restenosis within two years of angioplasty (Rymer and Jones, 2018). Thus, prevention of restenosis remains one of the greatest unmet needs in PAD patients.
Despite extensive translational research, preclinical studies have yet to translate to clinical trials due to several limitations of PAD animal models. These include the inconsistencies in the surgical approach used, the outcome measurements employed, the animal species, and the lack of concomitant conditions seen in PAD in humans (Krishna et al., 2016). There are two widely used models to investigate post-angioplasty restenosis – (1) wire-injury model and (2) balloon-injury model (Gasper et al., 2013; Takayama et al., 2015). They employ surgical techniques to induce endothelial denudation and arterial stretch in the FA. These models have provided insights into the cell biology of post-angioplasty restenosis, and a platform to test therapeutic compounds against neointimal hyperplasia. These models also provide a means to assess the effects on restenosis following endothelial denudation. The femoral wire-injury model requires an arteriotomy in the muscular branch of the FA to produce endothelial denudation. The balloon-injury model involves using an angioplasty balloon to overstretch an artery. Following either method of arterial injury, they recapitulate the pathological hallmark of PAD, neointimal hyperplasia and restenosis (Gasper et al., 2013; Takayama et al., 2015)
The femoral wire-injury model
Mechanistic insights of neointimal hyperplasia development were mostly derived from studies using the wire-injury model (Sata et al., 2000; Han et al., 2001; Kang et al., 2004; Wang et al., 2006; Chen et al., 2015; Afzal et al., 2016; Baek et al., 2017; Baek et al., 2018; Yang et al., 2018; Mori et al., 2019). The rat arterial injury model was first established in the late 1970s (Clowes et al., 1977) using a flexible wire (Lindner et al., 1993), and then subsequently used in mice. Originally, a mouse model of endothelial denudation was established in the common carotid artery (CA) with a flexible wire under a dissecting microscope (Lindner et al., 1993) but is now mainly induced in the FA (Sata et al., 2000). Although the larger CA provides easier access, the neointima formation tends to be less robust as compared to FA, likely due to ineffective denudation. Conversely, the FA wire-injury models are highly reproducible, particularly since the wire for inducing FA injury is commercially manufactured and available (Takayama et al., 2015). More importantly, given that the CA and FA are structurally different, there would be differences in the vascular remodeling response (Moser et al., 2016), and vascular injury at the FA would better reflect the femoro-popliteal lesions commonly seen in PAD patients (Shu and Santulli, 2018).
Briefly, a straight spring wire (0.38 mm diameter) is inserted into the FA for a minute to denude the endothelium and enlarge the vessel. After which, the wire is removed and the distal FA is ligated at the point of bifurcation to restore blood flow to the FA (Takayama et al., 2015). Two hours following the transluminal injury, fibrin and aggregated platelets are deposited at the site of wire insertion. After a week, immunohistochemistry revealed that hematopoietic cells accumulate, marking peak inflammatory cell infiltration, resulting in neointimal hyperplasia consisting of VSMCs (Shoji et al., 2004). Leukocyte infiltration at this time is largely mediated by myeloid-related protein (MRP)-14 as shown via immunohistochemical analysis, eventually contributing to neutrophil- and monocyte-dependent vascular inflammation and proliferation of VSMCs (Croce et al., 2009). Further histological analyses showed that tumor necrosis factor alpha (TNF-α) was important in the inflammatory fibroproliferation (Zimmerman et al., 2002). After four weeks, a concentric, homogenous neointimal lesion can be observed using hematoxylin and eosin staining (Sata et al., 2000), with accumulation of leukocyte and adhesion molecules on the denuded luminal surface as revealed using immunohistochemistry (Roque et al., 2000) (Figure 1). The VSMCs contributing to the neointimal hyperplasia are largely derived from bone marrow (BM)-derived progenitors (Han et al., 2001). Vessel injury activates stem cell factor (SCF, or Steel factor) that is critical for the mobilization of BM-derived progenitors to the site of injury, which in turn mediates the differentiation of c-Kit+ progenitor cells to VSMCs (Wang et al., 2006).
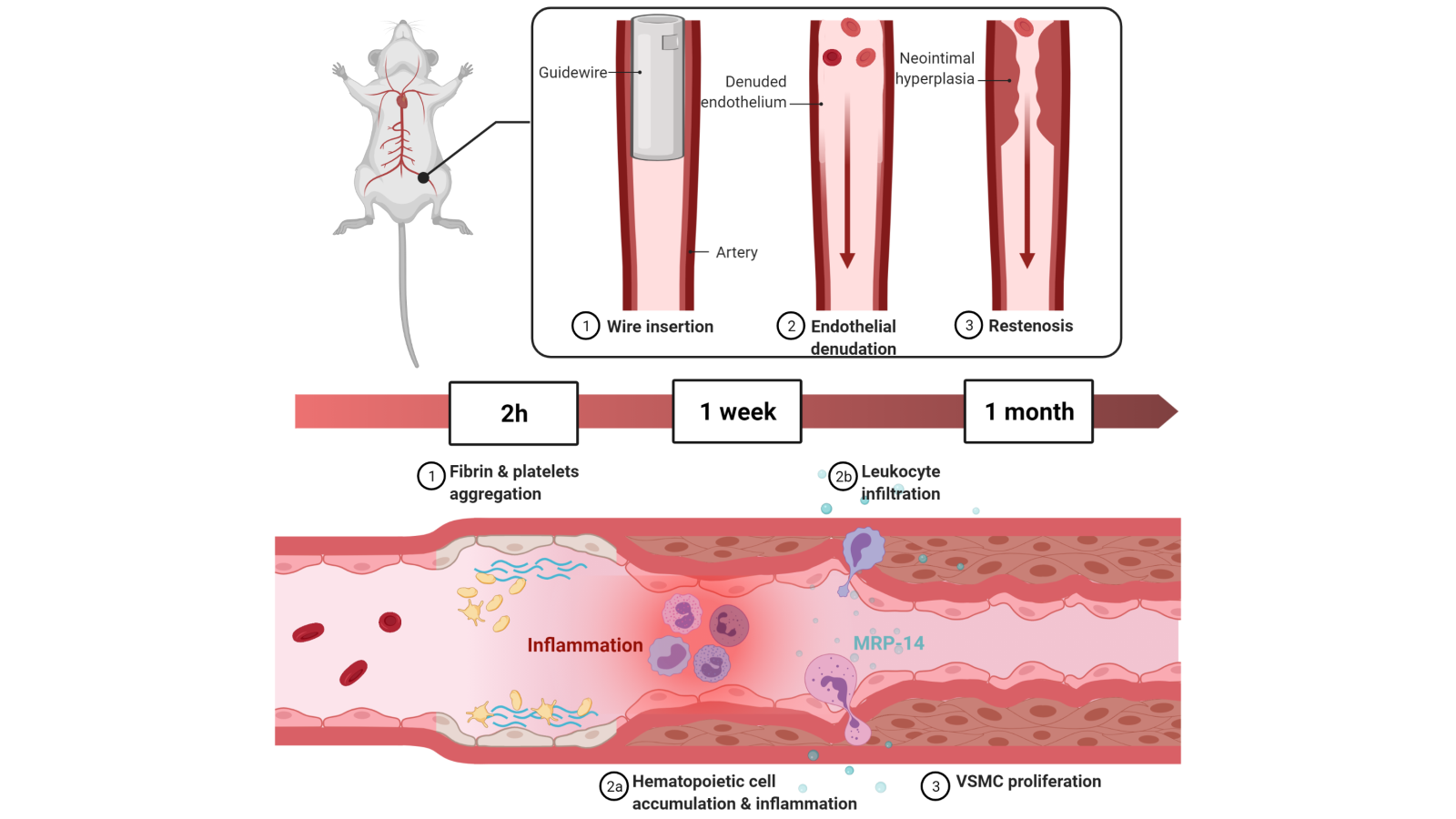
In a new window | Download PPT
Figure 1: The murine femoral artery (FA) wire-injury model involves insertion of a wire into the FA to induce endothelial denudation. Within two hours, fibrin and platelets aggregate at the site of vascular injury. Inflammation peaks at one week with accumulation of hematopoietic cells along with the development of neointimal hyperplasia. Neointimal hyperplasia is composed of vascular smooth muscle cells (VSMCs) that mostly originate from bone marrow (BM)-derived progenitors. Leukocyte infiltration at this point is largely mediated by myeloid-related protein (MRP)-14 that modulates neutrophil- and monocyte-dependent vascular inflammation and VSMC proliferation. After a month, a concentric homogenous neointimal lesion can be seen.
Benefits of this model
The impact of rodent models lies in the availability of transgenesis and tools for genetic manipulation, offering an attractive platform to investigate cellular and molecular events compared to large animal models (Curaj et al., 2015). Previous studies suggested that insulin and insulin receptors are largely involved in the increased restenosis risk in DM and insulin resistance (Bornfeldt et al., 1992). More recently, using genetic engineering (knockout) and wire-injury murine models, a study has elucidated a main player in VSMC proliferation in diabetic post-angioplasty restenosis. It demonstrated that the actions of insulin through the homozygous insulin receptor, not the previously thought insulin growth factor receptor, accelerated neointimal hyperplasia in DM and insulin resistance (Li et al., 2019).
Given the many genetic engineering strategies established in mice, the use of mice would allow for gain- and loss-of-function studies to provide mechanistic insights or elucidate potential drug targets. For instance, several microRNAs were found to confer protection against post-injury neointimal hyperplasia. In injured vessels, miR-34a expression is downregulated; gain/loss-of-function assays showed that miR-34a could reduce VSMC proliferation through the repression of Notch1 (Chen et al., 2015). Similarly, miR-214 was shown to reduce VSMC proliferation, migration, and actin polymerization through inhibition of NCK associated protein 1 (NCKAP1) – a mediator for lamellipodia formation and cell motility (Afzal et al., 2016). In addition, miR-22 was able to reverse pathological VSMC proliferation, and prevent post-angioplasty neointimal hyperplasia by modulating several target genes – MECP2, HDAC4, and EVI1 (Yang et al., 2018).
Rodent models have also provided valuable insights into the molecular mechanisms of post-angioplasty restenosis. Leukocytes were known to be important in the development of neointimal hyperplasia. Using the wire-injury model, 5-lipoxygenase in monocytes was found to contribute to neointimal hyperplasia development by modulating the monocyte-macrophage differentiation (MMD). The resulting macrophage infiltrated the neointima, contributing to post-injury vascular remodeling (Baek et al., 2017). Similarly, leukotriene signaling in monocytes was shown to be essential for MMD via high-mobility group box 1 (HMGB1), which in turn contributed to neointimal hyperplasia (Baek et al., 2018).
Rodent models, being widely available, provided a suitable platform for drug studies. Luseogliflozin, a sodium-glucose co-transporter 2 (SLGT2) inhibitor, was found to attenuate neointimal hyperplasia in mice fed a high-fat diet, in part through the suppression of macrophage platelet-derived growth factor-B (PDGF-B) (Mori et al., 2019). Since SGLT2 inhibitors are used to manage type 2 DM (Tat and Forest, 2018), the beneficial effects seen in neointimal hyperplasia is particularly exciting given the association of PAD with DM.
Lastly, the wire-injury model is relatively quick to generate, with the wire-injury procedure taking about 20 min, and the ensuing neointimal hyperplasia peaking after 3 to 4 weeks. Furthermore, as mentioned earlier, the commercially manufactured guidewires for FA wire-injury models provide greater robustness and high reproducibility of the model (Takayama et al., 2015).
Limitations of this model
Rodent models have inherent anatomical and physiological differences that may impact the interpretation of research findings. For instance, rodents do not develop atherosclerosis naturally, nor do they have strokes, myocardial infarction, or claudication (Kent and Liu, 2004). Structurally, murine arterial intima has only endothelium overlaying the internal elastic lamina, and lacks the smooth muscle cells or connective tissues found in human arterial intima. Murine tunica media is also thinner, and murine artery lacks the vasa vasorum (Lee et al., 2017). The small arteries of rodents also mean that it is impossible to test human-like endovascular interventions in these models. For such studies, the balloon-injury model would be more suitable. Moreover, although the techniques are highly reproducible, different studies have employed varying methodology (Ebert et al., 2021), making it hard to compare results and draw meaningful interpretations between studies. Also, since the wire-injury model is technically challenging, a single operator should perform the technique on all animals of a single trial to avoid large discrepancies and variations.
Future directions with this model
Many post-angiography restenosis studies have used the wire-injury model that was first established by Sata et al. (2000). This murine model has since seen some improvements. To better recapitulate advanced atherosclerotic plaques, more extensive vascular damage can be induced. This is achieved by using a bigger diameter wire with no hydrophilic coating, repeatedly inserting and retracting the wire ten-times before leaving it in the FA for one minute. The additional steps led to the breakdown and rupture of elastic lamina along with hyperplasia neointimal contributed by adventitial lineage cells (Nomura-Kitabayashi and Kovacic, 2018).
Given that the risk of restenosis following femoropopliteal intervention is dependent on the anatomical severity of the lesions (Iida et al., 2020), the absence of a preceding atherosclerotic lesion in most arterial injury studies may result in variations in the resultant neointimal hyperplasia. Animal models should mimic these anatomical severities in order to provide a better chance at translating from benchtop to bedside. A preceding atherosclerotic lesion in mice can be induced using diet or genetic modification (Lee et al., 2017) prior to arterial injury (i.e. double injury). Nonetheless, the murine atherosclerosis may differ from that in humans, particularly since the murine lesion lacks a thick fibrous cap (Lee et al., 2017). With the advent of blastocyst complementation, perhaps chimeric mice containing human blood vessels can be created to better represent the post-angioplasty restenosis. Though it has never been done, human blood vessels are able to make connections with mouse blood vessels (Tsukada et al., 2021). Furthermore, chimeric mice with pluripotent stem cell-derived vascular endothelial cells have also been successfully made (Hamanaka et al., 2018).
Balloon-injury model
In terms of the process of inducing vascular injury, the balloon-injury model is most similar to clinical settings (Figure 2). It uses larger animal models compared to mice, such as rats (Fingerle et al., 1990; Indolfi et al., 1995; Indolfi et al., 2001), rabbits (Griese et al., 2003), and swine (Gasper et al., 2013) due to easier access to the blood vessels. The porcine models are more clinically relevant for PAD as it induces the vascular injury at the FA instead of the carotid artery as seen in rats and rabbits. It should also be noted that the latex balloon used in rats significantly overstretches an artery as compared to PVC balloon angioplasty, which maintains a balloon-to-artery ratio of 1:2:1. Furthermore, the resulting lesion lacks several features to that seen in humans such as calcium deposits (Lafont and Faxon, 1998).
.png)
In a new window | Download PPT
Figure 2: The murine femoral artery (FA) wire-injury model involves insertion of a wire into the FA to induce endothelial denudation. Within two hours, fibrin and platelets aggregate at the site of vascular injury. Inflammation peaks at one week with accumulation of hematopoietic cells along with the development of neointimal hyperplasia. Neointimal hyperplasia is composed of vascular smooth muscle cells (VSMCs) that mostly originate from bone marrow (BM)-derived progenitors. Leukocyte infiltration at this point is largely mediated by myeloid-related protein (MRP)-14 that modulates neutrophil- and monocyte-dependent vascular inflammation and VSMC proliferation. After a month, a concentric homogenous neointimal lesion can be seen.
In 2013, Gasper et. al. (2013) established two techniques to create porcine models for FA restenosis. Briefly, the first model (i.e. single-injury model) involved using an angioplasty balloon to overstretch (40 to 60%) 4 centimeters of the proximal superficial FA. To induce great injury, three inflations, 30 seconds each, were done. The second model (double-injury model) was similar but diseased substrate was induced prior to the overstretch. First, endothelial denudation was achieved by using a compliant embolectomy balloon inflated to 2 atm, and dragged three times through the entire length of the target superficial FA. After two weeks, balloon angioplasty was performed to overstretch (by 20 to 30%) 4 centimeters of the proximal and distal superficial FA. Similar to the first model, three inflations, 30 seconds each, were performed (Gasper et al., 2013). The double-injury model resulted in a higher degree of neointimal hyperplasia without differences in the extent of medial fibrosis or inflammation as compared to the single-injury model. Using the single-injury model, it was found that nab-rapamycin was able to reduce lumen area stenosis, and medial fibrosis (Gasper et al., 2013). This double-injury model was recently adapted to investigate the effects of direct injection of sirolimus nanoliposomal formulation (Nanolimus) via infusion catheter (Bullfrog catheter) on the inflammatory response in post-angioplasty vascular injury. The study yielded promising results where Nanolimus led to significant reduction in neointima area, and luminal stenosis (Ang et al., 2020). Although the double-injury model requires an additional surgical procedure, and hence time, the model results in two injury sites per FA.
Benefits of this model
The main advantage of using swine compared to rodent is that the induced lesions closely resemble the post-angioplasty neointimal hyperplasia observed in humans, and their severity is proportional to the injury. Physiologically, swine has a similar circulating cholesterol make-up – 60% low-density lipoproteins, 38% high-density lipoprotein – compared to humans. Unlike rodents, swine can develop atherosclerosis spontaneously. Furthermore, swine have similar hemodynamic parameters as compared to humans (Lelovas et al., 2014). More importantly, the porcine model produces neointimal hyperplasia similar to human restenotic neointima with regards to cell size, density, and histopathological presentation (Gasper et al., 2013).
Limitations of this model
The procedures to induce balloon-injury models in swine require a catheterization laboratory with a dedicated team of anesthetists and anesthesiologist. Furthermore, the cost of maintaining large animals is significantly higher, with greater burden for husbandry and housing. Lastly, genetic engineering can be more challenging in swine as compared to mice.
Future directions with this model
Larger animal models such as swine provide greater vascular access to investigate promising endovascular interventions. Additionally, a recent study has shown the feasibility of creating chimeric swine with human endothelial cells (Das et al., 2020). Unfortunately, the use of large animal models may be hampered by the logistical issues as mentioned earlier. Small animal models could be used for initial proof-of-concept studies before translating it to larger animal models, and eventually into humans. With technological advancements, perhaps small animal models could be generated that better recapitulate human post-angioplasty restenosis.
Diabetic animal models of PAD
The prevalence of type 2 DM has been increasing in many parts of the world, in part due to rapid urbanization, unhealthy eating, and sedentary lifestyles. DM is a leading cause of human suffering and deaths (Khan et al., 2020). It is associated with increased risk of PAD (Fowkes et al., 2013), with a recent multicenter retrospective showing an inverse association between diabetes and dialysis with the severity of femoropopliteal lesions (Takahara et al., 2020). Importantly, DM is associated with higher risk and accelerated rate of post-angioplasty restenosis (Gilbert et al., 2004; Fujita et al., 2020; Pan et al., 2021). The increased risk is in part due to (1) metabolic perturbations leading to endothelial dysfunction, (2) increased inflammation and proliferation by insulin, or glucose, and (3) insufficient VSMC apoptosis (Lv et al., 2013). To investigate the effects of post-angioplasty restenosis in diabetes, rabbits (Sligar et al., 2019) or rodents are widely used with the latter being more popular. Diabetic rodent models have been widely used, and their advantages and disadvantages were extensively reviewed previously, (Goldberg and Dansky, 2006; Al-Awar et al., 2016). Studies on post-angioplasty restenosis in DM have used both, genetic models and chemically-induced models (Gonzalez-Navarro et al., 2007). However, due to the mechanism in which DM is induced, different models may yield differing results. For instance, balloon-injury caused increased neointimal hyperplasia (> 200%) in obese Zucker rats (experimental type II diabetes) compared to lean Zucker rats (Park et al., 2001). Conversely, post-angioplasty neointimal hyperplasia by wire-injury was attenuated significantly (~90%) in leprdb/db diabetic mice (experimental type II diabetes) compared to nondiabetic wild-type mice (Stephenson et al., 2003).
DM models are not limited to rodents. Several laboratories have successfully induced type 1-like diabetes with the injection of streptozotocin, which destroys a significant proportion of insulin-producing pancreatic β-cells in Yorkshire domestic pigs. Diabetes combined with hypercholesterolemia increases atherosclerosis two-fold compared with hypercholesterolemia alone (Bentzon et al., 2014). The progress in pig genomics research has recently made it possible to create mini-pig versions of the genetic atherosclerosis models that have proved efficient in mice. That study used a single bolus intravenous injection of streptozotocin (STZ) to create diabetic swine to investigate sirolimus-eluting stent implantation. Post-angioplasty neointimal hyperplasia was shown to persist for at least six months (Zhang et al., 2007).
Summary, conclusions and future perspectives
Animal models remain an indispensable tool to pave the way for medical advancements. The advent of angioplasty has helped many PAD patients. However, the often-ensuing restenosis due to neointimal hyperplasia may complicate the success of angioplasty. The wire-injury and the balloon injury animal models are widely accepted and have been used to uncover insights into the disease, and its therapy. Nonetheless, despite many years of research, post-angioplasty restenosis remains prevalent. Promising results from pre-clinical trials failed to translate into clinics largely due to the limitations of the animal models. Rodent models have inherent differences when compared to their human counterpart, whereas the use of porcine models requires extensive logistics. Nonetheless, both models have their own advantages. The rodent models allow for genetic engineering to elucidate potential drug targets while the porcine models offer similar-sized arteries to test human-like endovascular interventions. Thus, both of these models remain invaluable to the field of PAD research. In the future, we envision the use of murine chimeras to recapitulate PAD better, and hopefully improve the translatability of preclinical results to clinical trials.
The femoropopliteal lesions were previously classified by the Trans-Atlantic Society Consensus (TASC). A recent large multicenter prospective study has proposed a new angiographic risk score to better predict the 12-month restenosis risk following femoropopliteal interventions in clinical settings (Iida et al., 2020). Though reproducible, the extent of vascular injury in the animal models likely varies between operators, and hence, studies. Perhaps by classifying the animal models according to the risk score, the models would provide more translatable insights, especially to specific patient subgroups.
In conclusion, the use of more clinically relevant animal models of PAD will facilitate the identification and validation of novel treatment targets for preventing the onset and progression of PAD and result in improved outcomes in patients with PAD.
Acknowledgements
Derek Hausenloy is Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006). This article is based upon work from National Research Foundation Competitive Research Program (NRF CRP25-2020RS-0001) and COST Action EUCARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). Sauri Hernandez-Resendiz is supported by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Young Individual Research Grant (OF-YIRG)-[NMRC/OFYIRG/0078/2018].
Conflict of Interest
The authors declare no conflicts of interest.
References
Jasper Chua1,2†
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
Gustavo E. Crespo-Avilan1,2,3†
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 3Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
Elisa Liehn4,5,6
4Department of Intensive Care and Intermediate Care, University Hospital, RWTH Aachen University, Aachen, Germany. 5Department of Cardiology, Angiology and Intensive Medicine, University Hospital Aachen, Aachen, Germany. 6Human Genetic Laboratory, University for Medicine and Pharmacy, Craiova, Romania.
Sauri Hernandez-Resendiz1,2*
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
Derek J. Hausenloy1,2,7-9*#
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 7Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 8The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, United Kingdom.9Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
†Co-authors have contributed equally. *Joint senior authors
Corresponding author:
Prof Derek J. Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg
Correspondence should be addressed to Prof Derek J. Hausenloy (derek.hausenloy@duke-nus.edu.sg).

In a new window | Download PPT
Figure 1: The murine femoral artery (FA) wire-injury model involves insertion of a wire into the FA to induce endothelial denudation. Within two hours, fibrin and platelets aggregate at the site of vascular injury. Inflammation peaks at one week with accumulation of hematopoietic cells along with the development of neointimal hyperplasia. Neointimal hyperplasia is composed of vascular smooth muscle cells (VSMCs) that mostly originate from bone marrow (BM)-derived progenitors. Leukocyte infiltration at this point is largely mediated by myeloid-related protein (MRP)-14 that modulates neutrophil- and monocyte-dependent vascular inflammation and VSMC proliferation. After a month, a concentric homogenous neointimal lesion can be seen.
.png)
In a new window | Download PPT
Figure 2: The murine femoral artery (FA) wire-injury model involves insertion of a wire into the FA to induce endothelial denudation. Within two hours, fibrin and platelets aggregate at the site of vascular injury. Inflammation peaks at one week with accumulation of hematopoietic cells along with the development of neointimal hyperplasia. Neointimal hyperplasia is composed of vascular smooth muscle cells (VSMCs) that mostly originate from bone marrow (BM)-derived progenitors. Leukocyte infiltration at this point is largely mediated by myeloid-related protein (MRP)-14 that modulates neutrophil- and monocyte-dependent vascular inflammation and VSMC proliferation. After a month, a concentric homogenous neointimal lesion can be seen.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6620 | 69 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA