Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
A comparison of common risk factors between aortic disease and coronary artery disease mortality
Time:2021-12-28
Number:4987
Ariel F Ying1, Shi Hui Cheng1, Aizhen Jin2, Tze Tec Chong3, Derek J Hausenloy1,4,5,6,7, Woon-Puay Koh2,8
Author Affiliations


- 1Duke-National University of Singapore Medical School, Cardiovascular and Metabolic Disorders Program, Singapore.
- 2Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Programme, National University of Singapore, Singapore.
- 3Singapore General Hospital, Department of Vascular Surgery, Singapore.
- 4National Heart Research Institute Singapore, National Heart Centre, Singapore.
- 5The Hatter Cardiovascular Institute, University College London, London, UK.
- 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
- 7Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 8Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore.
Conditioning Medicine 2021. 4(5): 244-250.
Abstract
Although aortic disease and coronary artery disease (CAD) often co-exist, it remains unclear if established risk factors of atherosclerosis affect both diseases equally. To assess if established atherosclerosis risk factors affect mortality from CAD, thoracic aortic aneurysms and dissections (TAADs), and abdominal aortic aneurysms (AAAs) differently in an Asian population-based cohort, we used data from the Singapore Chinese Health Study, which recruited 63,257 middle-aged and older Chinese people residing in Singapore from 1993-1998. Information on lifestyle and medical history was obtained at recruitment. CAD, TAAD, and AAA mortality was determined via linkage to the death registry through 31 Dec 2018. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were derived from subdistribution hazard models to account for competing risk. Current smoking was a much stronger risk factor for AAA mortality (HR: 5.27, 95% CI: 2.87-9.68) than for TAAD (HR: 2.04, 95% CI: 1.11-3.77) or CAD mortality (HR: 1.87, 95% CI: 1.72-2.03) (Ps for heterogeneity ≤ 0.031). Conversely, hypertension was a stronger risk factor for TAAD mortality (HR: 2.91, 95% CI: 1.76-4.80) than for AAA (HR: 1.23, 95% CI: 0.72-2.10) or CAD mortality (HR: 1.60, 95% CI: 1.49-1.71) (P values for heterogeneity ≤ 0.022). Diabetes increased CAD mortality risk (HR: 3.20, 95% CI: 2.96-3.46), but reduced TAAD (HR: 0.22, 95% CI: 0.05-0.89) and AAA mortality risk (HR: 0.23, 95% CI: 0.05-0.94) (P value for heterogeneity ≤ 0.0003). In conclusion, in this cohort, smoking, hypertension, and diabetes affect the risk of TAAD, AAA, and CAD mortality differently, suggesting differences in the pathogenesis of these three conditions.
Keywords: Thoracic aortic aneurysm, Aortic dissection, Abdominal aortic aneurysm, Coronary artery disease, Mortality, Cohort
Abstract
Although aortic disease and coronary artery disease (CAD) often co-exist, it remains unclear if established risk factors of atherosclerosis affect both diseases equally. To assess if established atherosclerosis risk factors affect mortality from CAD, thoracic aortic aneurysms and dissections (TAADs), and abdominal aortic aneurysms (AAAs) differently in an Asian population-based cohort, we used data from the Singapore Chinese Health Study, which recruited 63,257 middle-aged and older Chinese people residing in Singapore from 1993-1998. Information on lifestyle and medical history was obtained at recruitment. CAD, TAAD, and AAA mortality was determined via linkage to the death registry through 31 Dec 2018. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were derived from subdistribution hazard models to account for competing risk. Current smoking was a much stronger risk factor for AAA mortality (HR: 5.27, 95% CI: 2.87-9.68) than for TAAD (HR: 2.04, 95% CI: 1.11-3.77) or CAD mortality (HR: 1.87, 95% CI: 1.72-2.03) (Ps for heterogeneity ≤ 0.031). Conversely, hypertension was a stronger risk factor for TAAD mortality (HR: 2.91, 95% CI: 1.76-4.80) than for AAA (HR: 1.23, 95% CI: 0.72-2.10) or CAD mortality (HR: 1.60, 95% CI: 1.49-1.71) (P values for heterogeneity ≤ 0.022). Diabetes increased CAD mortality risk (HR: 3.20, 95% CI: 2.96-3.46), but reduced TAAD (HR: 0.22, 95% CI: 0.05-0.89) and AAA mortality risk (HR: 0.23, 95% CI: 0.05-0.94) (P value for heterogeneity ≤ 0.0003). In conclusion, in this cohort, smoking, hypertension, and diabetes affect the risk of TAAD, AAA, and CAD mortality differently, suggesting differences in the pathogenesis of these three conditions.
Keywords: Thoracic aortic aneurysm, Aortic dissection, Abdominal aortic aneurysm, Coronary artery disease, Mortality, Cohort
Introduction
Diseases of the aorta are largely asymptomatic and often present only when they rupture or dissect. However, more than half of the patients may die before they ever reach a hospital (Laine et al., 2017), which explains the high mortality rates of up to 80-90% for these diseases (Guirguis-Blake et al., 2019; Kent, 2014). Although primary care screening programs have been shown to reduce abdominal aortic aneurysm (AAA) mortality rates in Western countries (Guirguis-Blake et al., 2019), such screening programs are not routinely carried out in most Asian countries, including Singapore, possibly due to a low prevalence of AAAs in the general Asian population (Chan et al., 2021). However, primary prevention aimed at risk factor reduction for aortic diseases has been found to be the most cost-effective way of reducing its impact in the general population (Wong and Zajac, 2018).
In order to reduce aortic disease mortality in the population by primary prevention, it is necessary to elucidate the risk factors implicated in the pathogenesis of aortic diseases. Depending on the anatomical involvement, aortic diseases can be divided into those that affect the thoracic aorta, those that affect the abdominal aorta, and those that affect both regions. The majority of aortic diseases, particularly in the abdominal aorta, are thought to be related to atherosclerosis (Isselbacher, 2005), whereby arterial luminal stenosis arising from plaque deposition induces compensatory changes in the media and extracellular matrix to normalize the lumen diameter and reduce shear stresses, resulting in aortic dilatation (Golledge and Norman, 2010). On the other hand, aortic diseases that affect the thoracic aorta may present as dissection or aneurysm, and have been associated with genetic or acquired disorders in connective tissues or extracellular matrix such as Marfan syndrome (Jondeau et al., 2012).
Interestingly, although risk factors for aortic diseases are similar to those of other atherosclerotic diseases, such as coronary artery disease (CAD), differences in the associations of these risk factors with aortic diseases and CAD have been reported. For example, although smoking has been reported to be associated with the risk of aortic diseases (Aune et al., 2018), some studies have reported that the risk smoking confers on aortic aneurysms is far greater than the risk it confers on CAD (Lederle et al., 2003; Pirie et al., 2013; Pujades-Rodriguez et al., 2015; Stoekenbroek et al., 2016). In addition, in meta-analyses, diabetes mellitus, a strong CAD risk factor, was found to be inversely associated with the risk of aortic diseases (D'Cruz R et al., 2019; Takagi & Umemoto, 2015). In fact, in a study using ultrasound measurements, the investigators have reported a lack of dose-response relationship between atherosclerosis and abdominal aortic diameter, and suggested that atherosclerosis may not be a causal event in abdominal aortic aneurysms, but instead develops in parallel with or is secondary to aneurismal dilatation (Johnsen et al., 2010).
In addition, epidemiological studies have suggested that the risk factors for disease in the thoracic aorta and the abdominal aorta may also differ, although findings have been conflicting (Cho et al., 2014; Ito et al., 2008; Landenhed et al., 2015). For instance, different studies have reported positive or null associations between hypertension and abdominal aortic aneurysms (Ito et al., 2008; Landenhed et al., 2015), or between smoking and thoracic aortic aneurysms (Cho et al., 2014; Landenhed et al., 2015). In addition, a study has found patients with thoracic aortic aneurysms to have reduced systemic atherosclerosis (Achneck et al., 2005), while patients with abdominal aortic aneurysms appeared to have increased subclinical atherosclerosis (Laughlin et al., 2011). Furthermore, comparatively few studies have been done in Asian populations (Lawlor et al., 2008; Shirakawa et al., 2017). Hence, it is useful to gain insights on how risk factors related to atherosclerosis affect diseases of the aorta differently from CAD, and to further differentiate between risk factors for thoracic aortic aneurysms and dissections (TAADs) and those for abdominal aortic aneurysms (AAAs). In the present study, we examined prospectively the associations between traditional risk factors of cardiovascular disease and the risk of mortality from TAAD, AAA, and CAD in a large population-based cohort of middle-aged and older Chinese men and women in Singapore.
Materials and Methods
Study population
The Singapore Chinese Health Study is a large population-based prospective cohort study that recruited 63,257 Chinese participants aged 45 to 74 years old from April 1993 to December 1998 (Hankin et al., 2001). Study participants belonged to either of the two major dialect groups of Chinese people in Singapore (Hokkiens or Cantonese), and they were permanent residents or citizens who resided in government-built housing estates, where 86% of all Singaporeans resided during that period. This study was approved by the Institutional Review Board at the National University of Singapore and informed consent was received from all study participants.
Assessment of exposure variables
At recruitment, participants were interviewed by trained interviewers using a standardized questionnaire to collect information on demographic factors such as education levels, lifestyle factors including smoking and alcohol use, weight and height, and medical history. Cigarette smoking was assessed via the question “Have you ever smoked at least one cigarette a day for one year or longer?”, with the options being “no” for never smokers, “yes, but I quit smoking” for former smokers and “yes, and I currently smoke” for current smokers. Alcohol intake frequency was assessed by asking for separate intake of beer, rice wine, grape wine and hard liquor, along with eight categories of frequency ranging from “never or hardly ever” to “two or more times a day”. Body mass index (BMI) was calculated as weight in kilograms (kg) divided by height in meters squared (m2), based on self-reported height and weight. Medical history was assessed by asking participants whether they have ever been diagnosed by a physician to have diabetes, hypertension, and/or CAD (no, yes). Using standard protocols, we subsequently validated the accuracy of the self-reported, physician-diagnosed diabetes (Odegaard et al., 2008) and hypertension (Talaei et al., 2016) in this cohort.
Ascertainment of mortality cases
Study participants were followed up via linkage of the cohort database with the Singapore Registry of Births and Deaths through 31 December 2018 to determine the date and primary cause of death. The primary cause of death was coded using the International Statistical Classification of Diseases and Related Health Problems Ninth Revision (ICD-9) up until 31 December 2011, and its tenth revision (ICD-10) from 1 January 2012 to 31 December 2018. CAD mortality was determined using ICD-9 codes 410-414 and ICD-10 codes I20-I25. Aortic disease mortality was determined using ICD-9 codes 441.0-441.4 and ICD-10 codes I71.0-I71.4, and then further classified based on the site of pathology (thoracic, abdominal, thoracoabdominal, or unspecified). Cases with ICD-9 codes 441.0-441.2 and ICD-10 codes I71.0-I71.2 were classified as TAAD mortality, while cases with ICD-9 codes 441.3-441.4 and ICD-10 codes I71.3-I71.4 were classified as AAA mortality.
As of 31 December 2018, only 57 participants (0.09%) in this cohort were known to be lost to follow-up due to reasons such as migration out of Singapore. This suggests that vital statistics are virtually complete in this cohort.
Statistical analysis
Person-years for each participant was calculated from the date of baseline interview to the date of death, loss to follow-up or 31 December 2018, whichever came first. Since the same risk factors were investigated for CAD mortality, AAA mortality, and TAAD mortality, we used the Fine-Gray subdistribution hazard model to account for competing risk among the three mortalities (Fine & Gray, 1999) with the strength of a given association measured by the corresponding hazard ratios (HR), 95% confidence intervals (CI), and p value.
All regression models was adjusted for age at interview (years), sex (whole cohort analysis), dialect group (Cantonese, Hokkien), year of baseline interview (1993-1995, 1996-1998), education level (no formal education, primary school, secondary school and above), smoking status (non-smoker, ex-smoker, current smoker), frequency of alcohol consumption (less than once a week, at least once a week), BMI (< 23.0 kg/m2, 23.0 to < 25.0 kg/m2, ≥ 25.0 kg/m2), as well as history of diabetes, hypertension, and CAD (none or prevalent at baseline). Tests for trend were performed by treating BMI as a continuous variable. P value for heterogeneity was performed based on the comparison between pairs of hazard ratios (Altman and Bland, 2003).
All analyses were performed using SAS, version 9.4 (SAS Institute, Inc. Cary, NC). All p values presented are two-sided, and p < 0.05 was considered statistically significant.
Results
Baseline characteristics of participants are presented in Table 1. After a mean of 19.5 (standard deviation 6.5) years of follow up, we identified 4284 participants who had died of CAD and 157 participants who had died of aortic diseases, of which 75 were TAAD cases and 75 were AAA cases. Seven aortic disease cases were either not specified (n = 6) or had thoraco-abdominal disease (n = 1), and were not included in the analysis. Compared to CAD and TAAD, AAA cases were more likely to be men, significantly older at baseline interview, and older at death (p < 0.050).
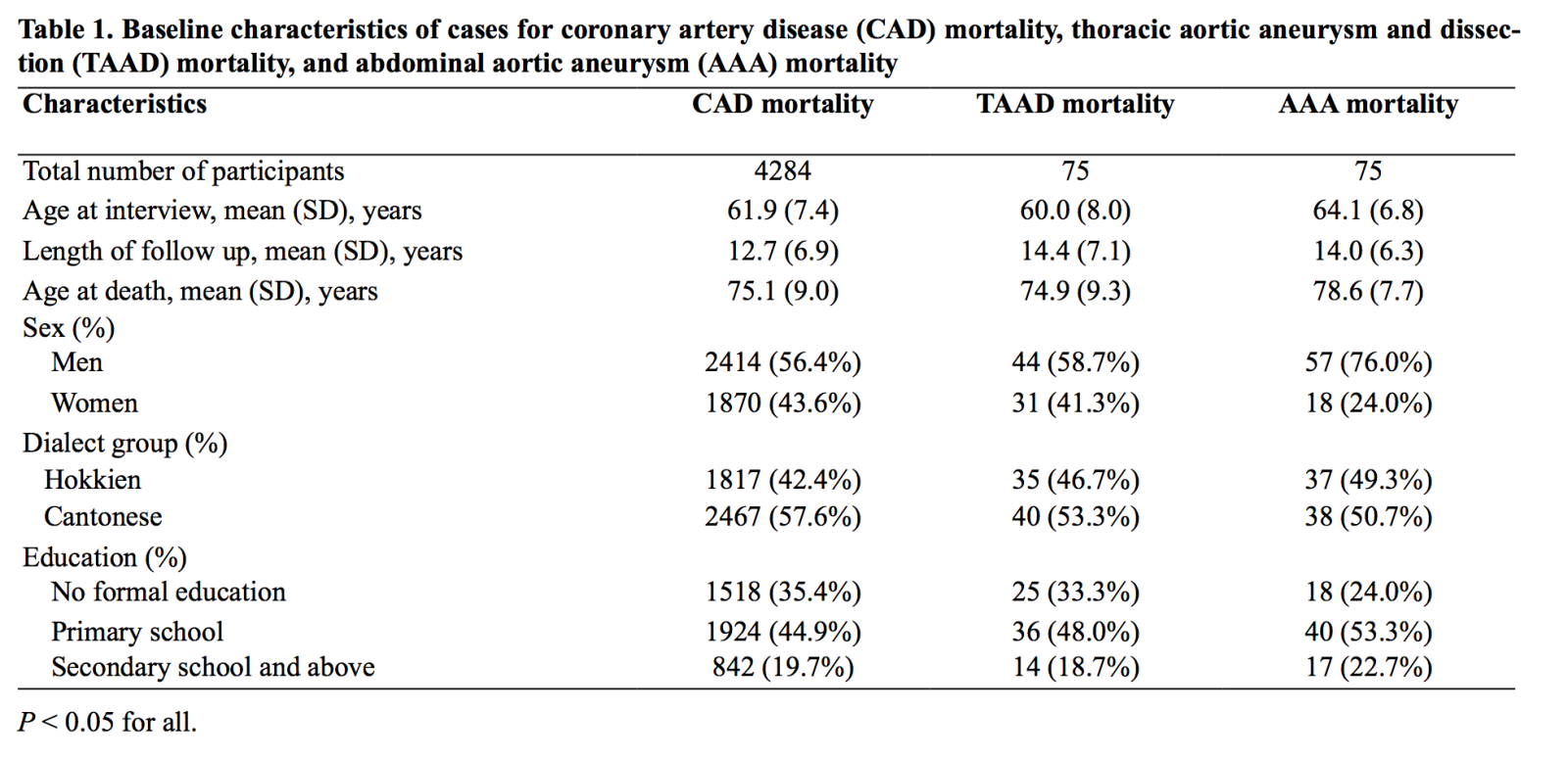
Table 2 compares the associations between risk factors of TAAD mortality, AAA mortality, and CAD mortality. Women were around half as likely as men to die from TAAD (HR, 0.49; 95% CI, 0.26-0.95), AAA (HR, 0.44; 95% CI, 0.23-0.83), and CAD (HR, 0.53; 95% CI, 0.49-0.58), without any significant difference in risk estimates among the three types of mortality (p value for heterogeneity ≥ 0.559). Compared to their leaner counterparts, those with BMI ≥ 23.0 kg/m2 had a modest increase in CAD mortality risk (p value for trend = 0.0001) and a non-statistically significant increase in TAAD mortality (p value for trend = 0.636) and AAA mortality (p value for trend = 0.235) (p value for heterogeneity ≥ 0.495). On the other hand, compared to less frequent drinking, consuming alcohol at least once a week conferred a modest reduction in risk of CAD mortality risk (HR, 0.80; 95% CI, 0.72-0.89), TAAD mortality risk (HR, 0.78; 95% CI, 0.36-1.71), and AAA mortality risk (HR, 0.72; 95% CI, 0.35-1.48) (p value for heterogeneity ≥ 0.786), although only the association with CAD reached statistical significance. Overall, our results suggested that the association between higher BMI and increased risk of aortic disease mortality, and the association between regular alcohol consumption and reduced risk of aortic disease mortality were similar to the corresponding associations between these two factors and risk of CAD mortality.
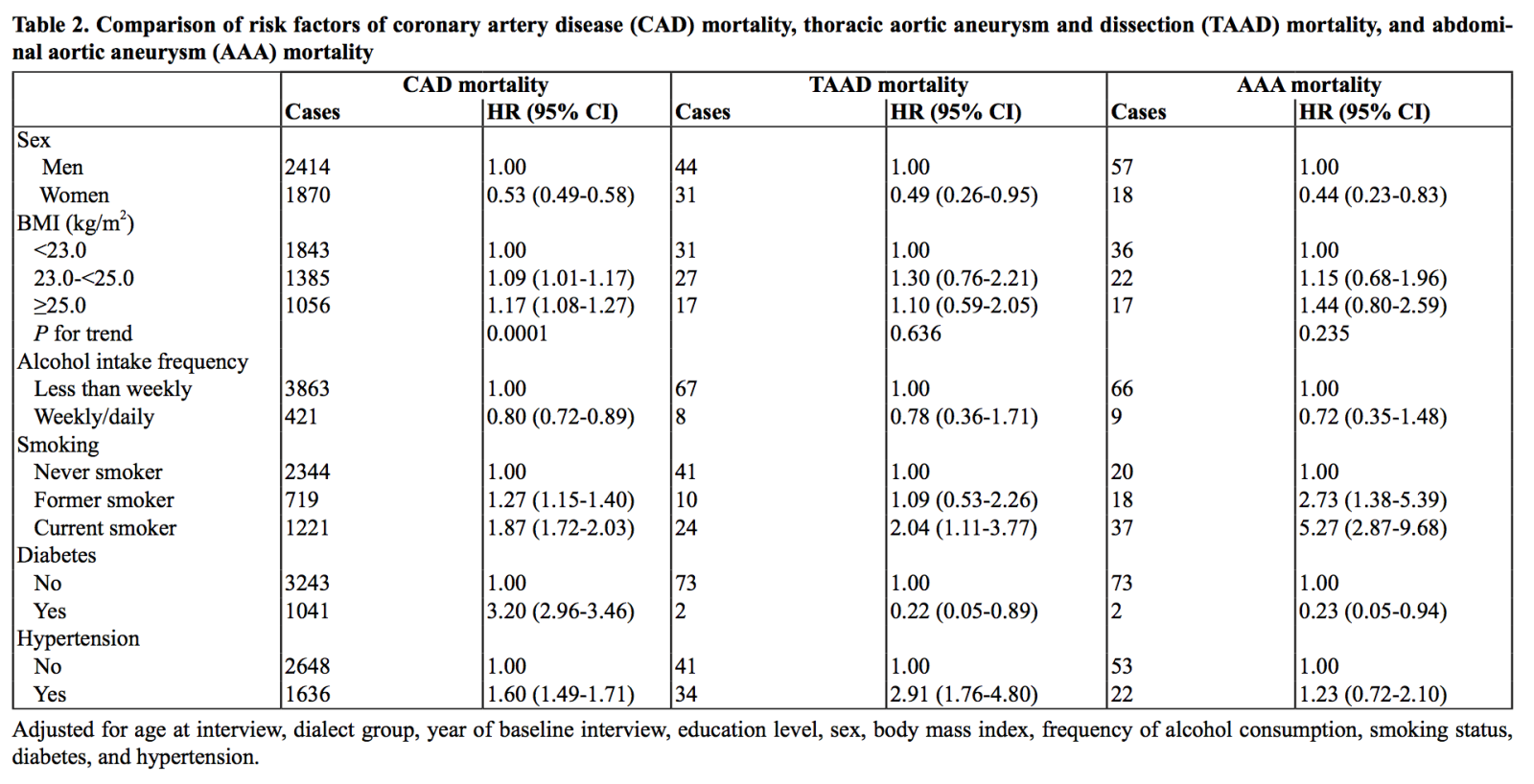
Compared to never smokers, current smokers had a much greater increase in risk for AAA mortality (HR, 5.27; 95% CI, 2.87-9.68) than for CAD mortality (HR, 1.87; 95% CI, 1.72-2.03) and TAAD mortality (HR, 2.04; 95% CI, 1.11-3.77) (p value for heterogeneity ≤ 0.031). Although this risk was attenuated in former smokers, this subgroup still had a greater risk of AAA mortality (HR, 2.73; 95% CI, 1.38-5.39) compared to that of CAD mortality (HR, 1.27; 95% CI, 1.15-1.40) (p value for heterogeneity = 0.029).
In the investigation of medical history at recruitment, hypertension was associated with a greater risk of TAAD mortality (HR, 2.91; 95% CI, 1.76-4.80) as compared to CAD mortality (HR, 1.60; 95% CI, 1.49-1.71) and AAA mortality (HR, 1.23; 95% CI, 0.72-2.10) (p value for heterogeneity = 0.020 for both). In contrast, diabetes was associated with an increase in risk of CAD mortality (HR, 3.20; 95% CI, 2.96-3.46) but a reduction in risk of both TAAD mortality (HR, 0.22; 95% CI, 0.05-0.89) and AAA mortality (HR, 0.23; 95% CI, 0.05-0.94) (p value for heterogeneity ≤ 0.0003).
Discussion
In this prospective population-based Asian cohort, although smoking, hypertension, and increased BMI were positive risk factors for CAD, AAA, and TAAD mortality, and drinking alcohol regularly was associated with reduced risk of mortality from all three diseases, there were significant differences in the risk estimates. Smoking was associated with a much greater risk of AAA mortality than CAD mortality, while hypertension was associated with a much greater risk of TAAD mortality than CAD mortality. In contrast, diabetes was associated with an increased risk of CAD mortality but a reduced risk of both TAAD and AAA mortality.
Comparison to other observational studies
In the examination of risk factors for TAAD, our study concurs with the observation that the male sex is a risk factor (Shen et al., 2020). In addition, most epidemiological studies also concur with our findings that diabetes does indeed confer a reduced risk for TAAD; indeed, a meta-analysis on ten studies reported an odds ratio (OR) of 0.77 (95% CI, 0.61-0.98; p = 0.03) for the association between diabetes and TAAD (D'Cruz R et al., 2019). Prospective studies on the associations between other risk factors and TAAD are scarce. To the best of our knowledge, only one study had prospectively examined the associations between alcohol and TAAD (Shirakawa et al., 2017), and it was in agreement with our results that light alcohol consumption conferred a reduced risk of TAAD, with a multivariate-adjusted HR of 0.16 (95% CI, 0.05-0.50). In the Swedish Malmö Diet and Cancer study (Landenhed et al., 2015), the authors prospectively examined the effects of smoking and hypertension on thoracic aortic aneurysms, aortic dissections, and abdominal aortic aneurysms, and reported a positive association between current smoking and the risk of both aortic dissection (HR, 1.91; 95% CI, 1.12-3.25; p = 0.02) and thoracic aortic aneurysm (HR, 2.20; 95% CI, 1.20-40.1; p = 0.01), although these risk estimates were lower than those for the risk of AAA (HR, 5.13; 95% CI, 3.49-7.54; p < 0.0001), which concurred with our results. Similarly, in two studies that measured the diameters in various regions of the aorta via computed tomography (Cho et al., 2014; Rogers et al., 2013), smoking was associated with a larger increase in diameter of the abdominal aorta than of the thoracic aorta. The Malmö Diet and Cancer study (Landenhed et al., 2015) also reported a positive association between hypertension and aortic dissection (HR, 3.37; 95% CI, 1.51-7.55) that was higher than that for AAA (HR, 2.21; 95% CI, 1.35-3.62) after multivariate-adjusted analysis. A strong association between hypertension and TAAD has also been reported by various other studies (Cho et al., 2014; Howard et al., 2013; Ito et al., 2008; Rogers et al., 2013), which is also our finding in the current study.
For the risk factors of AAA, our study also concurs with the general consensus that the male sex is a risk factor (Isselbacher, 2005; Shen et al., 2020). For the relationship between diabetes and AAA, most prospective cohort studies showed an inverse association, although several of them did not reach statistical significance, possibly due to the low prevalence of diabetes in their cohorts (1.4%-3.5%) (Iribarren et al., 2007; Wong et al., 2007) compared to our cohort (9.0%). Indeed, a meta-analysis of thirteen studies reported a negative association between diabetes and the risk of AAA (OR, 0.59; 95% CI, 0.52-0.67; p < 0.00001) (Takagi & Umemoto, 2015). Therefore, our findings support the general consensus that diabetes could indeed be a protective factor for AAA. Our finding of smoking being a stronger risk factor for AAA mortality than for CAD mortality is also consistent with most studies. In a systematic review of ten studies published in 2003, all the studies showed a stronger association between current smoking and aortic aneurysm mortality than CAD mortality in both men and women (Lederle et al., 2003). In recent large-scale prospective cohort studies, such as the Million Women Study (Pirie et al., 2013), the CALIBER program (Pujades-Rodriguez et al., 2015), and the EPIC-Norfolk Study (Stoekenbroek et al., 2016), the association between smoking and AAA incidence or mortality was approximately 1.5-fold to four-fold higher than the association between smoking and CAD incidence or mortality.
However, prospective studies on the effects of hypertension on AAA have been inconsistent. Although most cohort studies agree that hypertension is positively associated with AAA risk, with a recent meta-analysis of 21 cohort studies reporting a relative risk of 1.66 (95% CI, 1.49-1.85) (Kobeissi et al., 2019), two studies, which included non-fatal AAA, namely, the EPIC-Norfolk Study (Stoekenbroek et al., 2016) and the CALIBER program (Rapsomaniki et al., 2014), did not report any significant association between hypertension and AAA risk. Interestingly, sensitivity analysis in the CALIBER program that only included mortality cases revealed a 14% increase in risk of AAA for every 20 mmHg rise in systolic blood pressure, and a 76% increase in risk for every 10 mmHg rise in diastolic blood pressure, suggesting that hypertension may be associated with large AAAs that were fatal (Rapsomaniki et al., 2014).
Biological mechanisms
Our findings are supported by biological plausibility. The strong relationship between smoking and AAA could be explained by smoking enhancing metalloproteinases (MMPs) activity in the extracellular matrix by increasing expression of MMPs and downregulating MMP inhibitors, leading to extracellular matrix degradation (Newby, 2006). The latter, in turn, causes vascular smooth muscle cells (VSMCs) to migrate into the arterial lumen and proliferate to form atherosclerotic plaques, and disruptions of cell-matrix interactions necessary for VSMC survival, which could explain the scarcity of VSMCs in aortic aneurysm walls (Newby, 2006). Interestingly, while MMP-9 is produced by synthetically active VSMCs and fibroblasts in the thoracic aorta, MMP-9 is produced by macrophages in higher abundance in the abdominal aorta, which may explain why the thoracic aorta has been found to be more resistant to plaque formation induced by cigarette smoking than the abdominal aorta (Ruddy et al., 2008).
The stronger effect of hypertension on TAAD than on AAA mortality risk may also be explained by structural differences between the thoracic and abdominal aorta. The thoracic aorta is thicker, has more elastin content and is supplied by vasa vasorum, while the abdominal aorta is relatively thin with lesser elastin and is entirely avascular (Wolinsky & Glagov, 1969). Chronic hypertension has been found to limit the vasodilatory capacity of vasa vasorum, thus reducing blood flow to thoracic aorta media (Marcus et al., 1985). Interruption of vasa vasorum blood supply to the aortic wall may then result in reduced distensibility of the thoracic aorta and medial necrosis, both of which have been observed in TAAD (Stefanadis et al., 1995). Given these differences, the pathogenesis of TAAD and AAA may therefore be different, thus accounting for the difference in risk factors. Further research is necessary to characterize the pathogenesis of aortic dissection and aneurysms by anatomical location.
In terms of the protective effect of diabetes on aortic aneurysms, there are a few possible mechanisms. Firstly, diabetes can cause advanced glycation of collagen lattices which, in turn, induces cross-links that inhibit MMP-2 and MMP-9 secretion and reduce degradation of the aortic wall (Golledge et al., 2008). Furthermore, diabetes increases expression of plasminogen activator inhibitor-1 (PAI-1), which can then indirectly inhibit MMPs (Dua et al., 2010). Finally, diabetic medications, such as metformin, sulfonylurea, and empagliflozin, have also been found to have an inhibitory effect on the development of aortic aneurysms (Hsu et al., 2016; Shen et al., 2020).
Strengths and weaknesses
The strengths of our study include a large sample size, the prospective design, a long follow-up period, a minimal loss to follow-up, the accuracy of death certificate coding in Singapore, and the usage of competing risk analysis to account for the fact that these three diseases have similar risk factors. Nevertheless, we also acknowledge several limitations to our study. First, height, weight, and medical history were self-reported, which may have measurement errors and lead to non-differential misclassification. However, separate studies have validated the accuracy of self-reported diabetes and hypertension in this cohort (Odegaard et al., 2008; Talaei et al., 2016). Second, assessments of risk factors were only performed at baseline, and as such some non-differential misclassification due to later changes in comorbidities or smoking status may occur. However, the development of these diseases occurs over a long period of time, and as such newly diagnosed conditions will have less impact on their pathogenesis. In addition, non-differential misclassification can only lead to an underestimation of the true effect size of these risk factors and thus there will be no significant change to our results. Furthermore, we do not have data on several potential confounders such as lipid profiles or diabetes treatment. There is also the possibility of confounding from risk factors that were not included in our model, such as Marfan syndrome or systemic lupus erythematosus, but the confounding effect of such conditions is not expected to be significant due to their rarity in the general population. Despite the large cohort size, several subgroup analyses were underpowered due to the small number of observations in certain strata; for instance, only 18% of the participants consumed alcohol even once a month, and only 21.8% percent of our cohort participants were overweight or obese (defined as BMI ≥ 25 kg/m2). Hence, the associations of alcohol consumption or BMI with the risk of TAAD or AAA mortality did not reach statistical significance in our cohort, although the direction of these associations concurred with other studies.
Conclusions
In this prospective large population-based Asian cohort, we found distinct differences in risk estimates for TAAD mortality and AAA mortality as compared to those for CAD mortality. In particular, the strong association between hypertension and TAAD mortality suggests that a chronically elevated blood pressure may be the main pathogenesis underlying TAAD formation. More research is necessary to characterize the differential pathophysiology underpinning aortic diseases based on anatomical location, and risk modification to fine-tune primary prevention strategies may be useful to further reduce mortality in these highly lethal conditions.
Acknowledgements
We are grateful to Ms. Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study. We acknowledge the founding Principal Investigator of the Singapore Chinese Health Study, Mimi C. Yu. We also thank the Ministry of Health in Singapore for assistance with the identification of aortic disease and coronary artery disease cases via record linkage analysis with various databases.
Funding
This study was supported by the National Institutes of Health, USA (R01 CA144034 and UM1 CA182876). D.J.H. was supported by the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017), Centre Grant, and Collaborative Centre Grant scheme (NMRC/CGAug16C006). W.-P.K. was supported by the National Medical Research Council, Singapore (MOH-CSASI19nov-0001).
Conflict of Interest
The authors declare no conflicts of interest.
References
Ariel F Ying1
1Duke-National University of Singapore Medical School, Cardiovascular and Metabolic Disorders Program, Singapore.
Shi Hui Cheng1
1Duke-National University of Singapore Medical School, Cardiovascular and Metabolic Disorders Program, Singapore.
Aizhen Jin2
2Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Programme, National University of Singapore, Singapore.
Tze Tec Chong3
3Singapore General Hospital, Department of Vascular Surgery, Singapore.
Derek J Hausenloy1,4-7
1Duke-National University of Singapore Medical School, Cardiovascular and Metabolic Disorders Program, Singapore. 4National Heart Research Institute Singapore, National Heart Centre, Singapore. 5The Hatter Cardiovascular Institute, University College London, London, UK. 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan. 7Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Woon-Puay Koh2,8*
2Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Programme, National University of Singapore, Singapore.
8Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore.
Corresponding author:
Prof. Woon-Puay Koh
Email: kohwp@nus.edu.sg


Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4987 | 4 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA