International bi-monthly journal of cell signaling, tissue protection, and translational research.
Realizing the therapeutic potential of novel cardioprotective therapies: The EU-CARDIOPROTECTION COST Action - CA16225
Ioannou Andreadou1, Pavle Adamovski2, Monika Bartekova3, Christophe Beauloye4, Luc Bertrand5, David Biedermann6, Vilmante Borutaite7, Hans Erik Bøtker8, Stefan Chlopicki9, Maija Dambrova10, Sean Davidson11, Yvan Devaux12, Fabio Di Lisa13, Dragan Djuric14, David Erlinge15, Inês Falcao-Pires16, Eleftheria Galatou17, David García-Dorado18, Alfonso T. Garcia-Sosa19, Henrique Girão20, Zoltan Giricz21, Mariann Gyöngyösi22, Donagh Healy23, Gerd Heusch24, Vladimir Jakovljevic25, Jelena Jovanic26, Frantisek Kolar27, Brenda R Kwak28, Przemyslaw Leszek29, Edgars Liepinsh30, Sarah Longnus31, Jasna Marinovic32, Danina Mirela Muntean33, Lana Nezic34, Michel Ovize35, Pasquale Pagliaro36, Clarissa Pedrosa da Costa Gomes37, John Pernow38, Andreas Persidis39, Sören Erik Pischke40, Bruno K Podesser41, Fabrice Prunier42, Tanya Ravingerova43, Marisol Ruiz-Meana44, Rainer Schulz45, Alina Scridon46, Katrine H Slagsvold47, Jacob Thomsen Lønborg48, Belma Turan49, Niels van Royen50, Marko Vendelin51, Stewart Walsh52, Derek Yellon53, Nace Zidar54, Coert J Zuurbier55, Péter Ferdinandy56, Derek J Hausenloy57
Author Affiliations


- 1Faculty of Pharmacy, National and Kapodistrian University of Athens Panepistimiopolis, Zografou, 15771
- 2Private Health Institution "Dr. Adamovski,” General practice medicine, Bitola, Macedonia
- 3Institute for Heart Research, Centre of Experimental Medicine, Slovak Academy of Sciences, Bratislava, Slovakia
- 4Cliniques Universitaires Saint-Luc, Division of Cardiology, Brussels, Belgium; Université catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Pole of Cardiovascular Research, Brussels, Belgium
- 5Université catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Pole of Cardiovascular Research, Brussels, Belgium
- 6Institute of Microbiology, CAS, Center of Biotransformation and Biocatalysis, Vídeňská 1083 CZ-142 20, Praha 4, Czech Republic, EU
- 7Neuroscience Institute, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 8Department of Cardiology; Aarhus University Hospital; Aarhus N; Denmark
- 9Jagiellonian Centre for Experimental Therapeutics (JCET), Jagiellonian University; Chair of Pharmacology, Jagiellonian University Medical College, Krakow, Poland
- 10Latvian Institute of Organic Synthesis, Riga, Latvia; Riga Stradins University, Latvia
- 11The Hatter Cardiovascular Institute, University College London, 67 Chenies Mews, London, WC1E 6HX, UK
- 12Cardiovascular Research Unit, Luxembourg Institute of Health, Luxembourg City, Luxembourg
- 13Department of Biomedical Sciences and Neuroscience Institute of CNR, University of Padova, Via U. Bassi 58/B, Padova, Italy
- 14Institute of Medical Physiology “Richard Burian“ Faculty of Medicine, University of Belgrade, str. Visegradska 26/II, 11000 Belgrade, Serbia
- 15Dept of Cardiology, Clinical Sciences, Lund University, Lund, Sweden
- 16Department of Surgery and Physiology, Faculty of Medicine, Universidade do Porto, Porto, Portugal
- 17Program of Pharmacy, Department of Life and Health Sciences, School of Science and Engineering, University of Nicosia, Cyprus
- 18Vall d'Hebron University Hospital and Research Institute, Universitat Autónoma de Barcelona; CIBERCV
- 19Institute of Chemistry, University of Tartu, Ravila 14a, Tartu 50411, Estonia
- 20Faculty of Medicine - University of Coimbra, 3000-354 Coimbra, Portugal; Coimbra Institute for Clinical and Biomedical Research (iCBR)
- 21Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary
- 22Dept. of Cardiology, Medical University of Vienna, Austria
- 23Department of Vascular Surgery, Mercy University Hospital, Cork, Ireland
- 24Institute for Pathophysiology, West German Heart and Vascular Centre, University of Essen Medical School, Essen, Germany
- 25Department of Physiology, Faculty of Medical Sciences, University of Kragujevac, Serbia; 1st Moscow State Medical University IM Sechenov, Department of Human Pathology, Moscow, Russia
- 26Department of Cardiology, University Clinical Centre of the Republic of Srpska, Faculty of Medicine University of Banja Luka, Bosnia and Herzegovina
- 27Department of Developmental Cardiology, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic
- 28Department of Pathology and Immunology, and Department of Medical Specializations - Cardiology, University of Geneva, Geneva, Switzerland
- 29The Cardinal Stefan Wyszynski Institute of Cardiology, Warsaw, Poland
- 30Latvian Institute of Organic Synthesis, Riga, Latvia; Riga Stradins University, Latvia
- 31Department of Cardiovascular Surgery, Inselspital, Bern University Hospital and Department of Biomedical Research, University of Bern, Switzerland
- 32Department of Physiology, University of Split School of Medicine, Split, Croatia
- 33Department of Functional Sciences - Pathophysiology, Centre for Translational Research and Systems Medicine, "Victor Babeș" University of Medicine and Pharmacy, Timișoara, Romania
- 34Department of Pharmacology, Clinical Pharmacology and Toxicology, Faculty of Medicine University of Banja Luka, 14 Save Mrkalja Str, 78000 Banja Luka, Bosnia and Herzegovina
- 35Explorations Fonctionnelles Cardiovasculaires, Hôpital L. Pradel, Claude Bernard University and Hospices Civils de Lyon, Lyon, France
- 36Department of Clinical and Biological Sciences, University of Turin, Torino, Italy; National Institute of Cardiovascular Research, Bologna, Italy
- 37Cardiovascular Research Unit, Luxembourg Institute of Health, Luxembourg, Luxembourg
- 38Department of Medicine, unit of Cardiology, Karolinska Institutet, Stockholm, Sweden
- 39BioVista Inc. EU Offices, 34 Rodopoleos street, Elliniko, Athens 16777, Greece
- 40Department of Immunology and Clinic of Emergencies and Critical Care, Oslo University Hospital and University of Oslo, Oslo, Norway
- 41Ludwig Boltzmann Cluster for Cardiovascular Research at the Centre for Biomedical Research, Medical University of Vienna, Austria
- 42Institut MITOVASC, UMR INSERM U1083 and CNRS 6015, CHU Angers, University Angers, France
- 43Department of Cardiovascular Physiology and Pathophysiology, Head, Institute for Heart Research, CEM SAS, Bratislava, Slovakia
- 44Vall d'Hebron University Hospital and Research Institute, Universitat Autonoma de Barcelona; CIBERCV
- 45Institute of Physiology, Justus-Liebig University Giessen, Giessen, Germany
- 46Department of Physiology, University of Medicine and Pharmacy of Tirgu Mures, Romania; Center for Advanced Medical and Pharmaceutical Research, Tirgu Mures, Romania
- 47St. Olavs hospital, Trondheim University Hospital; Norwegian University of Science and Technology
- 48Department of Cardiology; Rigshospitalet; Copenhagen University Hospital; Denmark
- 49Department of Biophysics, Ankara University Faculty of Medicine, Ankara Turkey
- 50Department of Cardiology, Radboud University Medical Center, Nijmegen, the Netherlands
- 51Laboratory of Systems Biology, Department of Cybernetics, School of Science, Tallinn University of Technology, Estonia
- 52HRB Clinical Research Facility, Galway, Ireland. Funding Source Health Research Board Ireland
- 53The Hatter Cardiovascular Institute, University College London
- 54Faculty of Pharmacy, University of Ljubljana, Aškerčeva cesta 7, 1000 Ljubljana, Slovenia
- 55Laboratory of Experimental Intensive Care and Anesthesiology, Department of Anesthesiology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
- 56Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary; Department of Biochemistry, University of Szeged, Szeged, Hungary; Pharmahungary Group, Szeged, Hungary
- 57The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, St Bartholomew’s Hospital, London, UK; National Institute of Health University College London Hospitals. Biomedical Research Centre, London, UK; National Heart Research Institute Singapore, National Heart Centre, Singapore; Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore; Yong Loo Lin School of Medicine, National University Singapore, Singapore
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often follows are the leading causes of death and disability in Europe and worldwide. As such, new treatment strategies are needed to protect the heart against acute ischemia/reperfusion injury (IRI) in order to preserve cardiac function and prevent adverse left ventricular remodeling and HF – a strategy termed “cardioprotection.” Despite intensive experimental and clinical research since the discovery of the remarkable cardioprotective effect of ischemic preconditioning more than 3 decades ago, there are currently no effective cardioprotective therapies in clinical practice. The challenge has been to successfully translate novel cardioprotective therapies discovered in experimental studies into the clinical setting for patient benefit.
This EU-CARDIOPROTECTION COST Action CA16225 will address this challenge by setting up a pan-European research network of leading experts in experimental and clinical cardioprotection, to jointly develop innovative strategies for translating novel cardioprotective therapies into the clinical setting. This will be achieved through 4 main research objectives, each linked to the activities of a Working Group (WG): (1) WG1 New Targets: to use innovative strategies to discover novel targets for cardioprotection, given that many of the established cardioprotective targets have so far failed; (2) WG2 Combination Therapy: to investigate the effects of using combination therapy directed to multiple targets as an innovative cardioprotective strategy, given that single-targeted approaches to cardioprotection have so far failed; (3) WG3 Confounders: to use more clinically relevant animal AMI/HF models for testing novel cardioprotective therapies which take into account the confounding effects of co-morbidities and co-medications, given that many of the failed clinical studies have been based on therapies developed using juvenile healthy animal models; and (4) WG4 Consortium: to set up a European network of research centers (called the European Cardioprotection Consortium (ECC)) for multi-center randomized placebo-controlled testing of novel cardioprotective therapies in small/large animal AMI/HF models, and in AMI/HF patients, in order to improve the rigor of pre-clinical and clinical testing of novel cardioprotective therapies. In summary, the overall objective of the EU-CARDIOPROTECTION COST Action CA16225 will be to improve the translation of novel cardioprotective therapies into the clinical setting for patient benefit.
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often follows are the leading causes of death and disability in Europe and worldwide. As such, new treatment strategies are needed to protect the heart against acute ischemia/reperfusion injury (IRI) in order to preserve cardiac function and prevent adverse left ventricular remodeling and HF – a strategy termed “cardioprotection.” Despite intensive experimental and clinical research since the discovery of the remarkable cardioprotective effect of ischemic preconditioning more than 3 decades ago, there are currently no effective cardioprotective therapies in clinical practice. The challenge has been to successfully translate novel cardioprotective therapies discovered in experimental studies into the clinical setting for patient benefit.
This EU-CARDIOPROTECTION COST Action CA16225 will address this challenge by setting up a pan-European research network of leading experts in experimental and clinical cardioprotection, to jointly develop innovative strategies for translating novel cardioprotective therapies into the clinical setting. This will be achieved through 4 main research objectives, each linked to the activities of a Working Group (WG): (1) WG1 New Targets: to use innovative strategies to discover novel targets for cardioprotection, given that many of the established cardioprotective targets have so far failed; (2) WG2 Combination Therapy: to investigate the effects of using combination therapy directed to multiple targets as an innovative cardioprotective strategy, given that single-targeted approaches to cardioprotection have so far failed; (3) WG3 Confounders: to use more clinically relevant animal AMI/HF models for testing novel cardioprotective therapies which take into account the confounding effects of co-morbidities and co-medications, given that many of the failed clinical studies have been based on therapies developed using juvenile healthy animal models; and (4) WG4 Consortium: to set up a European network of research centers (called the European Cardioprotection Consortium (ECC)) for multi-center randomized placebo-controlled testing of novel cardioprotective therapies in small/large animal AMI/HF models, and in AMI/HF patients, in order to improve the rigor of pre-clinical and clinical testing of novel cardioprotective therapies. In summary, the overall objective of the EU-CARDIOPROTECTION COST Action CA16225 will be to improve the translation of novel cardioprotective therapies into the clinical setting for patient benefit.
Introduction
Acute myocardial infarction (AMI) and the heart failure (HF) that complicates it are the leading causes of death and disability in Europe and worldwide. The most effective treatment for limiting myocardial infarct (MI) size and preventing HF following AMI is timely myocardial reperfusion using primary percutaneous coronary intervention (PPCI). However, despite timely PPCI, mortality and morbidity following AMI remain significant, with 7% death and 22% hospitalization for HF at one year ( Cung et al., 2015 ). Accordingly, new treatments are required to reduce MI size, in order to preserve left ventricular (LV) systolic function and prevent the development of post-AMI HF – a treatment strategy termed “cardioprotection.”
After myocardial reperfusion, the most powerful intervention for reducing MI size in the experimental setting is ischemic conditioning, an endogenous cardioprotective phenomenon by which brief episodes of ischemia and reperfusion applied to the heart or a remote organ/tissue limit MI size (Hausenloy, 2013;Hausenloy and Yellon, 2016;Hausenloy et al., 2017b).
Intensive investigation of ischemic conditioning over the last 30 years (Hausenloy et al., 2016) has identified a large number of signaling pathways and therapeutic targets for cardioprotection (Hausenloy, 2013;Hausenloy et al., 2017a;Hausenloy et al., 2017b). However, despite this, no effective therapies for protecting the heart against acute ischemia/reperfusion injury (IRI) have been translated into the clinical setting for patient benefit. A large number of cardioprotective therapies have been investigated in AMI patients treated with PPCI including a growing number of high-profile clinical studies, but the vast majority of these have failed to show benefit in terms of reducing MI size and improving clinical outcomes.
The main challenge, therefore, is to improve the translation of novel cardioprotective therapies shown to be effective in the experimental setting into the clinical arena for patient benefit. Our EU-CARDIOPROTECTION COST Action (CA16225, 4 years’ duration Oct 2017 to Sept 2021) will address this challenge by setting up a pan-European network of research centers tasked with several key objectives, each of which will be addressed by four Working Groups (see Figure 1): (1) discovery of new therapeutic targets and innovative strategies for cardioprotection; (2) testing effects of combination therapy to target multiple signaling pathways both within and outside the cardiomyocyte; (3) investigating the effects of confounders (co-morbidities and co-medications) on cardioprotection; and (4) multi-center randomized controlled pre-clinical and clinical testing of novel cardioprotective therapies.
The reasons for the failure to translate novel cardioprotective therapies into the clinical setting for patient benefit are multiple and complex and have been reviewed in a number of recent papers (Ovize et al., 2010;Hausenloy, 2013;Lecour et al., 2014;Ibanez et al., 2015;Perrino et al., 2017;Hausenloy et al., 2017b;Heusch, 2017). Here we focus on several of the most important factors and outline how our EU-CARDIOPROTECTION COST Action will address these issues.
Therapeutic targets for cardioprotection: The majority of experimental studies have focused on targeting well-established signaling pathways and targets many of which have not proven to be beneficial in the clinical setting. Furthermore, the experimental approach has relied on a reductionist strategy focused on a single signaling pathway or target. As such, novel therapeutic targets and strategies need to be discovered in order to improve the translation of cardioprotection into the clinical setting, e.g. by taking into account unbiased “fishing” approaches by multi-omics technologies (Perrino et al., 2017).
To address this, the aim of Working Group 1 (NEW TARGETS) of our EU-CARDIOPROTECTION COST Action will be to discover novel signaling pathways and therapeutic targets within and outside the cardiomyocyte, and identify innovative strategies for cardioprotection.
Single-targeted cardioprotective therapies: The majority of experimental studies have used a single-targeted approach, directed to one signaling pathway or target within cardiomyocytes. However, a large number of different cardioprotective pathways underlie ischemic conditioning. Furthermore, the detrimental effects of acute IRI on the heart are complex, interdependent, and involve a number of players outside of the cardiomyocyte such as the coronary microvasculature, inflammatory cells, red blood cells, platelets, and extracellular vesicles (Sluijter et al., 2018;Hausenloy et al., 2017b;Heusch, 2016).
As such, the aim of Working Group 2 (COMBINATION THERAPY) of our EU-CARDIOPROTECTION COST Action will be to investigate combination therapy directed to multiple cardioprotective pathways and targets both within and outside the cardiomyocyte as an innovative approach to cardioprotection.
Experimental models with low translational value: The majority of experimental studies have used healthy juvenile animal MI models, which do not adequately reflect the clinical setting, given that most ischemic heart disease patients are middle-aged, have co-morbidities (such as diabetes, hyperlipidemia, hypertension), and are on co-medications (such as anti-platelet therapies, statins, beta-blockers, angiotensin converting enzyme inhibitors, and/or anesthetics). There are also obvious species differences in cardioprotective signaling between rodents, larger mammals and humans. These factors have been shown to confound the efficacy of cardioprotective therapies, and should be taken into consideration when evaluating novel cardioprotective therapies in experimental and clinical settings ( Ferdinandy et al., 2014 ).
As such, the aim of Working Group 3 (CONFOUNDERS) of our EU-CARDIOPROTECTION COST Action will be to identify the key co-morbidities and co-medications which confound cardioprotection, in order to improve the translational value of the animal MI models.
Therapies with inconsistent cardioprotective efficacy: Many of the cardioprotective therapies that have failed in the clinical setting did not show consistent and robust cardioprotection in the experimental setting. This can be attributed to methodological limitations of the pre-clinical studies including lack of randomization, non-blinded treatment allocation, non-blinded data analysis, lack of standardized animal AMI/HF models and acute IRI protocols, and the lack of rigor in statistical methods. Therefore, more rigorous testing of novel cardioprotective therapies in the experimental setting are required to ensure that only the most promising therapies are investigated in the clinical arena.
To address this, the aim of Working Group 4 (CONSORTIUM) of our EU-CARDIOPROTECTION COST Action will be to set up a European Cardioprotection Consortium (ECC) for multi-center experimental testing of new cardioprotective therapies in clinically relevant small/large animals, and for testing new cardioprotective therapies in proof-of-concept clinical studies in patients subjected to acute IRI including ST-segment elevation myocardial infarction (STEMI) and coronary artery bypass graft (CABG) patients.
Research Objectives of the COST Action
Working Group 1: New Targets
The majority of the experimental studies investigating novel cardioprotective therapies have focused on targeting well-established signaling pathways/targets, many of which have not proven to be beneficial in the clinical setting. In some respects, this may have been because the therapy had not been optimized in terms of the experimental setting (dose, acute versus chronic administration and timing of therapy), and in this regard, there is an opportunity to optimize the treatment approach. In other cases, in which treatments have failed, novel therapeutic targets and strategies need to be discovered in order to improve the translation of cardioprotection from the laboratory to the clinical setting. These new cardioprotective strategies should focus on (a) discovering new therapeutic targets in novel cardioprotective pathways within the cardiomyocyte and (b) other components of acute IRI outside of the cardiomyocyte such as the microvasculature, inflammatory cells, and platelets. Potential novel targets for cardioprotection include i) inflammation targets such as macrophages/lymphocytes, RNA/DNA, and inflammasome; ii) novel mechanisms of cell death such as necroptosis and pyroptosis; iii) extracellular matrix; iv) fibroblasts; v) endothelial cells and vascular smooth muscle; vi) platelets and vii) novel mitochondrial targets(Hausenloy et al., 2017b); and viii) novel insulin targets and zinc-transporters targets associated with sarcolemma, sarco(endo)plasmic reticulum and mitochondria in hyperglycemic, failing and aging heart (Tuncay et al., 2017;Tuncay et al., 2018;Olgar et al., 2018).
The use of innovative strategies such as multi-omics (transcriptomics, epigenetics, proteomics, and metabolomics) and innovative in silico network biology evaluation is another objective of our COST Action in order to identify new therapeutic targets for cardioprotection. The pathophysiology of the ischemic heart is very complex; therefore, large-scale, unbiased, global approaches capable of identifying multiple branches of the signaling networks activated in the ischemic/reperfused heart might be a preferred approach in the discovery of novel targets. Proteomics and metabolomics offer simultaneous readouts of hundreds of proteins and metabolites altered in the ischemic myocardium, and the possibility of integrating different -omics approaches gives new hope for a better understanding of the complicated molecular signaling activated by acute myocardial ischemia and reperfusion (Perrino et al., 2017;Varga et al., 2015). In general, WG1 will contribute to an improved knowledge of the sequence of molecular events triggered by ischemia-reperfusion that ultimately lead to tissue damage. This is required for the development of novel and effective therapeutic approaches. The identification of novel therapeutic targets may lead to the design, synthesis, and testing of novel chemical modulators in both cells and animals.
Expected Research Outputs of WG1
1. To identify at least 6 novel targets for cardioprotection either within the cardiomyocyte (newly discovered signaling pathways) or outside the cardiomyocyte (involving inflammatory cells, fibroblasts, the microvasculature, red blood cells, or platelets).
2. To identify at least 3 innovative strategies for identifying novel targets for cardioprotection such as epigenetics or multi-omics strategies (genomics, transcriptomics, metabolomics, lipidomics or proteomics).
Working Group 2: Combination Therapy
The majority of experimental studies have used a single-targeted approach, directed to a single signaling pathway and target within the cardiomyocyte. However, there exists a number of different cardioprotective pathways underlie ischemic conditioning. Furthermore, the detrimental effects of acute IRI on the heart are complex, interdependent, and involve a number of players outside of the cardiomyocyte such as the microvasculature, extracellular matrix, inflammatory cells, red blood cells and platelets. For example, it has been shown that multiple cardioprotective interventions (each with a different mechanism of action), – e.g., mild hypothermia, sodium/hydrogen exchange blocker plus a P2Y12 inhibitor, – reduced MI size to a greater extent beyond the one that achieved by a P2Y12 inhibitor alone (Yang et al., 2013b;Yang et al., 2012;Yang et al., 2013a;Yang et al., 2013c). Here we will utilize the joint expertise of different European network members to investigate combination therapy directed to multiple cardioprotective pathways and targets both within and outside the cardiomyocyte as an innovative treatment strategy for cardioprotection.
Expected Research Outputs of WG2
To identify at least 6 promising treatment strategies that are likely to have synergistic effects when administered in combination and that are directed to targets within the cardiomyocyte (newly discovered signaling pathways) or outside the cardiomyocyte (involving inflammatory cells, the extracellular matrix, the microvasculature, red blood cells, or platelets) for testing in pre-clinical acute myocardial IRI models.
Working Group 3: Confounders
Ischemic heart disease in humans is a complex disorder caused by, or associated with, cardiovascular risk factors and comorbidities, including hypertension, obesity, hyperlipidemia, diabetes, insulin resistance, heart failure, altered coronary circulation, and aging. These risk factors induce fundamental alterations in cellular signaling cascades that affect the development of IRI per se and responses to cardioprotective interventions. Moreover, some of the medications used to treat these risk factors, including statins, nitrates, and antidiabetic drugs, may impact cardioprotection by modifying cellular signaling (Ferdinandy et al., 2014).
The majority of experimental studies have used healthy juvenile animal MI models, which are of low translational value since they do not adequately reflect the clinical setting given that most CVD patients are middle-aged, have multiple co-morbidities and are on a variety of different co-medications. There are also obvious species differences in cardioprotective signaling between rodents, larger mammals, and humans. These factors have been shown to confound the efficacy of cardioprotective therapies and need to be taken into consideration when evaluating novel cardioprotective therapies in the experimental and clinical setting (Ferdinandy et al., 2014). Therefore, in this WG, we will utilize the joint expertise of the European network members to investigate key co-morbidities and co-medications that confound cardioprotection in order to improve the translational value of animal MI models.
Our aim will be to improve the pre-clinical evaluation of novel cardioprotective therapies identified in the experimental setting by investigating the effect of confounding factors such as co-morbidities (age, diabetes, hypertension, hyperlipidemia) and co-medications (anti-platelet agents, statins, beta blockers, anesthetics) on their cardioprotective efficacy. Importantly, the mechanisms through which these confounding factors interfere with cardioprotection elicited by ischemic conditioning are not clear and will also be investigated in this objective, as this will provide mechanistic insights into cardioprotection.
In order to achieve this goal, we will use more clinically relevant animal models that take into account co-morbidities and co-medications relevant to MI patients. Although studies have reported co-morbidities such as age, diabetes and left ventricular hypertrophy to attenuate the efficacy of cardioprotective therapies (Ferdinandy et al., 2014), the mechanisms underlying this effect are unknown and will be investigated in this objective. In addition, further studies are required to investigate the effect of co-medication such as anti-platelet agents, statins, anesthetics and their impact on the efficacy of new cardioprotective therapies.
Expected Research Outputs of WG3
1. To identify at least the 2 most important co-morbidities to consider when designing preclinical and clinical cardioprotection studies (factors include age, male sex, diabetes, hypertension, hypercholesterolemia).
2. To identify at least the 2 most important co-medications to consider when designing preclinical and clinical cardioprotection studies (co-medications include anti-platelet agents, statins, anesthetics, nitrates).
Working Group 4: Consortium
Many of the cardioprotective therapies that have failed in the clinical setting did not show consistent and robust cardioprotection in the experimental setting. This can be attributed to methodological limitations of the pre-clinical studies including lack of randomization, non-blinded treatment allocation, non-blinded data analysis, lack of standardized animal models and IRI protocols, and the lack of rigor in statistical methods. Therefore, more rigorous testing of novel cardioprotective therapies in the experimental setting is required to ensure that only the most promising therapies are investigated in the clinical arena. As such in this COST Action proposal, we will put in place a Consortium for (a) multi-center experimental testing of new cardioprotective therapies in clinically relevant small/large animal and human models of acute IRI; and (b) testing of new cardioprotective therapies in proof-of-concept clinical studies in patients subjected to acute IRI including STEMI and CABG patients.
The Consortium, which will be termed the European Cardioprotection Consortium (ECC), will allow multi-site testing of novel cardioprotective therapies in clinically relevant experimental small and large animal models and is, in part, modeled on the National Institute of Health-sponsored CAESAR collaborative network (Consortium for Preclinical Assessment of Cardioprotective Therapies) in the United States(Schwartz-Longacre et al., 2011;Lefer and Bolli, 2011;Jones et al., 2015). The ECC will improve upon the NIH CAESAR collaborative network by (a) building on the infrastructure and knowledge obtained from the 3 WGs, in identifying novel cardioprotective targets and innovative strategies; and (b) undertaking proof-of-concept clinical studies in STEMI and CABG patients.
Expected Research Outputs of WG4
1. To put in place a network of research centers across Europe that will constitute the ECC for multi-center randomized controlled pre-clinical and clinical testing of novel cardioprotective therapies.
2. To standardize acute myocardial IRI protocols across the consortium and select which small animal (mouse, rat, rabbit) and large animal (pig, dog) AMI/HF models to include in the ECC.
3. To identify at least 4 novel cardioprotective therapies from WG 1, 2, 3 and key confounders highlighted in WG3 for testing in our ECC.
4. To identify at least 2 novel cardioprotective therapies which have demonstrated consistent and robust cardioprotection in the ECC, for future testing in proof-of-concept clinical studies in STEMI and CABG patients.
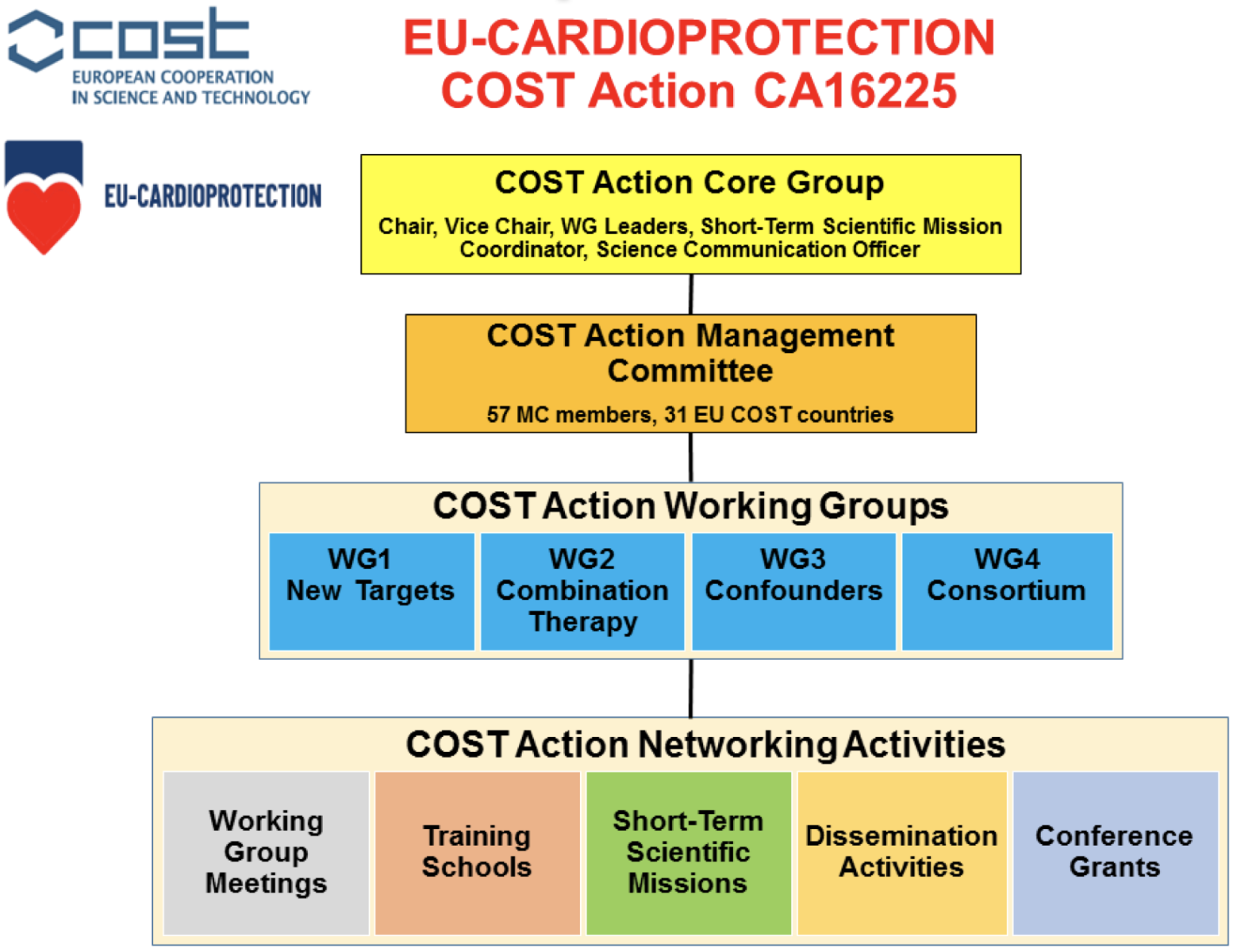
In a new window | Download PPT
Figure 1: Overview of the EU-CARDIOPROTECTION COST Action CA16225 organization and activities. The COST Action Core Group will oversee the EU-CARDIOPROTECTION COST Action Program (4 years’ duration, Oct 2017 to Sept 2021) in conjunction with the COST Action Management Committee (MC). The COST Action will utilize the COST Action Networking Activities to achieve the research objectives of the four Working Groups (WGs).
Expected impact of the EU-CARDIOPROTECTION COST Action
The immediate impact of the current project will be to create a European network of research centers to discover and disseminate novel therapeutic targets and to define strategies for cardioprotection to treat patients with co-morbidities and on co-medications. This project will establish networks and relationships with research users. The EU-Cardioprotection project involves users at all stages of research, including working with user stakeholder and participatory groups. The short-term impact will be achieved by exchanging ideas, knowledge, tools, and data of scientists working on different but related systems using various approaches.
The short-term scientific, technological and socio-economic impacts will provide: i) The knowledge basis for research scientists at all levels and industrial players to increase the number of successful innovative development projects aimed at discovering novel cardioprotective therapies. The COST Action will provide state-of-the-art, detailed protocols for measuring MI size in mice, rabbits, and pigs in a manner that is rigorous, accurate, and reproducible, thereby helping to best inform which therapies should be taken forward by industrial partners for further development and commercialization. ii) The capability of the COST Action members to jointly apply for a Horizon 2020 grant to set up the European Cardioprotection Collaboration network of research centers for the pre-clinical and clinical testing of novel cardioprotective therapies as outlined in WG4 of the project. iii) The ability to validate the efficacy and safety of current cardioprotective strategies such as ischemic postconditioning and remote ischemic conditioning in the treatment of AMI in the real world, i.e. in patients with major co-morbidities and co-medications. v) Data for early exclusion of candidate strategies unlikely to succeed in AMI patients.
The long-term scientific, technological and socio-economic impacts include: i) accumulation of the critical mass to understand problems with high levels of complexity such as cardioprotection -- problems that can only be solved by a concerted effort of multidisciplinary teams; ii) enabling researchers from less research intensive countries to interact and share ideas, resources and personnel with scientists from countries in which research enjoys a high priority iii) creation of new, and strengthening of existing, interactions among European scientists and international experts via our external advisory board; iv) setting up the ECC network of research centers for pre-clinical and clinical testing of novel cardioprotective therapies, which will lead to successful development of cardioprotective therapies, thereby solving an unmet need in the treatment of ischemic heart disease, which is currently the leading cause of morbidity and mortality in the EU; v) increasing the number and success rate of innovative development projects, creation of new jobs and increased competitiveness of innovative small-to-medium enterprises (SMEs); vi) heightening the impact of cardioprotection research on policy makers, regulatory bodies and national decision makers as well as on the private sector; vii) developing novel technologies and services that will impact on the growth of the EU’s knowledge-driven economy; and viii) contributing to healthy aging in the European population and providing a major impact on quality of life.
COST Action research network activities
The following Network Activities listed below will be used in our EU-CARDIOPROTECTION COST Action to deliver the main research objectives of our 4 WGs (see Figure 1).
WG Meetings: Bi-annual WG Meetings will allow the COST partners and others to disseminate and share knowledge relevant to the objectives of the 4 different, but closely inter-related, WGs. Invited speakers and chairs will be considered with respect to gender balance and geographical distribution. Early career investigators (ECIs) will be encouraged to chair sessions and present at the WG Meetings. We will invite leading international experts in preclinical and clinical cardioprotection to attend the meeting and provide expert input and advice to the relevant WGs. We will also invite international researchers who have expertise in setting up a similar cardioprotection research consortium in the U.S. – the NIH CAESAR Network -- to provide advice on setting up our ECC in WG4. Our first WG Meeting took place in October 2017 in Brussels at the COST Headquarters, and outlined the main objectives and activities of our EU-CARDIOPROTECTION Cost Actions. Our next WG meeting, which will be held in the Medical University of Vienna, Austria, in March 2018 (local organizers, Bruno Podesser and Mariann Gyöngyösi), will begin the implementation of strategies to achieve the WG objectives of the COST Action.
Training schools: In order to support the training and development of ECIs, training schools will be organized on a variety of topics relevant to cardioprotection research including (1) novel methods for discovering novel targets for cardioprotection and innovative cardioprotective strategies such as multi-omics approaches; (2) the use of combination therapies as a multi-targeted approach to cardioprotection; (3) clinically relevant animal AMI/HF based on co-morbidities (age, diabetes, hypercholesterolemia) and consideration of relevant co-medications (statins, nitrates, anesthetics, anti-platelet agents); and (4) guidelines for undertaking multicenter experimental studies and proof-of-concept clinical studies.
Short-term Scientific Missions (STSMs): In order to achieve long-lasting effects of this COST Action, ECIs will be supported in their networking activities by STSMs (duration of one week to 3 months and supported by a fixed grant from the COST Action). These will allow ECIs to learn new research techniques and gain valuable knowledge and experience from an institution or laboratory in another COST country. Increasing collaboration is important to make Europe more competitive. Exchanging ideas and knowledge across borders will lead to more successful projects. So far our EU-CARDIOPROTECTION COST Action has initiated 4 STSMs involving COST Member Countries and International Partner Countries (UK-Singapore, Greece-Germany, Slovakia-Hungary, Romania-Austria). We intend to initiate a further 8 STSMs each year for the duration of the COST Action.
Dissemination activities: A website providing access to the generated knowledge and annual newsletters, with mutual links to websites relevant to this COST Action has been set up at www.cardioprotection.eu. Scientific publications disseminating knowledge of the WG will be published as original articles, reviews and position papers in conjunction with other cardiovascular organizations and societies. Our COST Action will also sponsor joint scientific sessions at other European and international cardiovascular meetings including the International Society of Heart Research-European Section (ISHR-ES) meetings and ESC Working Group on Cellular Biology of the Heart.
Conference Grants: This will enable PhD students and ECIs from participating Inclusiveness Target Countries to attend international cardiovascular conferences that are not organized by our COST Action.
Summary of the EU-CARDIOPROTECTION COST Action
In summary, our COST Action will put in place a pan-European Research Network of leading experts in cardioprotection to jointly develop new initiatives and new strategies for finding innovative and more effective approaches to cardioprotection and for optimizing the pre-clinical and clinical evaluation of new cardioprotective therapies, so as to improve their translation into the clinical setting for patient benefit. The COST Action will co-ordinate and strengthen European research in the field of cardioprotection and accelerate scientific progress through the dissemination and sharing of new therapeutic targets among network members and industrial partners, thereby facilitating the discovery of new cardioprotective therapies. By utilizing the joint expertise of different European network members, we will investigate factors that confound the efficacy of new cardioprotective therapies, including comorbidities (such as age, diabetes, and hypertension) and co-medications (such as anti-platelet therapies, statins and beta-blockers). Finally, we will set up a European network of research centers for multi-center laboratory testing of new cardioprotective therapies using small and large animal models of acute IRI in order to select those therapies most likely to succeed in the clinical setting. All aspects of this COST Action proposal require a critical mass of partners covering a wide geographic distribution across Europe in order to deliver the objectives outlined in this proposal and improve clinical outcomes for AMI patients in Europe and worldwide.
Acknowledgements
This work was supported by the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action. DJH is the chair and PF is the vice-chair of the European Cooperation in Science and Technology (COST Action CA16225, EU-Cardioprotection).
CB and LB are supported by grants from the Fonds National de la Recherche Scientifique et Médicale (FNRS), Belgium, and Action de Recherche Concertée de la Communauté Wallonie-Bruxelles, Belgium, and by unrestricted grants from Astra Zeneca and Bayer. CB is Postdoctorate Clinical Master Specialist, and LB is Senior Research Associate of FNRS, Belgium. MB is supported by VEGA SR no. 2/0061/16. HEB is supported by The Danish Council for Strategic Research (11-108354), Novo Nordisk Foundation (Conditioning-Based Intervention Strategies – ConBis) and Trygfonden. VB is supported by grants from the Research Council of Lithuania and the Science Foundation of Lithuanian University of Health Sciences. SC is supported by the National Science Centre (Symfonia grant no. DEC-2015/16/W/NZ4/00070). DGD is supported by Grant PI17/01397 from Instituto de Salud Carlos III and the European Regional Development Fund (ERDF-FEDER). DD is supported by the Ministry of Education, Science and Technological Development of Republic of Serbia, grant number 175043. SMD is supported by The Department of Health’s NIHR Biomedical Research Centre and British Heart Foundation Project Grants (PG/16/85/32471 and PG/15/52/31598). YD is supported by the National Research Fund (grant # INTER/EUROSTARS/15/10282117) and the Ministry of Higher Education and Research of Luxembourg. PF is supported by grants from the Hungarian National Research, Development, and Innovation Office (OTKA K 109737, OTKA KH_17 125570, NVKP 16-1-2016-0017, and VEKOP-2.3.2-16-2016-00002). ZG is supported by the Bolyai Scholarship by the Hungarian Academy of Sciences. ATG-S is funded by the Estonian Ministry of Education and Research (grant IUT34-14). HG is supported by the European Regional Development Fund (ERDF) through the Operational Program for Competitiveness Factors (COMPETE) under the projects HealthyAging2020 CENTRO-01-0145-FEDER-000012- N2323 to CNC.IBILI and national funds through the Portuguese Foundation for Science and Technology [SFRH/SINTD/60112/2009;PEST-C/SAU/ UI3282/2011-2013; UID/NEU/04539/2013], PAC ‘NETDIAMOND’ POCI-01-0145-FEDER-016385. CPCG is supported by the Eurostars E! 9686 MIPROG project. GH is supported by the German Research Foundation (He 1320/18-3; SFB 1116 B8). DJH is supported by the British Heart Foundation (FS/10/039/28270); the National Institute for Health Research University College London Hospitals Biomedical Research Centre; Duke-National University Singapore Medical School; the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006); and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). FK is supported by The Czech Science Foundation (17-07748S and 18-03207S). BRK is supported by grants from the Swiss National Science Foundation (310030_162579 and 310030E_176050). FDL is supported by Leducq Transatlantic Network of Excellence; MIUR; University of Padova Strategico grant. DMM is supported by the university research grant PIII-C5-PCFI-2017/2018-01 and the French-Romanian bilateral cooperation project nr. 75 BM/2017. MRM is supported by grant PI15/01655 from Instituto de Salud Carlos III and grant 122/C/20150315-1121 from Marato TV3. LN is supported by Faculty of Medicine University of Banja Luka, Bosnia and Herzegovina. MO is supported by the OPeRa IHUB research program (ANR-10-IBHU-0004) within the “Investissements d’Avenir” operated by the French National Research Agency (ANR). PP is supported by the University of Turin, Ricerca Locale Ex-60% (Grants: PAGP_RIC_LOC_15_01; PAGP_RILO_16_01). SP is supported by The Research Council of Norway. BKP is supported by a grant from the Ludwig Boltzmann Society (REM 2017-209). JP is supported by the Swedish Research Council and Swedish Heart and Lung Foundation.
IFP is partially supported by Fundo Europeu de Desenvolvimento Regional (FEDER) through COMPETE 2020 – Programa Operacional Competitividade e Internacionalização (POCI), the project DOCnet (NORTE-01-0145-FEDER-000003), supported by the Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF), the project NETDIAMOND (POCI-01-0145-FEDER-016385), supported by European Structural and Investment Funds, Lisbon’s Regional Operational Program 2020. TR is supported by VEGA SR 2/0141/18, APVV-16-0263, MVTS SAS. RS is supported by the German Research Foundation CRC1213, B05. BT is supported by TUBITAK grants for SBAG-214S254 and SBAG-216S979. MV is supported by the Estonian Research Council (IUT33-7). DMY is supported by the BHF, MRC, Wellcome Trust, Rosetrees, Hatter Foundation. NZ is supported by the Slovenian Research Agency (Grant No. P1-0208, and Grant No. Z1-5458). CJZ is supported by Academic Medical Center and the Dutch Heart Foundation.
Conflict of interest
HEB is shareholder in CellAegis Inc. PF is the founder and CEO of Pharmahungary Group, a group of R&D companies focusing on services and novel technologies for cardioprotection and its comorbidities (www.pharmahungary.com). DES declares speaker fees from ZOLL. FP received research grants from Bayer and Boehringer. All other authors declare no conflict of interest.
References
Ioannou Andreadou1
1Faculty of Pharmacy, National and Kapodistrian University of Athens Panepistimiopolis, Zografou, 15771.
Pavle Adamovski2
2Private Health Institution "Dr. Adamovski,” General practice medicine, Bitola, Macedonia.
Monika Bartekova3
3Institute for Heart Research, Centre of Experimental Medicine, Slovak Academy of Sciences, Bratislava, Slovakia.
Christophe Beauloye4
4Cliniques Universitaires Saint-Luc, Division of Cardiology, Brussels, Belgium; Université catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Pole of Cardiovascular Research, Brussels, Belgium.
Luc Bertrand5
5Université catholique de Louvain, Institut de Recherche Expérimentale et Clinique, Pole of Cardiovascular Research, Brussels, Belgium.
David Biedermann6
6Institute of Microbiology, CAS, Center of Biotransformation and Biocatalysis, Vídeňská 1083 CZ-142 20, Praha 4, Czech Republic, EU.
Vilmante Borutaite7
7Neuroscience Institute, Lithuanian University of Health Sciences, Kaunas, Lithuania.
Hans Erik Bøtker8
8Department of Cardiology; Aarhus University Hospital; Aarhus N; Denmark.
Stefan Chlopicki9
9Jagiellonian Centre for Experimental Therapeutics (JCET), Jagiellonian University; Chair of Pharmacology, Jagiellonian University Medical College, Krakow, Poland.
Maija Dambrova10
10Latvian Institute of Organic Synthesis, Riga, Latvia; Riga Stradins University, Latvia.
Sean Davidson11
11The Hatter Cardiovascular Institute, University College London, 67 Chenies Mews, London, WC1E 6HX, UK.
Yvan Devaux12
12Cardiovascular Research Unit, Luxembourg Institute of Health, Luxembourg City, Luxembourg.
Fabio Di Lisa13
13Department of Biomedical Sciences and Neuroscience Institute of CNR, University of Padova, Via U. Bassi 58/B, Padova, Italy.
Dragan Djuric14
14Institute of Medical Physiology “Richard Burian“ Faculty of Medicine, University of Belgrade, str. Visegradska 26/II, 11000 Belgrade, Serbia.
David Erlinge15
15Dept of Cardiology, Clinical Sciences, Lund University, Lund, Sweden.
Inês Falcao-Pires16
16Department of Surgery and Physiology, Faculty of Medicine, Universidade do Porto, Porto, Portugal.
Eleftheria Galatou17
17Program of Pharmacy, Department of Life and Health Sciences, School of Science and Engineering, University of Nicosia, Cyprus.
David García-Dorado18
18Vall d'Hebron University Hospital and Research Institute, Universitat Autónoma de Barcelona; CIBERCV.
Alfonso T. Garcia-Sosa19
19Institute of Chemistry, University of Tartu, Ravila 14a, Tartu 50411, Estonia.
Henrique Girão20
20Faculty of Medicine - University of Coimbra, 3000-354 Coimbra, Portugal; Coimbra Institute for Clinical and Biomedical Research (iCBR).
Zoltan Giricz21
21Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary.
Mariann Gyöngyösi22
22Dept. of Cardiology, Medical University of Vienna, Austria.
Donagh Healy23
23Department of Vascular Surgery, Mercy University Hospital, Cork, Ireland.
Gerd Heusch24
24Institute for Pathophysiology, West German Heart and Vascular Centre, University of Essen Medical School, Essen, Germany.
Vladimir Jakovljevic25
25Department of Physiology, Faculty of Medical Sciences, University of Kragujevac, Serbia; 1st Moscow State Medical University IM Sechenov, Department of Human Pathology, Moscow, Russia.
Jelena Jovanic26
26Department of Cardiology, University Clinical Centre of the Republic of Srpska, Faculty of Medicine University of Banja Luka, Bosnia and Herzegovina.
Frantisek Kolar27
27Department of Developmental Cardiology, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic.
Brenda R Kwak28
28Department of Pathology and Immunology, and Department of Medical Specializations - Cardiology, University of Geneva, Geneva, Switzerland.
Przemyslaw Leszek29
29The Cardinal Stefan Wyszynski Institute of Cardiology, Warsaw, Poland.
Edgars Liepinsh30
30Latvian Institute of Organic Synthesis, Riga, Latvia; Riga Stradins University, Latvia.
Sarah Longnus31
31Department of Cardiovascular Surgery, Inselspital, Bern University Hospital and Department of Biomedical Research, University of Bern, Switzerland.
Jasna Marinovic32
32Department of Physiology, University of Split School of Medicine, Split, Croatia.
Danina Mirela Muntean33
33Department of Functional Sciences - Pathophysiology, Centre for Translational Research and Systems Medicine, "Victor Babeș" University of Medicine and Pharmacy, Timișoara, Romania.
Lana Nezic34
34Department of Pharmacology, Clinical Pharmacology and Toxicology, Faculty of Medicine University of Banja Luka, 14 Save Mrkalja Str, 78000 Banja Luka, Bosnia and Herzegovina.
Michel Ovize35
35Explorations Fonctionnelles Cardiovasculaires, Hôpital L. Pradel, Claude Bernard University and Hospices Civils de Lyon, Lyon, France.
Pasquale Pagliaro36
36Department of Clinical and Biological Sciences, University of Turin, Torino, Italy; National Institute of Cardiovascular Research, Bologna, Italy.
Clarissa Pedrosa da Costa Gomes37
37Cardiovascular Research Unit, Luxembourg Institute of Health, Luxembourg, Luxembourg.
John Pernow38
38Department of Medicine, unit of Cardiology, Karolinska Institutet, Stockholm, Sweden.
Andreas Persidis39
39BioVista Inc. EU Offices, 34 Rodopoleos street, Elliniko, Athens 16777, Greece.
Sören Erik Pischke40
40Department of Immunology and Clinic of Emergencies and Critical Care, Oslo University Hospital and University of Oslo, Oslo, Norway.
Bruno K Podesser41
41Ludwig Boltzmann Cluster for Cardiovascular Research at the Centre for Biomedical Research, Medical University of Vienna, Austria.
Fabrice Prunier42
42Institut MITOVASC, UMR INSERM U1083 and CNRS 6015, CHU Angers, University Angers, France.
Tanya Ravingerova43
43Department of Cardiovascular Physiology and Pathophysiology, Head, Institute for Heart Research, CEM SAS, Bratislava, Slovakia.
Marisol Ruiz-Meana44
44Vall d'Hebron University Hospital and Research Institute, Universitat Autonoma de Barcelona; CIBERCV.
Rainer Schulz45
45Institute of Physiology, Justus-Liebig University Giessen, Giessen, Germany.
Alina Scridon46
46Department of Physiology, University of Medicine and Pharmacy of Tirgu Mures, Romania; Center for Advanced Medical and Pharmaceutical Research, Tirgu Mures, Romania.
Katrine H Slagsvold47
47St. Olavs hospital, Trondheim University Hospital; Norwegian University of Science and Technology.
Jacob Thomsen Lønborg48
48Department of Cardiology; Rigshospitalet; Copenhagen University Hospital; Denmark.
Belma Turan49
49Department of Biophysics, Ankara University Faculty of Medicine, Ankara Turkey.
Niels van Royen50
50Department of Cardiology, Radboud University Medical Center, Nijmegen, the Netherlands.
Marko Vendelin51
51Laboratory of Systems Biology, Department of Cybernetics, School of Science, Tallinn University of Technology, Estonia.
Stewart Walsh52
52HRB Clinical Research Facility, Galway, Ireland. Funding Source Health Research Board Ireland.
Derek Yellon53
53The Hatter Cardiovascular Institute, University College London.
Nace Zidar54
54Faculty of Pharmacy, University of Ljubljana, Aškerčeva cesta 7, 1000 Ljubljana, Slovenia.
Coert J Zuurbier55
55Laboratory of Experimental Intensive Care and Anesthesiology, Department of Anesthesiology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Péter Ferdinandy*56
56Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary; Department of Biochemistry, University of Szeged, Szeged, Hungary; Pharmahungary Group, Szeged, Hungary.
Derek J Hausenloy*57
57The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, St Bartholomew’s Hospital, London, UK; National Institute of Health University College London Hospitals. Biomedical Research Centre, London, UK; National Heart Research Institute Singapore, National Heart Centre, Singapore; Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore; Yong Loo Lin School of Medicine, National University Singapore, Singapore.
*These two authors should be considered joint last authors.
Corresponding authors:
Professor Derek J. Hausenloy
Email: d.hausenloy@ucl.ac.uk

In a new window | Download PPT
Figure 1: Overview of the EU-CARDIOPROTECTION COST Action CA16225 organization and activities. The COST Action Core Group will oversee the EU-CARDIOPROTECTION COST Action Program (4 years’ duration, Oct 2017 to Sept 2021) in conjunction with the COST Action Management Committee (MC). The COST Action will utilize the COST Action Networking Activities to achieve the research objectives of the four Working Groups (WGs).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10938 | 64 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA