Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Coronary microvascular dysfunction: Implications in ischemic heart disease and therapeutic interventions
Time:2022-05-24
Number:10041
Sofia Lamprou1, Theano Dermintzoglou1, Dimitris Christos Kleitsiotis1, Ioanna Andreadou1
Author Affiliations


- 1Laboratory of Pharmacology, School of Pharmacy, National and Kapodistrian University of Athens, 15771, Athens, Greece.
Conditioning Medicine 2022. 5(1): 30-50.
Abstract
Ischemic heart disease (IHD) is the leading cause of morbidity and mortality worldwide. For many years, myocardial ischemia has been commonly recognized as the consequence of obstructive coronary artery disease (CAD). Nonetheless, a sizeable proportion of patients with ischemic symptoms demonstrate non-obstructed arteries during coronary angiography. Nowadays, a large body of evidence indicate that other mechanisms contribute to myocardial ischemia that are related to the epicardial segments of the coronary tree. In this context, coronary microvascular dysfunction (CMD) has been recently proposed as a major driver of IHD. CMD is a heterogeneous disease, which determines angina and ischemic episodes either in the absence or in the presence of obstructive CAD. Despite its high prevalence, patients with CMD are still underdiagnosed and under-treated, and thus, there is an unmet clinical need for treatments for CMD. In this review, we sought to evidence the relevance of CMD in the whole spectrum of IHD, including obstructive, non-obstructive, and chronic and acute syndromes. In this respect, we report key pathophysiological features of CMD, which mainly manifest as structural, functional, and extravascular alterations. Moreover, we discuss underlying molecular and mechanical effectors mediating its progression, namely inflammation, oxidative stress, shear stress, and autonomic dysregulation. Further we summarize available pharmacological interventions. The purpose of this review is to highlight the contribution of CMD in IHD, concluding with the perspective that elucidation of the underlying pathophysiology, as well as the proper assessment and stratification of CMD endotypes is imperative in order to move towards targeted pharmacological interventions.
Keywords: Ischemic heart disease, Coronary microvascular dysfunction, Pathophysiological alterations, Molecular and mechanical effectors, Pharmacological interventions, Oxidative stress
Abstract
Ischemic heart disease (IHD) is the leading cause of morbidity and mortality worldwide. For many years, myocardial ischemia has been commonly recognized as the consequence of obstructive coronary artery disease (CAD). Nonetheless, a sizeable proportion of patients with ischemic symptoms demonstrate non-obstructed arteries during coronary angiography. Nowadays, a large body of evidence indicate that other mechanisms contribute to myocardial ischemia that are related to the epicardial segments of the coronary tree. In this context, coronary microvascular dysfunction (CMD) has been recently proposed as a major driver of IHD. CMD is a heterogeneous disease, which determines angina and ischemic episodes either in the absence or in the presence of obstructive CAD. Despite its high prevalence, patients with CMD are still underdiagnosed and under-treated, and thus, there is an unmet clinical need for treatments for CMD. In this review, we sought to evidence the relevance of CMD in the whole spectrum of IHD, including obstructive, non-obstructive, and chronic and acute syndromes. In this respect, we report key pathophysiological features of CMD, which mainly manifest as structural, functional, and extravascular alterations. Moreover, we discuss underlying molecular and mechanical effectors mediating its progression, namely inflammation, oxidative stress, shear stress, and autonomic dysregulation. Further we summarize available pharmacological interventions. The purpose of this review is to highlight the contribution of CMD in IHD, concluding with the perspective that elucidation of the underlying pathophysiology, as well as the proper assessment and stratification of CMD endotypes is imperative in order to move towards targeted pharmacological interventions.
Keywords: Ischemic heart disease, Coronary microvascular dysfunction, Pathophysiological alterations, Molecular and mechanical effectors, Pharmacological interventions, Oxidative stress
Introduction
The term “ischemic heart disease” (IHD) refers to a pathological condition characterized by the mismatch between oxygen need and supply in the myocardium. Coronary artery disease (CAD) has been identified as the main culprit of myocardial ischemia, thus, the two terms are often used interchangeably (Jensen et al., 2020). However, a significant proportion of patients who undergo coronary angiography demonstrate normal epicardial coronary arteries, exhibiting few or no signs of obstruction (Patel et al., 2010). These patients represent a large clinical entity as up to half of all angina patients do not have obstructive epicardial coronary artery disease, even though they exhibit classic symptoms and signs of myocardial ischemia (Ford and Berry, 2019). However, invasive coronary angiography lacks the sensitivity to diagnose one major contributor to IHD, coronary microvascular dysfunction (CMD) (Ford and Berry, 2019).
Over the years, CMD has gained recognition as a relevant cause of IHD in the absence of coronary stenosis, as well as in ischemic syndromes with concurrent epicardial lesions (Godo and Shimokawa, 2021). Even though the microcirculation is not affected by atherosclerosis, disturbance of the blood flow in the microvessels is an established cause of myocardial ischemia (Sinha et al., 2020). Nonetheless, microvessels are not visible by routinely used imaging techniques and CMD had been under-recognized for many years, resulting in major knowledge gaps related to its pathophysiology (Berry and Duncker, 2020). However, CMD represents a burden both for patients’ health and the healthcare systems. Notably, it is estimated that CMD affects 50-65% of patients with non-obstructive CAD (Marinescu et al., 2015). Although underdiagnosed, CMD also manifests in patients with obstructive CAD. It has been demonstrated that approximately one-third of patients exhibiting signs of CMD during coronary endothelial function assessment will present with obstructive CAD after a 10-year follow-up period (Schächinger et al., 2000; Chen et al., 2016). This comes as no surprise as common pathophysiological alterations of CMD, such as endothelial and vasomotor dysregulation, are known to precede the formation of atherosclerotic lesions (Sechtem et al., 2020). Moreover, the presence of CMD might provide a plausible explanation as to the high prevalence of recurrent angina after successful revascularization. Of note, microvascular obstruction (MVO) was monitored in 56.9% of patients who received percutaneous coronary intervention (PCI) and was associated with increased risk of mortality and hospitalization (De Waha et al., 2017). A recent meta-analysis of observational studies also revealed a 4-fold increase in mortality and a 5-fold increase in major adverse cardiac events (MACE) for patients diagnosed with CMD, compared to individuals who demonstrated normal coronary microvascular circulation (Gdowski et al., 2020). These data reveal the need for reappraisal of the clinical and pharmacological management of IHD and in-depth preclinical and clinical research, focused on the smallest components of the coronary tree.
This review aims to highlight the pivotal role of CMD in the whole spectrum of IHD, encompassing both obstructive and non-obstructive syndromes. As treatment for CMD represents an unmet clinical need, we sought to demonstrate its key pathophysiological features, which are categorized into structural, functional and extravascular defects. Moreover, we summarize major molecular and mechanical effectors that drive the progression of CMD. By elucidating the underlying molecular mechanisms and pathophysiological alterations, we hope to comprehensively discuss the contribution of CMD to myocardial ischemia, in a variety of clinical settings. Finally, we summarize available pharmacological interventions, which we discriminated as conventional and novel. Given that data extracted from official guidelines are still scarce and the use of many drugs in CMD is empirical, we only include drugs that are evaluated on the clinical, or at least preclinical level.
Overview of coronary microvascular dysfunction (CMD)
Definition and clinical classification
CMD is defined as a disorder affecting the structure and/or function of the coronary microcirculation (Gdowski et al., 2020). Microcirculation is composed of vessels with a lumen diameter of <400 μm, ranging from arterioles to similar-sized venules and the capillaries (Taqueti and Di Carli, 2018). The long-established role of arterioles is the regulation of vascular resistance and hence, modulation of blood flow (Gutterman et al., 2016). However, structural and functional abnormalities documented in CMD impede the ability of the microcirculation to meet cardiac metabolic demands and result in decreased coronary flow reserve (CFR) values. CFR reflects the microcirculation's competence to increase coronary blood flow (CBF) above resting values in response to pharmacological vasodilation (Duncker et al., 2015).
CMD has been referred to by various terms in past, such as “cardiac syndrome X” or “chest pain with normal coronary arteries” (Herrmann et al., 2012). In 2007, Camici and Crea (2007) classified CMD into four main categories based on the underlying pathophysiology and clinical setting in which it manifests: (i) CMD in the absence of myocardial diseases and obstructive CAD, (ii) CMD in evident myocardial disease, (iii) CMD in obstructive CAD, and (iv) iatrogenic CMD. This classification has been recently revised according to the severity of CAD (Padro et al., 2020). Furthermore, a more simplistic classification has also been proposed in which CMD is divided into three main phenotypes: (i) without concurrent atherosclerosis, (ii) with non-obstructive atherosclerosis, and (iii) with obstructive atherosclerosis (Taqueti and Di Carli, 2018).
Diagnosis
Clinical features of CMD overlap with ones caused by obstructive CAD, and thus the clinical presentation alone is not sufficient for the diagnosis. However, the patient’s clinical presentation and possible comorbidities determine the assessments on which the diagnosis is based (Ong et al., 2020).
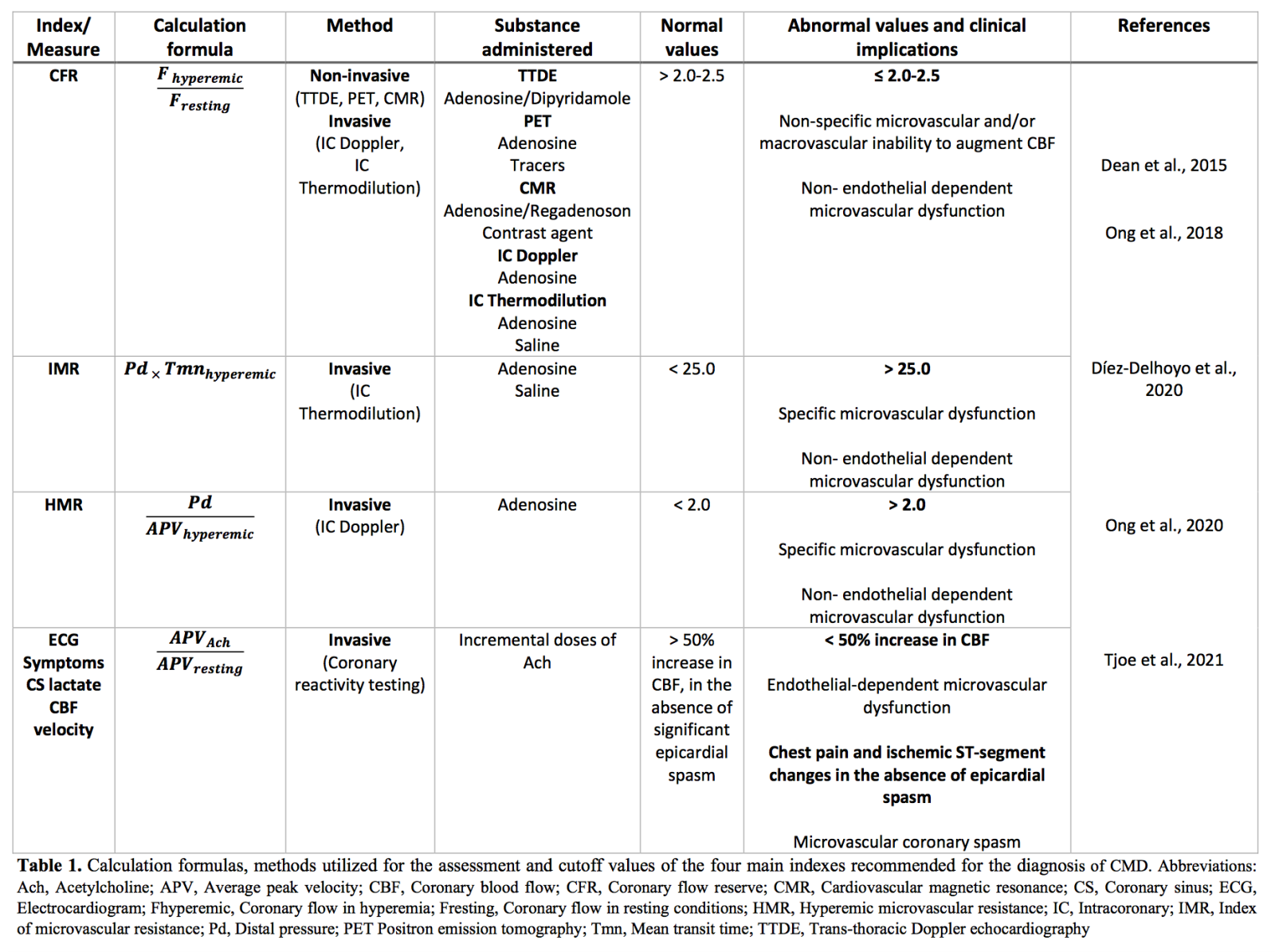
Considering that coronary microcirculation is beyond the resolution of currently available visualization techniques, diagnosis depends on the functional assessment of the coronary arteries using either invasive (e.g. coronary angiography, Doppler-flow wire, CFR, and index of microvascular resistance) or non-invasive methods (e.g. positron emission tomography, myocardial contrast echocardiography, cardiac computed tomography, and cardiac magnetic resonance) (Padro et al., 2020). The gold standards for the assessment are myocardial perfusion reserve (MPR) using positron emission tomography or cardiac magnetic resonance and its invasive equivalent, CFR. CFR is calculated as the ratio of maximum hyperemic coronary flow velocity to coronary flow velocity measured under resting conditions (Ong et al., 2020). The velocity can be measured via an intracoronary Doppler flow wire placed in the distal segment of the artery of interest or via temperature-sensitive diagnostic wire (Buono et al., 2021). Healthy subjects usually have high CFR values ranging from 2.5 – 5.0, due to the almost linear relationship between coronary blood flow and myocardial oxygen demand (Ong et al., 2020). The threshold value at which these CMD indicators define dysfunction varies across different studies (Marinescu et al., 2015). In general, a cutoff of CFR value of <2 is an accepted indication of abnormality, even though cutoff values depend on the patient characteristics and the diagnostic technique used (Xu et al., 2020). The main limitation of CFR and MPR is their inability to discriminate between epicardial and microvascular flow disruptions, especially in the presence of obstructive CAD (Shome et al., 2017; Xu et al., 2020). Moreover, CFR is calculated based on baseline coronary flow velocity and therefore, has a strong dependence on a patient’s resting hemodynamics. Contrarily, a parameter independent of baseline coronary flow is microvascular resistance (MVR). According to the technique used for coronary flow measurements, MVR is expressed either as the index of microvascular resistance (IMR), or as hyperemic microvascular resistance (HMR) (Díez-Delhoyo et al., 2020). Both CFR and IMR carry a significant prognostic value for clinical outcome of patients with CAD. In fact, a combination of low CFR and high IMR increases the risk of MACE independently of the extent of the epicardial stenosis (Lau et al., 2018).
Consequently, one main goal of CMD researchers is the extraction of reference values so as to facilitate the interpretation of results between distinct hemodynamic clinics (Masi et al., 2020). Moreover, due to the heterogeneity of the disease, the idea of integrated protocols assessing different mechanisms is intriguing. In line with this, an interventional diagnostic procedure (IDP), which includes acetylcholine coronary reactivity testing, as well as CFR and MVR assessment with adenosine using a dedicated wire should be adopted in the future (Ong et al., 2020).
At present, no standardized diagnostic algorithm is proposed. In 2019, the European Society of Cardiology (ESC) published guidelines for chronic coronary syndromes, which include recommendations for the diagnosis of microvascular angina (MVA). According to these guidelines, the possibility of a microcirculatory origin in angina should be explored by evaluating both microcirculatory conductance and arteriolar dysregulation. Impaired microcirculatory conductance is assessed by CFR and IMR measurements, while arteriolar dysregulation is evaluated by invasive acetylcholine provocation testing (Knuuti et al., 2020). Nonetheless, these guidelines specifically refer to the diagnosis of CMD in the absence of obstructive CAD, and therefore, do not address comprehensively the occurrence of CMD in a variety of other clinical settings.
A summary of the four main diagnostic indexes, accompanied with their calculation formulas, methods utilized for their assessment, and cutoff values is provided in Table 1.
Underlying pathophysiological alterations
CMD is driven and sustained by structural, functional, and extravascular alterations in the microcirculation of patients with cardiovascular risk factors. As a result, CBF through the microvascular compartment is impaired, potentially leading to sub-clinical or clinical myocardial ischemia (Taqueti and Di Carli, 2018).
Microvascular structural alterations result in increased vascular resistance due to narrower vessel lumen or decreased capillary density (i.e., capillary rarefaction) (Beltrame et al., 2009). Such structural defects are often documented in hypertension (Dabiri Askari et al., 2009) and hypertrophic cardiomyopathy (Camici et al., 2020). Reductions in the vascular lumen diameter could be attributed to intraluminal obstruction (e.g. microemboli), increased vascular size (e.g. adverse vascular remodeling), or extravascular compression (e.g. ventricular hypertrophy) (Beltrame et al., 2009). Vascular remodeling primarily occurs due to smooth muscle cell hypertrophy and fibrosis, resulting in reduced wall/lumen ratio and increased stiffening of the vascular wall. In fact, ageing is a common risk factor for the development of arterial stiffness (Scioli et al., 2014). This is corroborated by findings emerging from a preclinical study on normocholesterolemic rabbits, in which ageing was correlated with a fourfold increase in myocardial interstitial collagen (Orlandi et al., 2004). In patients, the remodeling has a wide distribution in the coronary microcirculation and usually affects the whole left ventricle (LV) (Kaski et al., 2018). These structural changes occur irrespective of CAD and induce gradual reductions in CFR, mimicking the effects of flow-limiting epicardial stenosis (Camici et al., 2015; Kaski et al., 2018).
Functional abnormalities underlying the pathophysiology of CMD arise from impaired dilation and/or constriction of the coronary pre-arterioles and arterioles (Camici et al., 2015). The mechanism of coronary vasomotor dysfunction may be endothelium-dependent or endothelium-independent (Crea et al., 2021). Endothelium-derived relaxing factors (EDRFs) include vasodilatory prostaglandins (e.g. PGI2), nitric oxide (NO), and endothelium-derived hyperpolarizing factors (EDHF). The contribution of each factor to the regulation of vascular tone depends on the size of the vessel of interest. NO participates mainly in the vasodilation of large conduit vessels, such as aorta or epicardial coronary arteries, whereas its importance decreases as vessel size decreases. Inversely, in smaller vessels, including resistance arteries, small mesenteric arteries, and coronary microvessels, EDHF plays a more critical role in vasodilation (Shimokawa and Godo, 2020). However, a recent comparative study indicated that both NO- and EDHF-mediated digital vasodilation measured in fingertip arterioles tended to decrease in patients with microvascular angina (Ohura-Kajitani et al., 2020). The endothelium-independent mechanism relies on myocyte tone and functional abnormalities in vascular smooth muscle cells (VSMCs) in CMD patients (Taqueti and Di Carli, 2018). As proof, patients with cardiovascular risk factors, such as diabetes, demonstrate attenuated vasodilator response to adenosine and dipyridamole, which exert their effects on the VSMCs of resistive vessels (Yokoyama et al., 1997; Di Carli et al., 2003). Microvascular spasm is also incorporated in the spectrum of vasomotor disturbances of CMD and could lead to myocardial ischemia in the absence of increased cardiac workload, triggering rest angina (Kaski et al., 2018). Herein, microvascular spasm alone could lead to clinically overt ischemia. In fact, it has been reported that intracoronary acetylcholine infusion in rest angina patients induces ischemic electrocardiographic (ECG) changes and myocardial lactate production without concurrent epicardial stenosis or spasm (Mohri et al., 1998).
Last but not least, the efficient perfusion of the myocardium is influenced by extravascular forces, as the coronary vasculature interacts closely with cardiomyocytes, as well as with the extracellular matrix (Westerhof et al., 2006). In fact, unlike other organs, perfusion in the heart occurs during diastole, as with systole the vasculature is compressed by the adjacent cardiomyocytes and therefore, flow is impeded (Carabello, 2006). Wave intensity analysis allows quantification of the forces that regulate flow and pressure within the coronary circulation (Broyd et al., 2017). The balance between accelerating and decelerating waves is described by the term coronary perfusion efficiency (Sinha et al., 2020). By utilizing this technique, a study indicated that patients with CMD have decreased perfusion efficiency during exercise and adenosine-induced hyperemia (Rahman et al., 2019). The main discrimination with exercise between “normal CFR” and one regarded as CMD was related to the microcirculation-derived backward waves (Rahman et al., 2019), which are considered the dominant source of diastolic coronary flow in healthy individuals (Silva et al., 2013). According to Rahman et al. (2019), patients with CMD are predisposed to ischemia as reduced perfusion efficiency indicates that a greater amount of energy is needed to augment CBF.
Mechanical, cellular, and molecular effectors of CMD
Oxidative stress and inflammation
The endothelial barrier lining both macro- and microvessels is a major cellular target for risk factor-induced pathology (Granger et al., 2010). The normal vasomotor and protective functions of the endothelium are compromised under its exposure to inflammatory, prothrombotic, and oxidative stimuli, which lead to the activation of endothelial cells (Crimi et al., 2009). Indeed, patients with microvascular spasm have activated endothelial cells significantly more often than patients with epicardial spasm or normal individuals (Lindemann et al., 2018).
Oxidative stress is considered the hallmark of pathophysiology of diseases related to most cardiovascular risk factors (Granger et al., 2010), especially diabetes (Andreadou et al., 2021) and ageing (Scioli et al., 2014). A recent study linked microvascular endothelial dysfunction (as monitored through impaired acetylcholine-induced vasodilation) with elevated levels of reactive oxygen species (ROS) produced by NADPH oxidase in obese individuals (La Favor et al., 2016). The deleterious effects of ROS are manifested through minimized NO bioavailability, either by reducing its synthesis and/or by increasing its oxidative inactivation (Tousoulis et al., 2014). Shear-stress induced NO-mediated vasodilation is a predominant vasodilatory mechanism in small coronary arteries and arterioles. However, flow-induced vasodilation was impaired in coronary arterioles of aged rats. The reduced vasodilatory capacity was attributed to enhanced superoxide anion and peroxynitrite (ONOO-) production, impaired endothelial nitric oxide synthase (eNOS) and SOD activity, and upregulated inducible NOS (iNOS) and NADPH expression (Csiszar et al., 2002). Apart from vasodilation, NO exerts anti-inflammatory, anti-apoptotic, anti-proliferative, and anti-thrombotic properties, while it also promotes angiogenesis. When NO is depleted, vasodilation is induced through other EDRFs, such as H2O2, which is a key compensatory mechanism of vasodilation in the microcirculation (Gutterman et al., 2016; Shimokawa and Godo, 2020). However, chronic release of H2O2 promotes inflammation, smooth muscle cell proliferation, activation of endothelial cells, and thrombosis (Gutterman et al., 2016). Furthermore, vasoconstriction is exacerbated when NO levels are diminished because endothelial cells start to secrete vasoconstrictors (endothelin, prostaglandin, and thromboxane) (Vancheri et al., 2020). In general, increases in oxidative stress impair proper endothelium-dependent relaxation and abolish the protective, non-vasomotor NO functions (Vanhoutte et al., 2017).
At the same time, many pro-inflammatory enzymes (e.g. phospholipase A2) and transcription factors (e.g. nuclear factor kappa-B, NF-κΒ) are susceptible to oxidative activation (Granger et al., 2010). As a result, oxidative stress enhances the biosynthesis of lipids (e.g. platelet activation factor, leukotrienes), adhesion molecules (e.g. vascular adhesion molecule-1, intracellular adhesion molecule-1, P-selectin, E-selectin) and cytokines (e.g. IL-8) (Tousoulis et al., 2014). Therefore, sites of endothelial dysfunction are also characterized by the presence and adhesion of leukocytes, primarily macrophages and T-lymphocytes (Tousoulis et al., 2014). Leucocyte-endothelial cell adhesion has been monitored in postcapillary venules in diabetes (Smolock et al., 2011) and hypercholesterolemia (Stokes, 2006). In fact, it has been proposed that hypercholesterolemia-induced leucocyte-endothelial adhesion is mediated through ROS-stimulated production of adhesion molecules, as previously described (Stokes, 2006). Taken together, oxidative stress and inflammation emerge as concurrent underlying events in endothelial dysfunction and, in fact, reinforce each other.
Consequently, chronic inflammation is an emerging risk factor for CMD. In line with this concept, a study implicated that C-reactive protein (CRP), an indicator of inflammation, measured by a high sensitivity assay (hs-CRP), correlates with symptoms and ECG signs of myocardial ischemia in microvascular angina patients (Cosín-Sales et al., 2003). Likewise, another study, which enrolled patients with cardiac syndrome X indicated a negative correlation between CRP levels and CFR (Recio-Mayoral et al., 2013). Therefore, CRP, or other mediators of the inflammatory process might have prognostic, or even therapeutic value for CMD.
Platelet – endothelium interactions
Platelets also bind to vascular endothelium and promote its activation. This is corroborated by experimental data where co-incubation of cluster of differentiation (CD)40L+ platelets with EC monolayers resulted in enhanced expression of endothelium-derived adhesion molecules and IL-8, a neutrophil chemoattractant cytokine (Stokes and Granger, 2012). In contrast to their interaction with large vessels, platelets are able to bind to intact inflamed microvessels without concurrent exposure of extracellular matrix components (Padro et al., 2020). The adhesion molecules utilized by platelets to attach to microvessels have been identified. P-selectin glycoprotein ligand-1 (PSGL-1), glycoprotein (GP)IIb/IIIa-fibrinogen-ICAM-1, and vWF-GPIba interactions served as major adhesion pathways. Moreover, binding of platelets to neighboring leukocytes increased superoxide concentrations and low shear rates significantly increased platelet-endothelium interactions (Tailor et al., 2005). Adherence to the endothelium makes platelets effectors of the inflammatory process, since they release a cornucopia of factors from their granules, either by synthesizing new bioactive molecules (e.g. ROS and thromboxane) or shedding existing molecules from their surface (e.g. CD40L) (Stokes and Granger, 2012).
Hemodynamic forces
The flow-dependent physical forces, which act upon and are sensed by the endothelium are main regulators of its homeostasis. These forces include wall shear stress (WSS), a tangential force, induced by blood flow on the arterial wall and tensile stress induced by blood pressure (Urschel et al., 2021). Coronary blood flow is also regulated by mechanical forces induced by pulse pressure, which is the difference between systolic and diastolic blood pressure. Indeed, enhanced perfusion pulsatility in basal as well as high output conditions, such as exercise, leads to increase in coronary flow in vivo (Pagliaro et al., 1999). It has also been demonstrated in canine experimental models that activation of intermediate- and small-conductance potassium-calcium channels, as well as adenosine and activation of ATP-sensitive potassium channels (KATP), contribute to modulation of pulsatile flow and endothelial-dependent coronary vasodilation (Pagliaro et al., 1999; Paolocci et al., 2001). Regarding the contribution of WSS, specific receptors located on the endothelial glycocalyx surface translate WSS into biochemical signals that regulate vascular tone, platelet activation, leukocyte adhesion, and endothelial permeability. Shear stress is a physiological signal, which could lead to release of EDRFs and consequent vasodilation. On the other hand, when abnormal mechanical forces are present, ECs could sense and translate them into cellular signaling events that initiate vascular damage (Padro et al., 2020). These contradictory effects of WSS depend on its type and magnitude. Steady laminar flow prompts ECs to enhance production of NO, prostacyclin, plasminogen activator, and several other vasodilatory or anti-inflammatory mediators. In contrast, disturbed turbulent flow predisposes endothelium to functional abnormalities and atherosclerosis, because it promotes endothelial apoptosis, permeability, and inflammation (Abe and Berk, 2014). Furthermore, low WSS is related to plaque progression, constrictive remodeling and atherogenesis, whereas high WSS is related to increased necrotic core area and expansive remodeling (Hung et al., 2016). A study managed to determine the association between low endothelial shear stress (ESS) and microvascular endothelial dysfunction in patients with non-obstructive coronary atherosclerosis. It was observed that coronary arteries with abnormal microvascular endothelial function exhibited significantly lower ESS compared to normal ones. Furthermore, preexisting microvascular dysfunction was associated with increased likelihood of transition to epicardial dysfunction, since low ESS may contribute to inflammation, atherosclerosis progression, vasoconstriction and, eventually, to epicardial abnormalities, too. Therefore, microvascular endothelial dysfunction acts both as a marker and a contributor to diffuse atherosclerosis (Siasos et al., 2018).
Autonomic dysregulation
Autonomic dysregulation results in enhanced sympathetic activation, which manifests as increased vasoconstriction (Padro et al., 2020). In patients with atherosclerotic risk factors subjected to glucose administration, it was proved that acute hyperglycemia (AHG) enhanced CMD, evidenced by decreased CFR, and CMD was significantly correlated with increased sympathetic tone caused by AHG. The investigators drew the conclusion that even though AHG does not affect coronary blood flow in healthy patients, it disrupts coronary microvascular function in subjects with a diseased coronary arterial bed. AHG could also induce sympathetic activation directly and/or via the induction of hyperinsulinemia and oxidative stress. Increased adrenergic tone causes coronary vasoconstriction and thus increased myocardial oxygen demand, exacerbating existing endothelial dysfunction (Takei et al., 2007). Even though healthy endothelium resists α-adrenergic vasoconstriction via shear stress- or α2-adrenergic-mediated mechanisms, dysfunctional endothelium is susceptible to adrenergic constrictor stimuli (Heusch et al., 2000). Intense vasoconstriction, mediated via α1 or α2 adrenoreceptors of VSMCs of macro- and microcirculation, can eventually mitigate perfusion and induce myocardial ischemia (Pries and Reglin, 2017). In a past study, cardiac nerve function was evaluated via metaiodobenzylguanidine (MIGB) scintigraphy, an indicator of adrenergic innervation of the heart. It was show that global and regional abnormalities were present in 75% of the non-obstructive coronary artery disease (INOCA) patients in contrast to only 42% of the control group. Therefore, abnormal function in cardiac sympathetic nerve endings could be the cause of the imminent angina. Cardiac MIBG defects may also reflect increased cardiac spillover of norepinephrine, which competes with MIGB in an antagonistic manner for uptake at nerve terminals. This could explain several clinical findings in these patients, including increased adrenergic signaling, reduction of CFR and heterogeneous myocardial perfusion patterns (Lanza et al., 1997).
A schematic representation of the four main mechanical, molecular, and cellular effectors contributing to CMD development is illustrated in Figure 1.
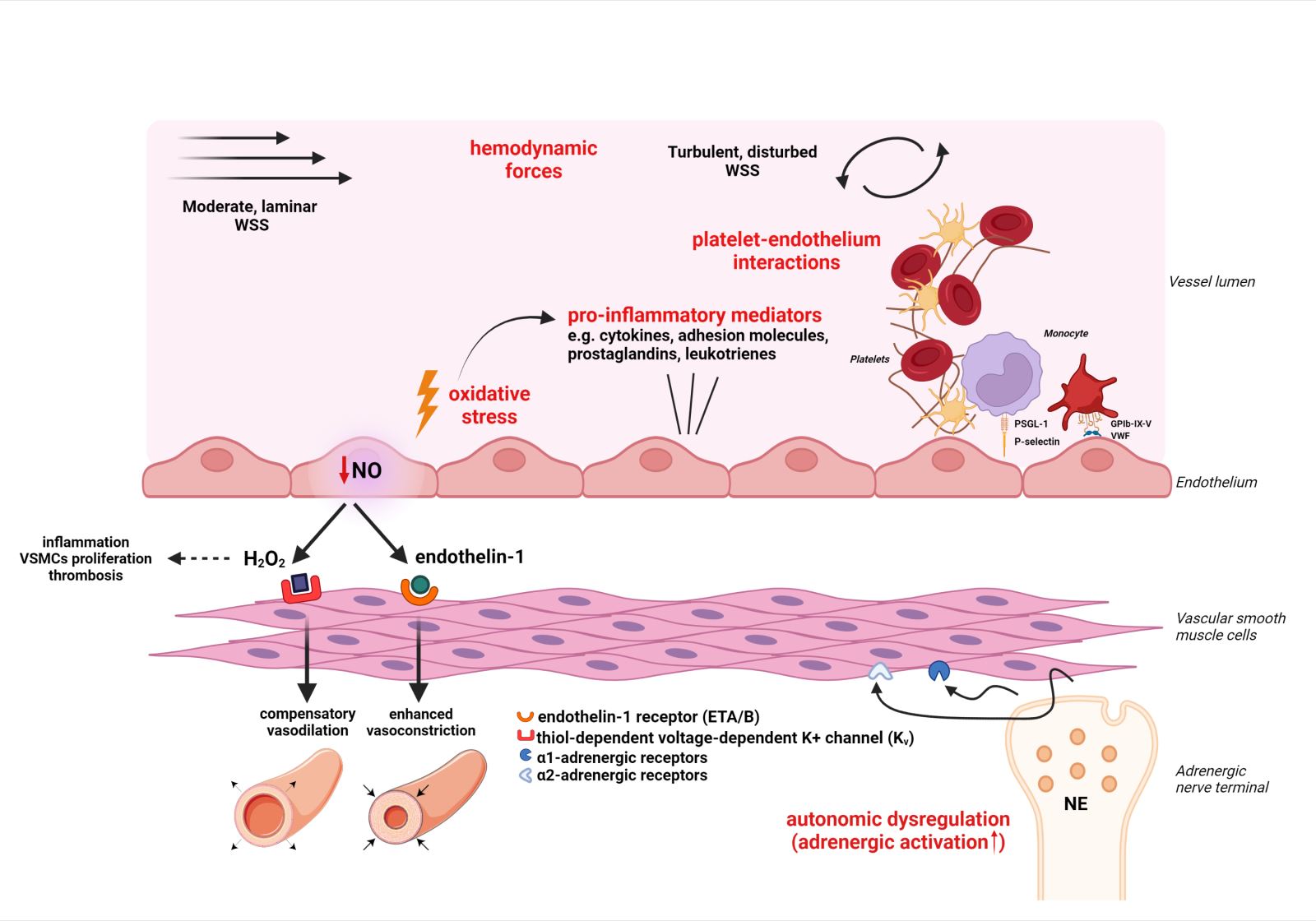
In a new window | Download PPT
Figure 1: Schematic representation of four main mechanical, cellular and molecular effectors of coronary microvascular dysfunction (see respective module). These are noted in red color and include: i. Oxidative stress and inflammation ii. Platelet – endothelium interactions iii. Hemodynamic forces iv. Autonomic dysregulation. Abbreviations: EDRF, Endothelium-derived relaxing factors; eNOS, endothelial NO synthase; ET, Endothelin; GPIb-IX-V, Glycoprotein Ib-IX-V; NE, Norepinephrine; PSGL-1, P-selectin glycoprotein ligand-1; ROS, Reactive oxygen species; VSMC, Vascular smooth muscle cell; VWF, von Willebrand factor; WSS, Wall shear stress Created with BioRender.com
Coronary microvascular dysfunction as a relevant cause of myocardial ischemia
Transient myocardial ischemia could be the consequence of various causes such as obstructive CAD, epicardial coronary artery spasm, elevated LV intramyocardial compression of the coronary intramural network, and reduced diastolic time when metabolic vasodilation is no longer able to increase CBF (Levy et al., 2019). In addition to conventional ischemic causes, CMD is now recognized as a significant contributor to myocardial ischemia. CMD could act alone or in combination with the aforementioned mechanisms to impair CBF (Crea et al., 2014). CMD induces myocardial ischemia by compromising the ability of the coronary microcirculation to augment CBF above resting values in response to increased myocardial metabolic demands or by initiating coronary microvascular spasm (Kaski et al., 2018). These mechanisms are driven by the detrimental structural and/or functional microvascular defects that underlie the pathophysiology of CMD.
Coronary microvascular dysfunction in coronary syndromes underlying ischemic heart disease
Ischemic heart disease (IHD) is a multifactorial, chronic and progressive disease, but it can unexpectedly initiate an acute episode. For many years, CAD has been considered as the main underlying pathological process driving IHD. Because of its dynamic nature, CAD can result in a variety of clinical conditions, often simply categorized as chronic coronary syndromes (CCS), or acute coronary syndromes (ACS). Before the introduction of the term CCS by the 2019 ESC guidelines, it was described as stable ischemic heart disease or stable coronary artery disease. ΑCS encompass unstable angina pectoris and myocardial infarction (MI), which is further divided into ST-segment elevation myocardial infarction (STEMI) and non-STEMI (Jensen et al., 2020; Neumann et al., 2020).
Coronary microvascular dysfunction in non-obstructive chronic coronary syndromes
Conditions defined by the presence of symptoms and signs of ischemia without significant artery stenosis (<50% of the lumen diameter) are termed INOCA (Merz et al., 2017). INOCA is associated with higher risk of MACE and all-cause mortality compared with individuals without IHD (Jespersen et al., 2012). MVA, defined as the clinical manifestation of myocardial ischemia due to CMD without obstructive CAD, is present in 52% of patients presenting with INOCA (Ford et al., 2019).
The specific mechanisms underlying CMD in non-obstructive CAD are still poorly understood, but endothelial dysfunction is considered a significant contributor. Indeed, endothelial dysfunction is present in approximately two-thirds of patients with non-obstructive CAD (Shaw and Anderson, 2016). Traditional cardiovascular risk factors are held accountable for the endothelium-dependent pathophysiology (Crea et al., 2014). In agreement with this, a swine model with multiple comorbidities - diabetes, hypercholesterolemia, and chronic kidney disease - demonstrated impaired myocardial blood flow and oxygen delivery during exercise, in the absence of significant epicardial atherosclerosis. According to the authors, these perturbations were mainly attributed to diminished NO bioavailability, a functional alteration of CMD, as no signs of arteriolar remodeling and fibrosis, structural alterations of CMD, were observed (Sorop et al., 2020a). Despite the high prevalence of functional abnormalities in this population, structural defects are also observed in animal models. For instance, in Ossabaw swine models with metabolic syndrome, blunted responses to adenosine and capillary rarefaction have been documented (Li et al., 2012). Accordingly, isolated coronary arterioles obtained from the same animal model demonstrated increased myogenic tone, capillary rarefaction, and decreased hyperemic flow capacity due to inward remodeling (Trask et al., 2012).
Altogether, these studies indicate that characteristic structural and functional defects of CMD on the coronary arterioles are capable of decreasing CFR values, in the absence of significant epicardial stenosis. Whether these defects act synergistically or independently in CMD-induced ischemia, remains to be elucidated.
Coronary microvascular dysfunction in obstructive chronic coronary syndromes
In patients with obstructive CCS, the correlation between the degree of stenosis and CFR markedly varies between individuals, indicating the existence of other possible contributors in myocardial ischemia (Levy et al., 2019). Results obtained from two major clinical trials, Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA), also corroborate the idea of multiple contributors in the ischemic process.
In the COURAGE trial, 34% of patients who were subjected to PCI exhibited persistent angina in 1-year follow-up. The angina prevalence of those patients was similar to the one observed in patients who did not undergo revascularization (Boden et al., 2007). Furthermore, the most recent trial, ISCHEMIA, provided a new insight into the “stenosis centric” hypothesis of IHD and concluded that an initial invasive strategy did not minimize the risk of ischemic episodes nor death from any cause, compared to an initial conservative strategy (Maron et al., 2020). So far, no randomized data have indicated a favorable impact of PCI on “hard” outcomes, such as MI and death (Tsigkas et al., 2021). In summary, a growing body of evidence indicates that additional mechanisms, including CMD, might initiate the pathological processes of ischemia and angina in patients with CCS.
There is also the notion that coronary epicardial and microvascular dysfunction reinforce each other. Alongside reductions in CBF, obstruction of epicardial arteries might also induce structural and functional alterations in distal microvessels. These include microvascular inward remodeling distal to a stenosis, vasomotor tone alterations, and coronary arteriolar and capillary rarefaction (Padro et al., 2020; Vancheri et al., 2020). In this respect, enhanced vasoconstriction in response to endothelin-1 (ET-1) was observed in isolated coronary arterioles obtained from swine with chronic stenosis. The same study documented a loss of ETB receptor-mediated vasodilation in vessels derived from the occluded LAD area, known to be mediated by NO and PGI2, whereas bradykinin vasodilation was preserved (Sorop et al., 2008). The latter might indicate a shift from NO to EDHF-mediated vasodilation, a compensatory mechanism that is enabled under oxidative stress and is documented in patients with CAD (Gutterman et al., 2016).
The concept that these microvascular defects persist after successful revascularization is validated by studies performed either on humans (Uren et al., 1993) or animal models (Kelly et al., 2011). Compared to other functional and structural abnormalities, capillary rarefaction develops quite rapidly. It is detected even after 90-min left anterior descending (LAD) artery occlusion in an experimental MI swine model (Vilahur et al., 2017).
Coronary microvascular dysfunction in non-obstructive acute coronary syndromes
ACS with normal and near normal epicardial arteries represent a heterogeneous clinical entity with a multitude of possible underlying causes, which are not always apparent. Within this condition, MI with non-obstructive CAD (≤50% stenosis of an epicardial coronary artery) is termed MINOCA. Patients with MINOCA are more often women, young individuals, and present with fewer traditional risk factors, when compared to patients with MI and CAD (Safdar et al., 2018).
Among the microvascular causes of MINOCA, coronary microvascular spasm accounts for about 25% of the cases. Moreover, microvascular spasm seems to be the underlying cause of persistent angina in 36% of MINOCA individuals. Mechanistically, endothelin and catecholamines, which are potent vasoconstrictors, are probably implicated. They reduce microvascular blood flow and thus, evoke transient myocardial ischemia (Vidal-Perez et al., 2019). In compliance with this theory, increased coronary microvascular constriction, along with impaired vasodilation was observed in a study that enrolled patients with non-obstructive acute coronary syndrome. Both vasomotor irregularities of the coronary microcirculation were persistent at 12-month follow-up (De Vita et al., 2019).
Coronary microvascular dysfunction in obstructive acute coronary syndromes
Although it is generally assumed that epicardial atherosclerosis precedes CMD (Crea et al., 2014), new insights into the pathophysiology suggest a bidirectional relationship between these two. Lerman et al. (2007) proposed a primary role of the coronary microcirculation in thrombus formation. According to this theory, microvascular dysfunction impairs CBF, which in turn alters the effect of shear stress on upstream coronary arteries (Lerman et al., 2007). As mentioned, changes in shear stress initiate inflammatory processes on the endothelium (Godo and Shimokawa, 2017), an initial and crucial step in thrombus formation on epicardial arteries (Lerman et al., 2007). Evidence that dyslipidemia adversely affects the microcirculation prior to the development of evident atherosclerosis also corroborate this theory. Pathophysiological processes underlying this effect encompass aggravated arginase activity, increased production of ROS and enhanced recruitment of inflammatory cells (Padró et al., 2018). Nonetheless, our current knowledge regarding the profound role of CMD in lesion formation is limited due to lack of data on microvascular function prior to an ACS.
Coronary microvascular dysfunction and coronary no-reflow phenomenon
Prompt mechanical reperfusion of the infarct-related artery by urgent PCI is the standard of care for acute MI (Crea et al., 2014). However, in a sizeable proportion of patients, re-opening of the artery does not translate into adequate myocardial reperfusion due to insufficient perfusion of the downstream capillaries supplying the myocardium (O’Farrel et al., 2017). This condition is known as no-reflow phenomenon and is related to microvascular defects caused by abrupt disruption of blood flow followed by its acute restoration during revascularization (Vancheri et al., 2020).
Chronic occlusion of a coronary artery severely impacts the function and architecture of downstream microvessels. Characteristic findings include endothelial swelling, myocyte edema and neutrophil infiltration. Coronary reperfusion exaggerates these defects; neutrophils and microthrombi obstruct the microvessels, ROS and complement activation initiate inflammation, and adjacent myocytes contract (Ito, 2014). Additionally, PCI itself could contribute to microvascular damage by causing microembolization of plaque debris, which obstruct the microvessels and initiate perivascular inflammatory reactions (Niccoli et al., 2017). At the same time, microembolization increases coronary artery resistance and initiates micro-infarcts (Ito, 2014).
As a result, MVO impedes the healing response of the affected myocardium, leading to thinning and enlargement of the necrotic zone. In fact, no-reflow is associated with increased prevalence of complications after MI, including adverse LV remodeling, heart failure, reduced LV ejection fraction, increased infarct size, and death (O’Farrell and Attwell, 2014). A recent pooled analysis of seven randomized trials enrolling STEMI patients who received primary PCI, revealed that MVO was present in 56.9% of patients after PCI, and the presence and extent of MVO was strongly associated with mortality and hospitalization for heart failure within 1 year (De Waha et al., 2017).
An emerging theory regarding the no-reflow phenomenon focuses on the coronary pericytes. Previous animal studies have documented narrowing of brain capillaries evoked by adjacent pericytes after a stroke (Peppiatt et al., 2006; Yemisci et al., 2009). Following this perspective, a recent study in rats by O’Farell et al. (2017) suggested that pericytes are partially responsible for diminished blood flow in cardiac capillaries after an ischemic episode. In their experiments, even after restoration of the occlusion, 40% of the capillaries remained blocked. Capillary occlusion colocalized with pericytes, where lumen diameter was 37% decreased. Treatment with adenosine, a relaxant for pericytes, decreased capillary blockage by 25% and enhanced perfusion volume by 57% (O’Farrel et al., 2017).
Pharmacological approaches for coronary microvascular dysfunction treatment
At the end of the 20th century, several studies predicted a favorable long-term prognosis for patients with INOCA (Kemp et al., 1986; Lichtlen et al., 1995). Nonetheless, over the years, a growing body of multilayered evidence indicates that patients with non-obstructive CAD have a high MACE risk, compared to reference groups (Gulati et al., 2009; Jespersen et al., 2012). More than half of INOCA patients concurrently have CMD, which acts as a significant contributor to the ischemic process (Vancheri et al., 2020). Therefore, these individuals need targeted pharmacotherapy, which aims either to alleviate ischemia symptoms or to prevent future cardiac-related events. However, INOCA patients are not still treated properly and clinical management guidelines fail to address this population as sufficient evidence-based data and large-scale randomized controlled trials are largely missing (Merz et al., 2020).
Conventional pharmacotherapy for coronary microvascular dysfunction management
Current clinical management of INOCA patients largely relies on use of conventional medication for concurrent treatment of comorbidities contributing to high CVD risk (e.g. diabetes, dyslipidemia), or alleviation of existing symptoms, such as anginal chest pain, and patients are usually matched to the most relevant therapy according to their clinical phenotype (Buono et al., 2021). Thus, a significant portion of drugs conventionally used in various diseases are simultaneously tested for favorable effects regarding, specifically, coronary microvascular dysfunction.
According to a European Association of Percutaneous Cardiovascular Interventions (EAPCI) Expert Consensus Document on INOCA, angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin-receptor blockers (ARBs), beta-blockers, calcium channel blockers, ranolazine, and trimetazidine are among the drugs commonly administered to patients presenting with INOCA along with microvascular angina. As far as vasospastic angina (VSA) is concerned, the same expert group recommends administration of calcium channel blockers, nitrates, or nicorandil to this patient group (Kunadian et al., 2021). In addition, the 2019 ESC guidelines for diagnosis and management of chronic coronary syndromes suggested that choice of treatment should depend on the mechanism or molecular pathway mainly responsible for MVA and patients, presenting CFR <2.0 or IMR ≥25 units and a negative acetylcholine provocation test, should be treated with β-blockers, ACE-Is, and statins, in combination with lifestyle changes, such as healthy diet and weight (Knuuti et al., 2020).
In fact, statins alone (e.g. pravastatin), or in combination with ACE-I (e.g. atorvastatin plus ramipril) have shown positive results when they are administered to normolipidemic INOCA patients for three or six months respectively by counteracting oxidative stress and exerting anti-inflammatory properties, thus protecting the endothelium and microvascular architecture (Kayikcioglu et al., 2003; Pizzi et al., 2004). Statins, in particular, are known for their pleiotropic effects, as they regulate inflammation, reduce lipid-rich plaque cores, inhibit the formation of foam cells, promote fibrous-cap thickening, and decrease platelet reactivity (Pinal-Fernandez et al., 2018). Recently, fluvastatin was administered in INOCA patients in addition to the calcium channel blocker, diltiazem. After a 3-month combination treatment, CFR was improved, NO levels were increased, and endothelin-1 was decreased in comparison to treatment of each agent alone (Zhang et al., 2014). Furthermore, in a recent systematic review and meta-analysis, it was concluded that statin treatment in patients with and without established cardiovascular disease was associated with significant improvement in coronary and peripheral endothelial function (Reriani et al., 2011). Another systematic review and meta-analysis, evaluating the effect of oral statins on coronary microvascular function, showed that these drugs can enhance CFR in patients with ischemia symptoms and no evident coronary stenosis, an improvement shown irrespective of the duration of the follow-up period (Yong et al., 2020).
Quinapril, was proved beneficial in the large TREND clinical study, ameliorating acetylcholine-induced constrictive responses and endothelial dysfunction in normotensive patients. ACE-I inhibition impeded the contractile effect of angiotensin II and augmented NO release, preceded by decreased bradykinin catabolism (Mancini et al., 1996). In a more recent randomized controlled trial conducted in women with non-obstructive CAD and CFR values <3.0, the patient group that received quinapril had augmented CFR values after 16 weeks of treatment compared to placebo. This increase correlated with improved angina symptoms. This improvement might implicate reduced blood pressure values and decreased Ang-II-induced vasoconstriction. Moreover, the protective effect in individuals with lower CFR values may indicate that the renin angiotensin aldosterone system is more implicated in women with severe CMD (Pauly et al., 2011).
Intensive medical therapy (IMT) is being investigated in a large-scale clinical trial as a promising intervention that will reduce MACE incidence in INOCA patients. This treatment regimen includes high intensity statins plus ACEIs or ARBs (Beltrame et al., 2021). The Women's IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR) trial is a multicenter, prospective, randomized, open-label with blinded-endpoint analysis (PROBE designed) clinical trial, which will compare IMT vs. usual clinical care in women with INOCA in terms of MACE occurrence rate over a 3-year follow-up time period (NCT03417388) (Handberg et al., 2021). Hopefully, the results will provide knowledge regarding the effectiveness of IMT and its possible inclusion in future guidelines and insights into the best way to improve cardiovascular health in this undertreated, underdiagnosed population.
Nebivolol, a third-generation beta-blocker, combines highly selective β1-blocking activity with NO-mediated vasodilating properties, thus comprising a widely used antianginal agent, which decreases myocardial oxygen consumption, increases diastolic coronary filling time, and simultaneously improves endothelial functionality (Aldiwani et al., 2021; Buono et al., 2021). Moreover, intracoronary nebivolol provoked a significant rise in CFR in CAD patients by increasing maximal coronary flow (Togni et al., 2007). Results from the phase 4, interventional NIRVANA clinical study, regarding the effect of nebivolol on angina presented in women with CMD, are eagerly anticipated (NCT01665508).
Calcium channel blockers decrease microvascular tone, possess vasodilatory properties and mitigate coronary vasospasm; hence, they could improve CFR in CMD patients. Nonetheless, a case-controlled study concluded that intracoronary diltiazem did not affect CFR (Sütsch et al., 1995). Similarly, amlodipine failed to contain the frequency of angina symptoms, even though it improved quality of life scores in INOCA patients (Lanza et al., 1999). However, patients receiving verapamil or long-acting nifedipine in a randomized, double-blind, placebo-controlled study expressed fewer episodes of angina, diminished consumption of nitrates, and prolonged exercise tolerance duration. These beneficial effects are unlikely to solely depend on coronary vasodilation, as improved exercise tolerance was not associated with an increased pressure-rate product (Cannon et al., 1985).
Ranolazine, a potent inhibitor of late sodium current (INa) channels in cardiomyocytes, is an anti-anginal drug, which decreases sodium-triggered intracellular calcium concentration, improves left ventricular diastolic tension and relaxation, and therefore, should enhance CBF (Hasenfuss and Maier, 2008; Dermintzoglou and Andreadou, 2021). However, its effects on the CMD population are somewhat contradictory. For example, a mechanistic trial among patients with evidence of CMD found that 2-week ranolazine treatment did not ameliorate angina, as measured by the Seattle Angina Questionnaire (SAQ) (Merz et al., 2016). In contrast, a pilot trial employing women with evident INOCA showed that 4-week ranolazine treatment improved angina, as assessed by the same questionnaire (Mehta et al., 2011). Another randomized trial indicated that patients with CFR<2,5 and non-obstructive CAD presented improved angina and perfusion with ranolazine, suggesting a possible implication of the INa channels in the management of CMD (Rambarat et al., 2019). It should be noted that the above studies come with limitations, due to the exclusive use of subjective measures for the evaluation of angina symptoms, such as patient-centered questionnaires. This is in contrast to the use of more valid and objective measures, including CFR values, for functional assessment of the coronary arteries, as previously mentioned in other clinical trials.
Trimetazidine inhibits free fatty acid oxidation and shifts cardiac metabolism towards glucose utilization (Marzilli et al., 2019). Data obtained from 23 randomized trials suggest that trimetazidine, administered as monotherapy or in combination with conventional anti-anginal drugs, is effective in treating stable angina. Compared to placebo arms, trimetazidine reduced weekly angina attacks, consumption of nitrates, and improved exercise tolerance (Ciapponi et al., 2017). A randomized study, employing 60 symptomatic patients with MVA showed similar results. The addition of trimetazidine to standard treatment improved symptoms, quality of life, and exercise tolerance (Leonova et al., 2017). Nonetheless, a recent randomized, double-blinded, placebo-controlled, event-driven trial of trimetazidine, when co-administered to patients already receiving optimal pharmacotherapy after successful PCI, did not impact the recurrence of angina or the outcome (Ferrari et al., 2020).
Short-acting nitrates, a fundamental drug category for patients with angina pectoris, do not exert the same effects on patients with MVA. A study conducted by Russo et al. (2013) found no improvement on exercise stress test (EST) parameters, probably due to less prominent nitrate-induced vasodilation in the microcirculation. Furthermore, it was proved that steady-state nitroglycerin infusion could induce selective dilation of coronary arterial microvessels of diameter greater than 200 microns (Kanatsuka et al., 1992). This may explain why nitrates do not exert sufficient relief of angina symptoms in MVA patients (Aldiwani et al., 2021).
Alternative therapeutic interventions and novel pharmacological approaches
Upregulation of thromboxane A2 synthase (TXAS)/thromboxane A2 (TXA2)/thromboxane prostanoid (TP) receptor signaling pathway can result in augmentation of platelet reactivity, aggregation, and arterial constriction, thus aggravating vascular damage (Merz et al., 2020). Therefore, inhibition of the aforementioned axis is considered a promising pharmacological intervention, inducing protection against microvascular injury and malfunction. Aspirin, a potent COX2 inhibitor, leads to reduced biosynthesis of TXA2. It was demonstrated that this inhibition might confer protective effects on the microvascularate in an ischemia-reperfusion preclinical model involving TXAS-, TXA2-, or thromboxane receptor (TP)- depleted mice. Inhibition of the TXAS/TXA2/TP axis, either by aspirin treatment or by genetic depletion of the encoding genes, induced protection of the microvasculature against oxidative injury in cardiac arteries (Chiang et al., 2018).
Phosphodiesterase (PDE) type 3 inhibitor, cilostazol, is used for intermittent claudication and secondary prevention after stroke, coronary stent restenosis, and PCI (Merz et al., 2020). PDE3 inhibition enhances intracellular levels of cyclic monophosphate adenosine (cAMP), and therefore, it exerts vasodilatory and antiplatelet properties (Liu et al., 2001). These effects were examined in VSA patients. Compared to the other two groups receiving no antiplatelet agent or aspirin, the one receiving cilostazol exhibited increased CFR and flow-dependent coronary dilation, due to increased cAMP-mediated NO production (Watanabe et al., 2003). Additionally, a prospective, multicenter study indicated that cilostazol effectively reduces angina frequency and VSA symptoms when administered to individuals whose symptoms are insufficiently controlled by conventional treatment (Yoo et al., 2013).
Furthermore, inhibition of a different PDE isoform, PDE5, augments NO production by diminishing cyclic monophosphate guanosine (cGMP) breakdown, thus contributing to vascular smooth muscle cell relaxation (Buono et al., 2021). The PDE5 inhibitor, sildenafil, was administered to women with INOCA and significantly improved CFR values, an effect mainly displayed by those with CFR <2.5 (i.e. CMD patients) (Denardo et al., 2011). On the contrary, a previous study suggested that sildenafil does not improve peripheral endothelium-mediated vasomotor activity or fibrinolytic function and, therefore, it cannot counteract the diffuse vascular abnormalities observed in CAD patients (Robinson et al., 2006). These preliminary data collectively highlight the need for additional larger, randomized, double-blinded trials employing PDE inhibitors to further investigate their potential use in CMD patients.
Rho-kinase (ROCK) is a small guanosine triphosphate-binding protein, which modulates the phosphorylation of contractile myofilaments and, therefore, regulates the vasoconstriction force. Upregulated ROCK signaling evokes VSMC's hypercontraction (Merz et al., 2020). Elevated ROCK activity in circulating neutrophils could also be clinically valuable for the prognostic stratification of VSA patients (Nihei et al., 2018). When administered intracoronary, fasudil, a potent ROCK inhibitor, ameliorates the acetylcholine-induced coronary vasospasm and resultant myocardial ischemia without affecting systemic hemodynamics or baseline CBF (Masumoto et al., 2002). Except for epicardial coronary spasm, fasudil was also protective against coronary microvascular spasm in 11 out of 13 pretreated patients (Mohri et al., 2003). Therefore, ROCK inhibition might be an emerging pharmacological target for CMD.
Endothelin receptor antagonists address ET-1, a small peptide formed mainly in the endothelium, which augments vasoconstriction through ETA or ETB receptor activation on VSMCs (Corban et al., 2020). The Coronary Microvascular Angina (CorMicA) trial results indicated that resistance arteries dissected from individuals with MVA had enhanced vasoconstriction responses to ET-1 (Ford et al., 2018b). In parallel, it was also demonstrated that long-term administration of atrasentan, a selective ETA receptor antagonist, improved percent change of CBF in response to acetylcholine (i.e. improved microvascular endothelial function) in patients with endothelial dysfunction and non-obstructive CAD compared to the placebo group (NCT0027149) (Reriani et al., 2010). Furthermore, 2-week treatment with the ETA receptor antagonist, darusentan, enhanced myocardial perfusion homogeneity at rest, indicating that in CMD patients, diffuse, heterogenous perfusion patterns in coronary circulation may be caused by ET-1 activity (NCT00738049) (Johnson and Gould, 2013). The Precision Medicine With Zibotentan in Microvascular Angina (PRIZE) is an ongoing, randomized, double-blind, placebo-controlled, crossover trial examining the effects of zibotentan, an oral ETA receptor antagonist, in patients with MVA (NCT04097314). The primary endpoint is exercise tolerance, evaluated by the Full Bruce protocol (Morrow et al., 2020).
Xanthine derivatives (e.g. theophylline, aminophylline, paraxanthine, pentoxifylline) may exert beneficial effects on CMD patients. These molecules block adenosine-A2 receptors (A2R) on VSMCs. The primary effect of adenosine in coronary microcirculation is to induce vasodilation and hyperemia and, thus, these agents inhibit adenosine-mediated vasodilation (Zhang et al., 2021). It is speculated that in CMD patients, these agents may prevent excessive dilation in well-perfused regions and move blood towards poor-perfused ones in the microvasculature (Aldiwani et al., 2021). Indeed, the anti-anginal effect of aminophylline observed in a study was attributed to CBF redistribution towards ischemic regions (Crea et al., 2014). Furthermore, xanthine derivatives may obtain analgesic properties, as adenosine is a major effector of ischemic chest pain, acting upon cardiac nerve pain fibers, and intravenous administration of aminophylline has indeed exhibited reduction of the severity of adenosine- and exercise-induced chest pain (Crea et al., 1990; Merz et al., 2020). Orally administered aminophylline in a randomized trial increased patients’ exercise threshold, but ST-segment changes were comparable to the placebo group (Elliott et al., 1997). It is important to note that AR-mediated actions implicate both coronary vasculature and cardiomyocytes, making it challenging to separate vascular effect from cardioprotection (Zhang et al., 2021).
The ion channel inhibitor with indication in CCS, ivabradine, diminishes the hyperpolarization-activated current (If) current in the sinoatrial nodal tissue, resulting in decreased diastolic depolarization and, eventually, lower heart rate. The contribution of ivabradine to IHD is based on improved exercise tolerance, reduced onset of ischemia during physical exercise, reduced angina symptoms, and nitrate use (Koruth et al., 2017). Ivabradine proved beneficial when administered to patients with stable CAD, as indicated by improved CFR and hyperemic coronary flow velocity. These effects were preserved even after heart rate correction, suggesting improved microvascular function (Skalidis et al., 2011). In a different study, patients with microvascular angina received ivabradine, ranolazine, or placebo for 4 weeks and both drugs exhibited significant improvement in symptoms, even though neither of them had effects on coronary microvascular function (Villano et al., 2013).
Metformin, an antidiabetic drug and insulin sensitizer, was evaluated in a double-blind, randomized, placebo-controlled trial in nondiabetic women with INOCA. Metformin recipients demonstrated decreased body weight, insulin resistance, and significantly improved endothelium-dependent microvascular activity, assessed by acetylcholine infusion. Endothelium-independent responses remained unaffected by metformin. Metformin was also beneficial for exercise-induced ST-segment depression and chest pain incidence (Jadhav et al., 2006). Another class of antidiabetics is sodium-glucose cotransporter 2 inhibitors (SGLT2i), which has exhibited the ability to decrease CVD risk, hospitalization, and mortality for heart failure in high-risk diabetes mellitus subjects, as indicated by findings of the EMPA-REG OUTCOME clinical trial. Such encouraging outcomes from recent large, randomized clinical trials have suggested the existence of direct effects of empagliflozin on the myocardium (Fitchett et al., 2016). For example, 6-month empagliflozin treatment decreased LV mass, a strong indicator of cardiovascular events, in patients with type 2 diabetes and CAD, in the EMPA-HEART CardioLink-6 trial (Verma et al., 2019). Therefore, effect of this class of drugs on CMD currently represents a promising research field. In fact, endothelial SGLT2 inhibition in murine aorta tissue led to a decrease in hyperglycemia-induced endothelial dysfunction and, in particular, impairment of acetylcholine-mediated vasodilation. These effects are probably mediated by protection of the endothelium from excess ROS production and glucose uptake (El-Daly et al., 2018; Venu et al., 2019). Recently, 10-week treatment with empagliflozin also improved coronary microvascular function, as well as cardiac contractile function, in ob-/ob- mice, a model of early diabetes. Empagliflozin-treated mice showed that may ameliorate NO-mediated endothelial function (Adingupu et al., 2019). Last but not least, 6-hour treatment of cardiac microvascular endothelial cells (CMECs) with empagliflozin in vitro before their co-incubation with cardiomyocytes led to improved contractile and diastolic function in cardiomyocytes. Empagliflozin pre-treatment also improved the mechanical properties of cardiomyocytes and retained the beneficial effect of CMECs on those cells, even if CMECs were pre-incubated with the proinflammatory cytokine, tumor necrosis factor (TNF)-α. Moreover, empagliflozin ameliorated impaired microvascular function, as indicated by low mitochondrial ROS levels and cytoplasmic ROS accumulation, as well as increased NO bioavailability within CMECs (Juni et al., 2019).
Anti-inflammatory drugs are assessed as positive therapeutic interventions in coronary microvascular dysfunction, because inflammatory cascades, mediated by several cytokines and chemokines, are a driving force accelerating endothelial dysfunction, one of the main effectors of CMD. In fact, 30-day administration of anakinra, an interleukin (IL)-1 receptor antagonist, in rheumatoid arthritis patients led to improvement of vascular function, indicated by increased CFR and flow-mediated dilation of the brachial artery (Ikonomidis et al., 2008). Similar results were also evident after 3-month administration of anakinra or tocilizumab – an IL-6 receptor antagonist – in a larger, randomized trial, recruiting patients with rheumatoid arthritis (Ikonomidis et al., 2019). Canakinumab is another fully human antibody that binds to IL-1β, decreasing its activity, and has been tested in the large, randomized, double-blind, placebo-controlled Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) trial. Canakinumab was administered every three months to patients with previous MI and high CRP levels and resulted in significant lower MACE rates after a median 3.7-year follow-up period (Ridker et al., 2017). Furthermore, a single injection of canakinumab in chronic gout patients led to a significant decrease in arterial stiffness and intima media thickness of carotid arteries after 120 days, indicating beneficial effects on vascular function (Eliseev et al., 2016. Αnti-TNF-α agents, such as the competitive inhibitor etanercept, have been evaluated in patients suffering autoimmune diseases to treat their CMD resulting from chronic, systemic inflammation (Sagris et al., 2021). For instance, 4-month treatment with etanercept significantly improved CFR by 11% in psoriasis patients compared with baseline values. Therefore, downregulation of production and activity of cytokines involved in psoriatic lesions as well as myocardial and endothelial dysfunction could prove a beneficial therapeutic strategy (Ikonomidis et al., 2017).
Nervous system-derived factors are “druggable” pharmacological targets for CMD since INOCA patients may experience symptoms of ischemia due to transient coronary constriction, reflected in the peripheral microvasculature. Neuropeptide Y (NPY) is co-released with norepinephrine from perivascular sympathetic nerves. NPY is a potent vasoconstrictor that provokes transient ischemia due to microvascular constriction and is elevated during exercise in angina patients (Merz et al., 2020). Nonetheless, selective NPY Y1 receptor blockade did not confer any favorable results on ischemic parameters or exercise tolerance, even though it attenuated the exercise-induced rise in blood pressure (Gullestad et al., 2003). It was also shown that NPY participated in the differentiation of mesenchymal stem cells (MSC) in the endothelium into cardiomyocytes, following the transplantation of MSCs in the infarcted heart, possibly contributing to myocardial repair after ischemic injury (Wang et al., 2010). These findings might open a new research field in order to pursue cell-based cardiac repair and remodeling during CMD and after an ischemic episode. However, further studies are necessary to determine the appropriate dose-response and effects of NPY inhibitors on microcirculation.
A novel therapeutic intervention, which is being tested in INOCA patients with CMD, is stem cell therapy, including bone marrow-derived autologous CD34+ stem cells. These cells possess angiogenic properties, as they are able to promote neovascularization and vascular repair in the microvasculature (Rai et al., 2021). They can transdifferentiate into endothelial cells, migrate towards ischemic regions and improve perfusion and integrity of the damaged myocardium (Sietsema et al., 2019). For instance, in a swine model of MI, intracoronary transplantation of the stem cells into infarcted hearts led to increased expression of growth factor genes, improved cardiac function, augmented vessel density, and subsequently extensive cardiac repair (Zhang et al., 2007). Recently, during the ESCaPE-CMD trial, intracoronary infusion of autologous CD34+ stem cells in INOCA patients with endothelial-independent CMD resulted in enhancement of CFR, as well as improvement of angina symptoms, as assessed by SAQ score (NCT03508609). There is also an ongoing phase 2, randomized, double-blinded, placebo-controlled trial, which is currently recruiting INOCA patients. They will receive a single intracoronary infusion of autologous CD34+ cells, and safety and efficacy of the intervention regarding treatment of CMD will be examined (Rai et al., 2021) (NCT04614467).
Furthermore, extracellular vesicles (EVs) are being investigated for possible therapeutic effects on cardiac microvascular dysfunction, due to their pivotal role as intercellular communication mediators among various cell types, such as cardiac or endothelial cells, and their cardioprotective capacity (Rezaie et al., 2019; Prakash et al., 2020). Data derived from preclinical studies are particularly encouraging. Exosomes, a subcategory of EVs of endosomal origin, which are derived from multipotent MSCs, have been recently studied in a rat myocardial ischemia – reperfusion injury preclinical model regarding their role as mediators of cardiac tissue repair and their regenerative potential in CMD (Yin et al., 2021). In addition, treatment of microvascular endothelial cells, under hypoxia-reoxygenation conditions, with MSC-derived exosomes enhanced their regenerative capacity and viability in vitro, whereas administration of such exosomes to rats subjected to ischemia/reperfusion injury led to improved cardiac function and regression of fibrosis (Wang et al., 2021). In 2018, the ESC working group published a position paper regarding the emerging role of EVs in the diagnosis and therapy of IHD. However, in the consensus statement it is highlighted that several regulatory, technical, and safety issues need to be explicitly addressed, in order to advance from preclinical to clinical studies (Sluijter et al., 2018). Nonetheless, a promising area for research regarding EVs, is their prognostic relevance as biomarkers in patients with CVD. Microvesicles, derived from activated platelets or endothelial cells, were found to be significantly increased in CVD patients, who exhibited low CFR values, a common indicator of microvascular dysfunction. CFR values also correlated negatively with several cardiovascular biomarkers, including NF-κB essential modulator, resistin, B-cell activating factor, insulin-like growth factor-binding protein 7, which were measured in the lysates of EVs isolated from patients plasma, and could potentially exhibit proinflammatory or proatherogenic properties, thus exacerbating CMD (Bryl-Górecka et al., 2021)
Remote ischemic conditioning (RIC) is currently being investigated as a possible non-pharmacological therapeutic intervention for CMD, due to promising findings suggesting that coronary microcirculation is a molecular target of RIC-induced cardioprotection (Heusch, 2016). In brief, RIC refers to the application of short, non-detrimental cycles of ischemia and reperfusion in a tissue, organ, or vascular bed distal to the heart, but has the extraordinary ability to mitigate myocardial reperfusion injury (Heusch et al., 2015). In a clinical trial, enrolling healthy young individuals and patients presenting chronic heart failure with reduced ejection fraction, one-week, twice-a-day RIC treatment, by 4 cycles of 5-minute inflation and deflation of a blood pressure arm cuff, induced significant enhancement of CFR in both subject groups, indicating a role in coronary microcirculation improvement (Kono et al., 2014). Similar results were also obtained in a more recent clinical trial, which recruited patients with suspected CAD, referred for coronary angiography. Indexes of coronary microvascular functionality were significantly improved after remote ischemic preconditioning. In fact, CFR was augmented and IMR was decreased to a significant extent after RIC compared with sham treatment or values measured before RIC (Lau et al., 2018). Last but not least, it was proposed that RIC represents a promising therapeutic approach with regards to 5-fluorouracil – induced coronary microvascular vasospasm, which often precedes global, non-segmental myocardial ischemia and contributes to diffuse anticancer-related cardiotoxicity. Due to the cardioprotective, as well as vasculoprotective properties of RIC, and its positive effects on CMD indicators, it could represent a beneficial, non-invasive strategy (Chong et al., 2019).
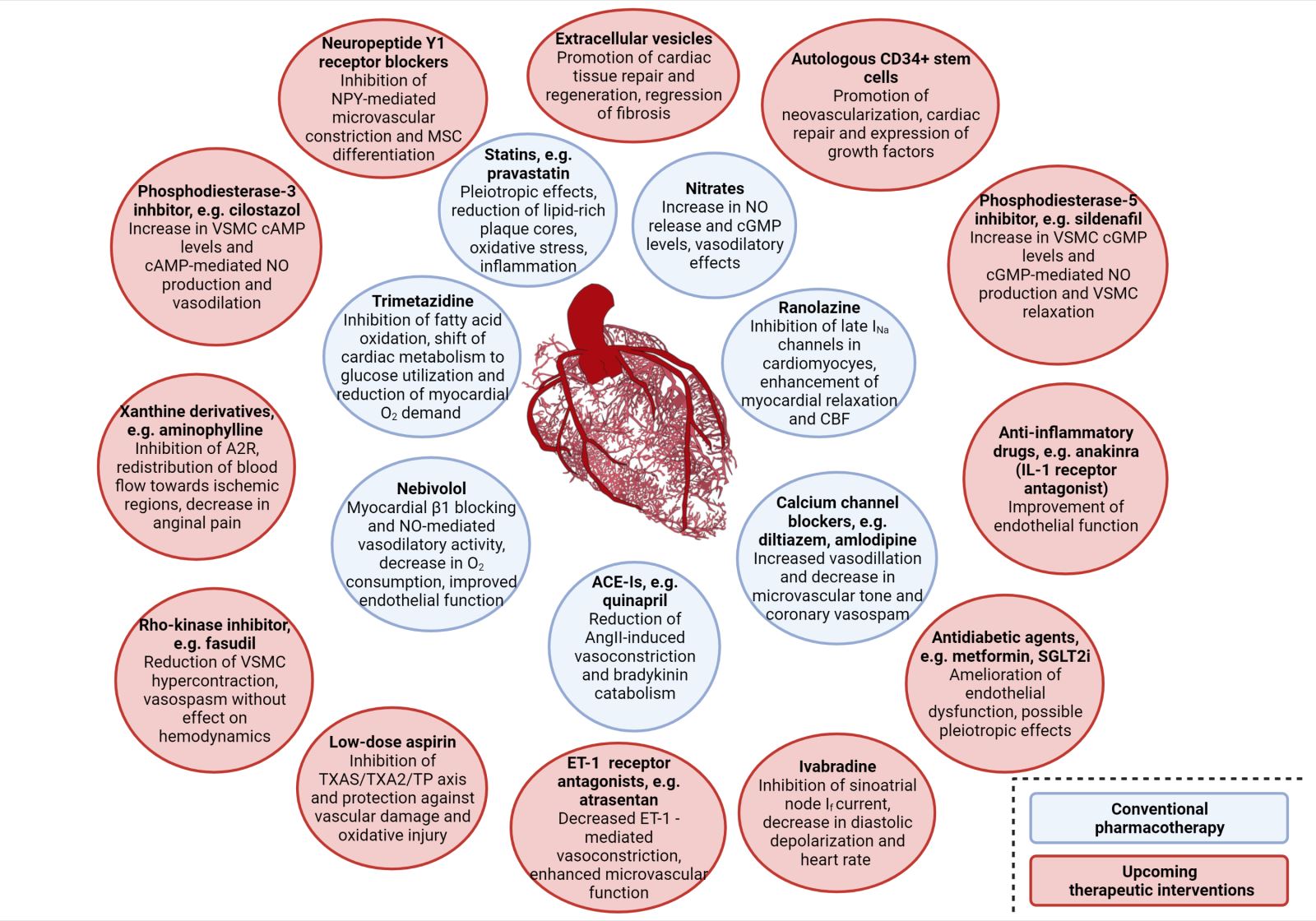
A summary of the clinical trials and their main findings regarding the aforementioned alternative therapeutic approaches is depicted in Table 2. Moreover, a schematic diagram of all the pharmacological approaches, conventional and novel, along with their main mechanism of action regarding improvement of CMD, is presented in Figure 2.
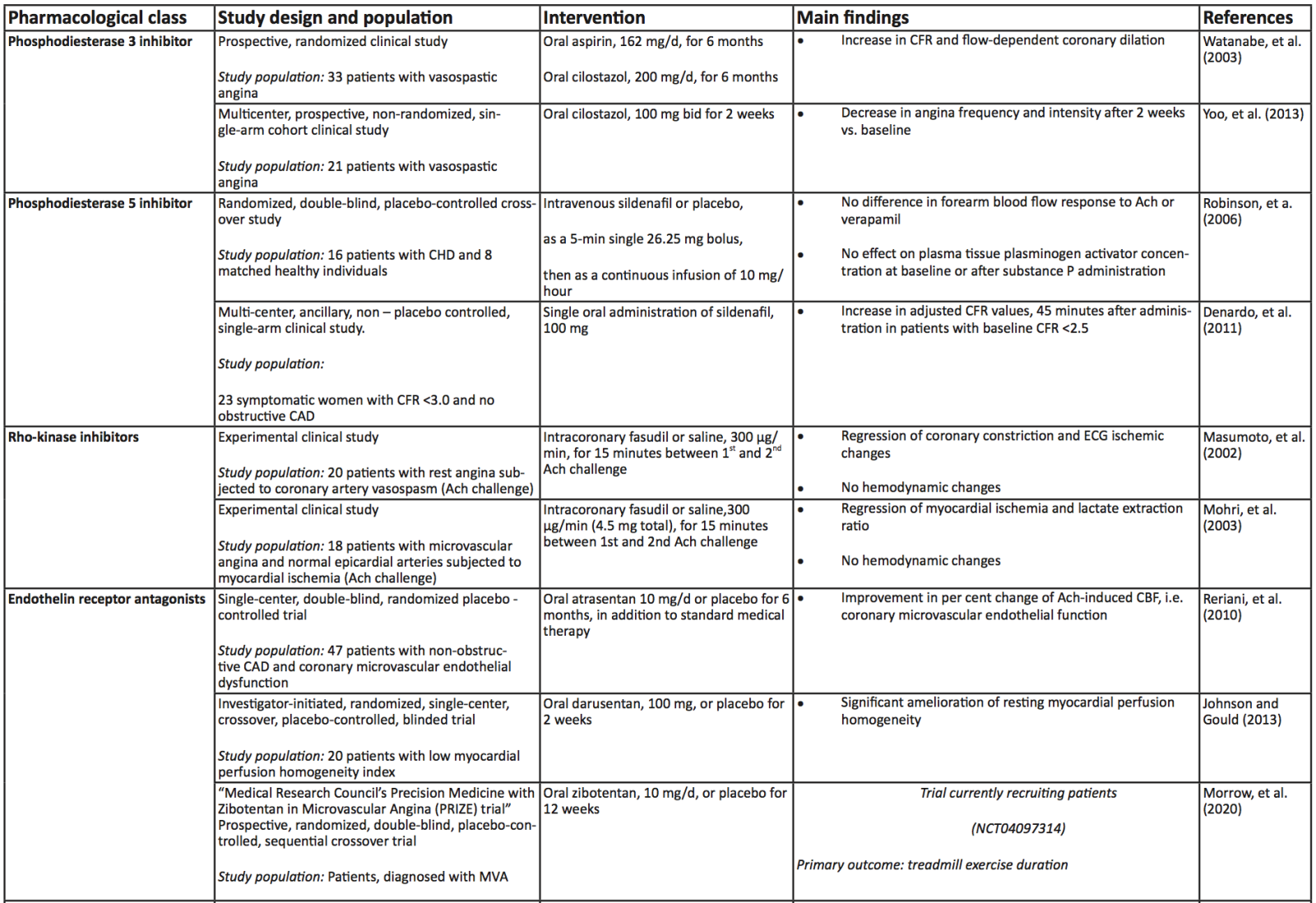
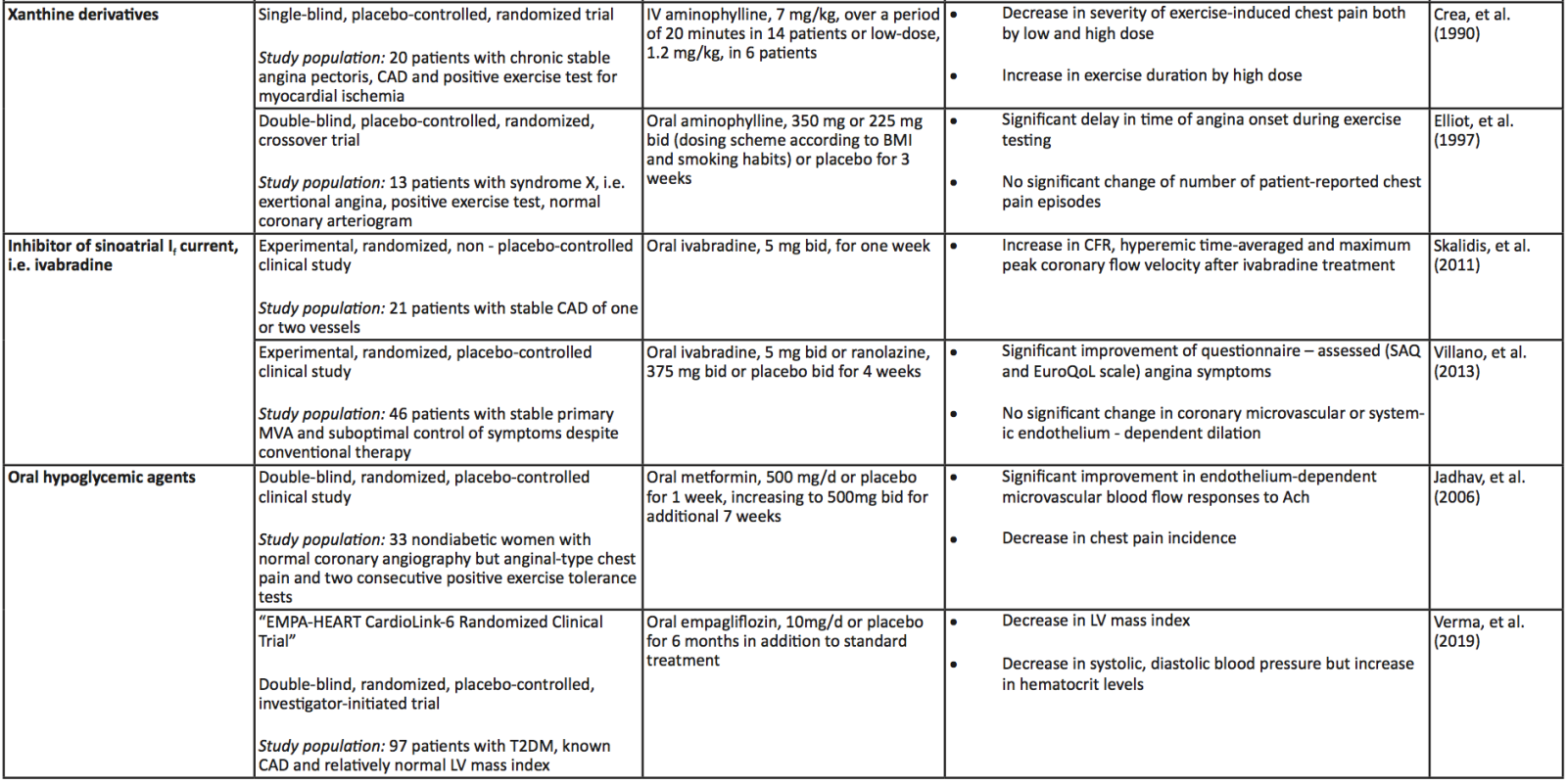
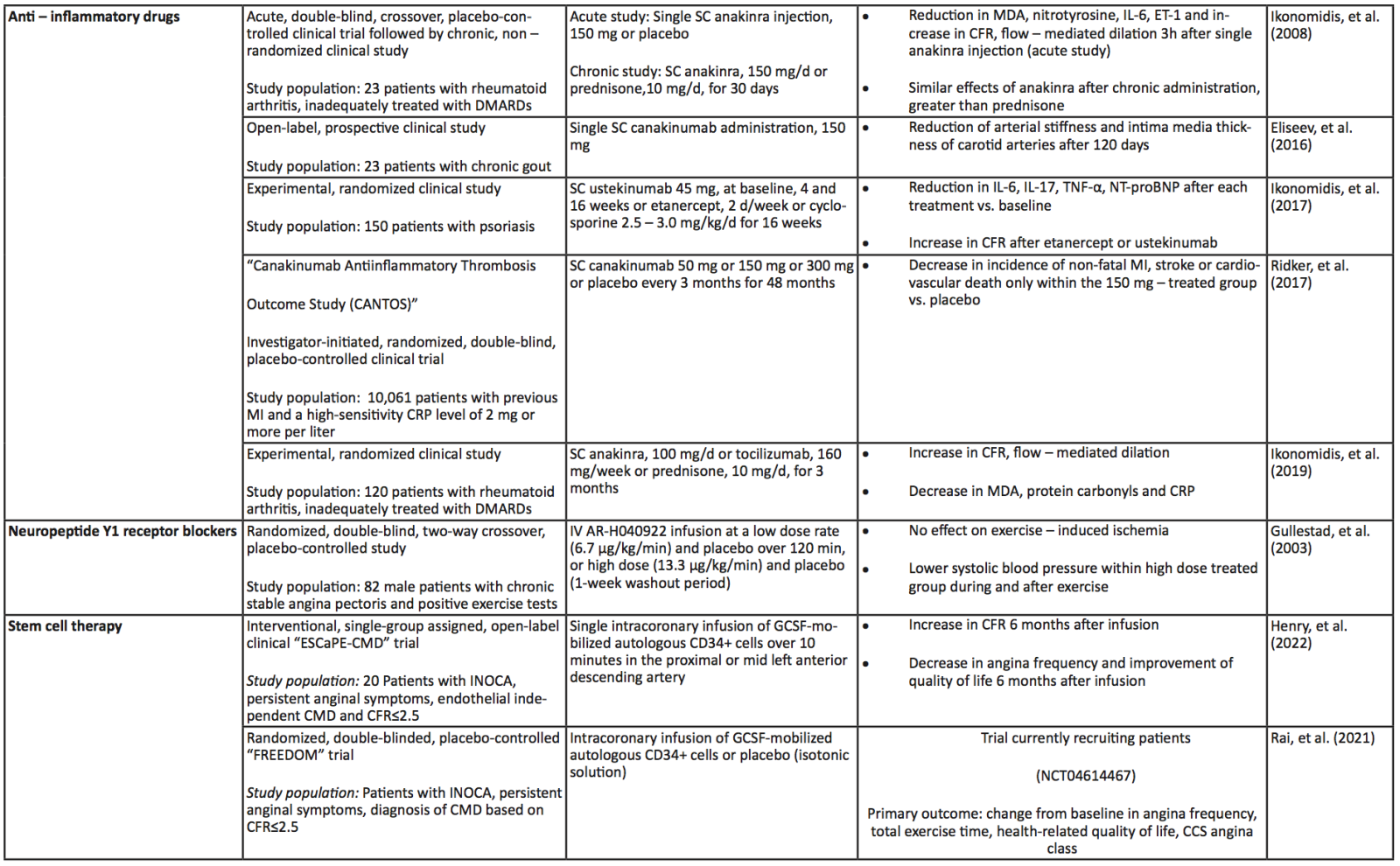
Table 2. Summary of the aforementioned (see respective module) clinical trials investigating the role of upcoming, alternative therapeutic interventions in coronary microvascular dysfunction and ischemia with non – obstructive coronary artery disease. Abbreviations: Ach, Acetylcholine; CAD, Coronary Artery Disease; CBF, coronary blood flow, CCS, Canadian Cardiovascular Society; CD, Cluster of Differentiation; CFR, Coronary Flow Reserve; CHD, Coronary Heart Disease; CMD, Coronary Microvascular Dysfunction; CRP, C-Reactive Protein; DMARDs, Disease-Modifying Antirheumatic Drugs; ECG, Electrocardiogram; GCSF, Granulocyte Colony-Stimulating Factor; HF, Heart Failure; IL, Interleukin; IMR, Index of Microcirculatory Resistance; INOCA, Ischemia with No Obstructive Coronary Arteries; IV, Intravenous; MDA, Malondialdehyde; MI, Myocardial Infarction; MVA, Microvascular Angina; NT-proBNP, N-Terminal pro B-type Natriuretic Peptide; RIC, Remote Ischemic Conditioning; SAQ, Seattle Angina Questionnaire; EuroQoL, European Quality of Life; LV, Left Ventricle; SC, Subcutaneous; T2DM, Type 2 Diabetes Mellitus
In a new window | Download PPT
Figure 2: Schematic diagram of the referred therapeutic interventions for clinical management of coronary microvascular dysfunction, including their respective mechanism of action. Conventional pharmacotherapy options are included in blue circles, whereas alternative and novel pharmacological approaches are included in red ones. Abbreviations: A2R, Adenosine receptor 2; ACE-Is, Angiotensin converting enzyme inhibitors; cAMP, cyclic adenosine monophosphate; CBF, Coronary blood flow; cGMP, cyclic guanosine monophosphate; ET-1, Endothelin-1; IL- 1, Interleukin-1; NPY, Neuropeptide Y; SGLT2i, Sodium-glucose transport protein 2 inhibitors; TP, Thrombospondin; TXA2S, Thromboxane A2 synthase; VSMC, Vascular smooth muscle cell Created with BioRender.com
Concluding remarks and future perspectives
In light of recent cardiovascular research progress, it appears that IHD is not solely the outcome of deficits in the macrocirculation. Instead, CMD also affects the smallest components of the coronary vascular tree by inducing characteristic structural and/or functional abnormalities. If severe enough, these abnormalities are capable of impeding myocardial perfusion. Therefore, CMD plays a cardinal role in IHD by diminishing CFR values or by inducing microvascular spasm.
CMD could be the next frontier of cardiovascular research in IHD, but for now, the scientific knowledge regarding its pathophysiology and underlying molecular mechanisms remains to be elucidated. The current literature indicates that coronary microvascular deficits are strongly associated with endothelial dysfunction and normal vasomotor mechanisms are dysregulated. However, unravelling of new, key pathogenic processes is of utmost importance in order to move towards targeted therapeutic interventions. For this purpose, animal models are instrumental, because coronary microvascular structure and function, as well as the progression of CMD, can be thoroughly examined. Moreover, the influence of risk factors, genetic predisposition, sex, and age in the pathogenesis of CMD should also be evaluated. Sorop et al. (2020b) recently published an insightful review, which summarizes all the available animal models of CMD in the setting of metabolic derangements for the study of INOCA (Sorop et al., 2020b). Nonetheless, as we demonstrated in our review, CMD might also manifest concurrently with epicardial lesions. Given that animal models used for the study of CAD have limited translational capacity (Liao et al., 2017), we acknowledge that the interplay between CMD and CAD might be challenging to study on a preclinical level.
The lack of scientific knowledge regarding CMD also reflects on the clinical setting. Proper assessment of the microcirculation and stratification of patients with CMD is a crucial step for the selection of optimal pharmacological interventions. Results obtained from the CorMicA (British Heart Foundation Coronary Microvascular Angina) study highlighted the importance of interventional diagnostic procedure (IDP) in INOCA patients in order to receive stratified medical therapy, tailored to their clinical characteristics, as discriminated by the applied diagnostic protocol. Patients who received pharmacotherapy according to their IDP results exhibited significant decrease of angina symptoms, as well as improved quality of life after one year, thus proving that patient outcomes could be greatly ameliorated if diagnostic interventions become an indispensable tool for clinicians to personalize treatment accordingly (Ford et al., 2018a).
Moreover, Rahman et al. (2020) proposed the discrimination of two distinct endotypes of CMD based on the physiological properties exhibited during assessment. According to this study, both endotypes present impaired CFR values, but the etiology of the impairment is based on the endotype. Patients with functional CMD have increased baseline flow - an effect probably mediated by enhanced eNOS activity - whereas patients with structural CMD have reduced hyperemic flow, probably due to dysregulated endothelium-dependent vasodilation. This discrimination should not be confused with the structural and functional defects we described on our review, as these endotypes are categorized according to the functional assessment and do not describe pathophysiological alterations of the microcirculation. Furthermore, the authors suggest that patients with structural CMD might benefit from therapies aiming to decrease afterload and myocardial oxygen demands (Rahman et al., 2020). Drug categories, which achieve these results, are beta-blockers, ACE-Is and ARBs, which are currently used for the management of CMD patients. Investigating whether this classification might confer additive value in the stratification of CMD patients, as well as choosing the optimal pharmacotherapy should be assessed in placebo-controlled randomized trials.
The lack of large, randomized, controlled trials has resulted in profound lack of guidelines for the clinical management of CMD patients, which currently remain under-treated. In the literature, there are many reviews that summarize currently used and potentially useful pharmacological approaches for the CMD population. In our review, we include only drugs whose use in CMD is supported by clinical or, at least, preclinical models, and could have a mechanism of action, relative to the pathophysiology of CMD. Hence, we sought to summarize only evidence-based pharmacological interventions. Nonetheless, as long as the data are derived from preclinical models or relatively small, intermediate outcome trials, they are not eligible to be included in upcoming guidelines. However, many of the novel treatments discussed in this review offer promising choices for the design of future large clinical trials. Pending the results of such trials, the medical community could evaluate available pharmacological options based on systematic reviews and meta-analyses, such as the one by Yong et al. (2020), which investigated the impact of oral cardiovascular medications on CFR in individuals with non-obstructive CAD.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work has been supported by the EU-CARDIOPROTECTION COST (European Cooperation in Science and Technology-Action) (CA16225).
Dr. Ioanna Andreadou, who serves on the Editorial Board of Conditioning Medicine, did not participate at any level in the editorial review of this manuscript.
References
Sofia Lamprou1
1Laboratory of Pharmacology, School of Pharmacy, National and Kapodistrian University of Athens, 15771, Athens, Greece.
Theano Dermintzoglou1
1Laboratory of Pharmacology, School of Pharmacy, National and Kapodistrian University of Athens, 15771, Athens, Greece.
Dimitris Christos Kleitsiotis1
1Laboratory of Pharmacology, School of Pharmacy, National and Kapodistrian University of Athens, 15771, Athens, Greece.
Ioanna Andreadou1
1Laboratory of Pharmacology, School of Pharmacy, National and Kapodistrian University of Athens, 15771, Athens, Greece.
Corresponding author:
Andreadou Ioanna
Email: janandread@pharm.uoa.gr
Professor Derek J. Hausenloy is the handling editor of this article.

In a new window | Download PPT
Figure 1: Schematic representation of four main mechanical, cellular and molecular effectors of coronary microvascular dysfunction (see respective module). These are noted in red color and include: i. Oxidative stress and inflammation ii. Platelet – endothelium interactions iii. Hemodynamic forces iv. Autonomic dysregulation. Abbreviations: EDRF, Endothelium-derived relaxing factors; eNOS, endothelial NO synthase; ET, Endothelin; GPIb-IX-V, Glycoprotein Ib-IX-V; NE, Norepinephrine; PSGL-1, P-selectin glycoprotein ligand-1; ROS, Reactive oxygen species; VSMC, Vascular smooth muscle cell; VWF, von Willebrand factor; WSS, Wall shear stress Created with BioRender.com

In a new window | Download PPT
Figure 2: Schematic diagram of the referred therapeutic interventions for clinical management of coronary microvascular dysfunction, including their respective mechanism of action. Conventional pharmacotherapy options are included in blue circles, whereas alternative and novel pharmacological approaches are included in red ones. Abbreviations: A2R, Adenosine receptor 2; ACE-Is, Angiotensin converting enzyme inhibitors; cAMP, cyclic adenosine monophosphate; CBF, Coronary blood flow; cGMP, cyclic guanosine monophosphate; ET-1, Endothelin-1; IL- 1, Interleukin-1; NPY, Neuropeptide Y; SGLT2i, Sodium-glucose transport protein 2 inhibitors; TP, Thrombospondin; TXA2S, Thromboxane A2 synthase; VSMC, Vascular smooth muscle cell Created with BioRender.com
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10041 | 72 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA