International bi-monthly journal of cell signaling, tissue protection, and translational research.
Stem cells and gene engineered stem cells for ischemic stroke treatment
Zimeng Wu1, Ka Huen Cheng2, Rubing Shi3, Yongting Wang3
Author Affiliations


- 1Department of Natural Sciences, University College London, London, UK.
- 2Department of Biochemical Engineering, University College London, London, UK.
- 3Med-X Research Institute and School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China.
Abstract
As one of the leading causes of mortality and disability globally, the mortality of patients with acute stroke has been reduced in many countries, and increasingly more stroke survivors are living with a disability. Currently, there is no proven neurorestorative treatment for chronic stroke. Stem cell therapies have emerged as potential restorative treatments for stroke. In this review article, we summarize recent clinical trials of stem cell treatment for stroke, review the cell types used in these studies, and discuss potential mechanisms of action, and recent preclinical methods for optimizing stem cell treatment efficacy in animal models.
Keywords: Stem cells, Ischemic stroke
Abstract
As one of the leading causes of mortality and disability globally, the mortality of patients with acute stroke has been reduced in many countries, and increasingly more stroke survivors are living with a disability. Currently, there is no proven neurorestorative treatment for chronic stroke. Stem cell therapies have emerged as potential restorative treatments for stroke. In this review article, we summarize recent clinical trials of stem cell treatment for stroke, review the cell types used in these studies, and discuss potential mechanisms of action, and recent preclinical methods for optimizing stem cell treatment efficacy in animal models.
Keywords: Stem cells, Ischemic stroke
Introduction
Of the over 13 million stroke cases each year worldwide, only a small percentage of patients can receive effective acute treatment. Currently, acute treatment of ischemic stroke includes emergency IV thrombolytic treatment and endovascular treatment (EVT). Emergency treatment of hemorrhagic stroke focuses on controlling the bleeding and reducing pressure in the brain caused by the excess fluid. After emergency treatment, stroke rehabilitation therapy focuses on helping the stroke survivor become as independent as possible. To date, there are no effective therapeutics targeting the chronic phase after stroke aside from the recently approved vagus nerve stimulation (VNS) paired with rehabilitation (Dawson et al., 2020).
Stem cell therapy emerged as a potential treatment to promote chronic recovery after stroke. Stem cell treatment has been shown to improve stroke recovery through multiple mechanisms of action, such as their ability to differentiate into functional cell types and exerting paracrine functions. At the same time, risk associated with stem cell treatment, such as potential tumorigenesis and graft-versus-host disease (GVHD), remains a concern for its clinical translation. Other challenges of stem cell treatment include limited efficacy, large scale production required for allogenic transplantation, ethical issues of embryonic stem cells (ESC), and bioavailability at the target organ. Although many challenges remain to be overcome, in recent years progress has been made in both clinical and preclinical studies using stem cells to treat ischemic stroke. In this review, we summarize recent clinical trials using stem cell treatment for stroke, review the cell types used in these studies, and discuss current preclinical methods for optimizing stem cell treatment efficacy in animal models.
Clinical trials of stem cells therapy for ischemic stroke
Summary
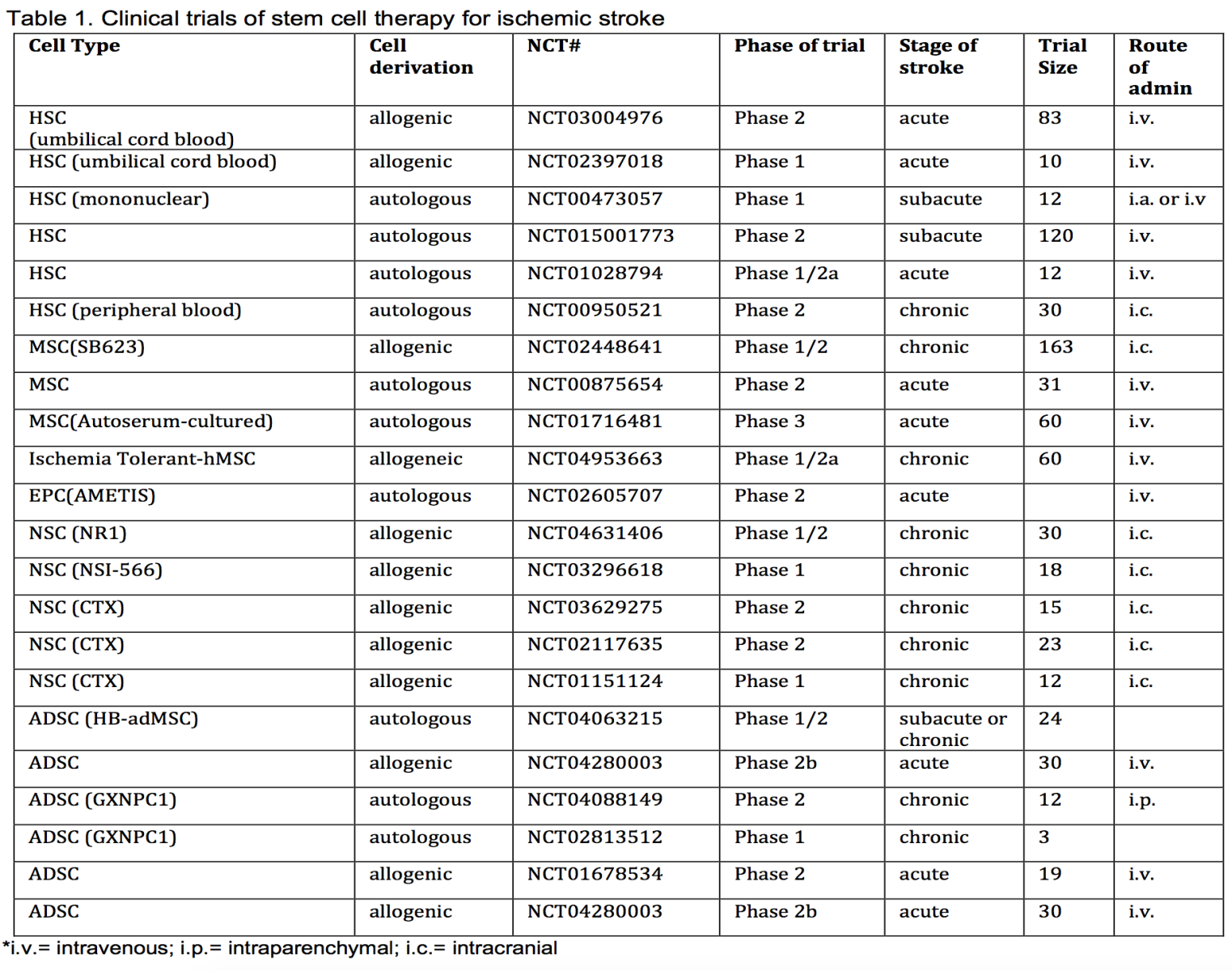
In Table 1, we summarize clinical trials that used stem cell treatment for ischemic stroke that have been registered at clinicaltrials.gov and are either completed or recruiting patients. We exclude studies that were terminated early or never recruited patients. In this table, we list the types of cells used, source of the cells, study registration number, the phase of the study, stage of stroke, trial size, and the routes of administration. We hope that this table will serve as a quick reference of recent clinical trials.
Types of stem cells used in clinical trials for ischemic stroke treatment
Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are self-renewing multipotent stem cells that can differentiate into all types of blood cells. While most of them reside in the bone marrow (BM) niche, HSCs can mobilize to peripheral blood under the stimulation of certain factors such as granulocyte colony-stimulating factor (G-CSF) (Demetri and Griffin, 1991), and certain diseases such as ischemic stroke (Dunac et al., 2007). HSC transplantation has been routinely used to treat immune deficiency-related diseases for decades. As for ischemic stroke, HSCs have been reported to promote neuroplasticity and functional recovery in pre-clinical models (Shyu et al., 2006). To confirm the safety and efficacy in human patients, a phase II study intracerebrally implanted autologous peripheral blood-derived HSCs (PBHSC) into 15 chronic stroke patients within 6-60 months of stroke onset (Chen et al., 2014). Prior to peripheral blood collection, patients were subcutaneously given 15 μg/kg G-CSF for five consecutive days, mobilizing HSCs and potentially amplifying PBHSC yield. Over a 12-month post-treatment period, Chen et al. (2014) observed significant improvement in stroke severity scores in the PBSC-treated cohort, consistent with improved neurological and functional recovery.
Autologous bone marrow transplantation has been a focus of many clinical trials. These studies isolate bone marrow from the patient and use immunosorting to obtain the necessary cells for transplantation. Aldehyde dehydrogenase (ALDH) was one of the first markers used to identify HSCs and hematopoietic progenitor cells (HPCs), with HSCs expressing the highest levels of ALDH (ALDH-bright, ALDH-br). (( Kastan et al., 1990). BM-derived ALDH-br cells transplanted into patients were reported to improve functional recovery in ischemic heart failure (Perin et al., 2012). Thus, Savitz et al. (2019) investigated autologous BM-derived ALDH-br cell transplantation in ischemic stroke treatment via intracarotid infusion of 8 million cells in 29 patients. However, there was no significant difference found between groups that received the ALDH-br cell treatment and placebo on the modified Rankin scale. It is worth noting that ALDH-br is expressed by not only hematopoietic stem cells but also mesenchymal and neural progenitor cells.
A more commonly used marker is the cell surface marker CD34. Immunosorted CD34+ cells have been intra-arterially transplanted in acute ischemic stroke studies. Moniche et al. (2012) transplanted 160 million cells into 10 patients within 5-9 days after stroke onset. They found no significant improvement in neurological function after 180 days of transplantation, while beta nerve growth factor (bNGF), a known marker for neurological recovery (Luan et al., 2019), increased significantly in the treatment group ). Banerjee et al. (2014) transplanted cells within 7 days of stroke onset in 5 patients and reported reductions in lesion volume after 6 months. This was also the first study in humans to show that intra-arterial infusion of autologous CD34+ cells for severe ischemic stroke was both safe and feasible.
Allogeneic BM transplantation has had limited success partly due to immunological complications such as graft rejection, GVHD, and delayed immune reconstitution. HSCs derived from umbilical cord blood (UCB-HSC) have served as an alternative to allogeneic BM transplantation as GVHD after UCB-HSC transplantation is lower compared to that of BM transplantation (Kim and Broxmeyer, 2011). Human leukocyte antigen (HLA) disparity between donor and recipient is the main risk factor in the development of GVHD (Park and Seo, 2012) and the number of mismatched HLA class I antigens was shown to correlate with GVHD development and low engraftment kinetics (Matsuno et al., 2009). Although the mechanisms of reduced GVHD in patients transplanted with UCB are not well understood, it has been suggested to be due to the phenotypic immaturity of T cells from UCB (Beck and Lam-Po-Tang, 1994). T cells from UCB show greater immune tolerance (Barker et al., 2001), and impair T-helper 1 (Th1) differentiation and allogeneic T cell activation (Chen et al., 2006). Thus, unlike other allogeneic sources of HSCs, HLA-matching is not required for transplantation. In a phase 1 study, non-HLA matched allogeneic UCB-HSC was intravenously administered to 10 patients within 3-9 days of stroke onset (Laskowitz et al., 2018). The study observed improvements in neurological and functional evaluations of patients over a 12-month post-infusion period. Although there was no control group for comparison, Laskowitz et al. (2018) established the safety and feasibility of a single intravenous administration of allogeneic UCB-HSC, augmenting the well-reviewed safety and efficacy of UCB-HSCs as a blood donor graft (Zhou et al., 2012), and supporting a phase II placebo-controlled study. More recently in a placebo-controlled phase II study (NCT03004976), allogeneic UCB-HSCs were intravenously administered to patients within 3-10 days of stroke onset. This on-going study aims to evaluate the changes in neurological symptoms, quality of life, and cognitive status of 83 patients for up to one-year post-infusion.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are known for their multipotency and can be isolated from a wide range of sources such as bone marrow, adipose tissue, cord blood, and fetal tissue. They can differentiate into multiple types of cells such as osteocytes, adipocytes, and chondrocytes (Bronckaers et al., 2014). These cells showed immune-modulatory properties and have been shown to protect blood-brain barrier integrity and to stimulate endogenous neurogenesis and angiogenesis in stroke animal models (Maltman et al., 2011; Guo et al., 2012; Tang et al., 2014). Among the various sources of MSCs, bone marrow, adipose tissue, and umbilical cord have been widely used in clinical trials.
Bone marrow-derived MSCs (BMSCs) are most commonly applied in clinical and pre-clinical studies. In 2005, Bang et al. (2005) for the first time applied autologous BMSCs intravenously in acute stroke patients and examined neurological recovery. The results in neuroimaging, as well as neurological scores, showed a gradual improvement in BMSCs-treated patients in the 12-month follow-up. However, the enrollment remained small with only 5 patients for the treatment group and 25 for the control. After this preliminary study, Bang and colleagues continued to conduct a long-term follow-up study of intravenous BMSC transplantation on a larger scale, with 16 patients treated with BMSCs and 36 for control (Lee et al., 2010). They confirmed the long-term safety and proved the beneficial effects of autologous intravenous BMSC transplantation for acute stroke patients. Subsequently, the team conducted the STARTING-2 trial, a phase III study that further applied intravenous BMSCs to chronic stroke patients. Unfortunately, no significant improvements in 90-day outcomes were established (Chung et al., 2021).
A phase 2 clinical trial (NCT02448641) was completed recently using modified human BMSC (SB623) as therapy for ischemic stroke. The SB623 cells have been transfected with a plasmid encoding the Notch-1 intracellular domain (NICD). Preclinical studies showed that gene transfection with the Notch-1 intracellular domain followed by growth factor treatment stimulated neuron-like differentiation of SB623 cells (Yasuhara et al., 2009). Following this, 18 patients were surgically transplanted with SB623 cells in the phase 1/2a clinical trial and showed a significant improvement from baseline for the European Stroke Scale score, Fugl-Meyer (F-M) total score, and F-M motor scale score 24 months post-treatment (Steinberg et al., 2016; Steinberg et al., 2019). Although seven patients experienced nine serious adverse events, they were resolved without sequelae and no patients withdrew due to these adverse events. Furthermore, no dose-limiting toxicities or deaths led to the conclusion that the 2-year study was safe and improvements in clinical outcomes were observed. These studies suggest that genetic engineering of stem cells could potentially serve as a platform approach to systematically augment stem cell function.
Adipose and umbilical cord-derived MSCs have also been shown to be safe in phase I/II clinical trials for acute ischemic stroke patients (Diez-Tejedor et al., 2014; Kim, 2018). Despite efficacy proven in clinical trials for autologous MSC treatments, the long culture time between cell collection and first injection remains a problem for timely treatment. In the recent 5 years, 8 out of 11 trials newly registered in clinicaltrials.gov have applied allogeneic MSCs in acute and chronic ischemic stroke treatments, with more attention on the choice of MSC sources, dosage, and injection routes.
Endothelial Progenitor Cells
Endothelial Progenitor Cells (EPCs) were first identified from human peripheral blood in 1997. They are the precursor of vascular endothelial cells and participate in angiogenesis and vascularization (Asahara et al., 1997). In pre-clinical models, EPCs have shown promising effects in protecting the neurovascular unit and helping restore neurological function after ischemic stroke by repairing the vascular endothelium and its secretory function (Liao et al., 2017). Most of the clinical studies with EPCs in ischemic stroke patients are observational, focusing on the dynamic change and related function of EPC after stroke onset. Up to date, only 2 interventional trials have been registered in clinicaltrials.gov, both conducted by the same team. The studies used autologous EPC by intravenously transplanting the cells to acute stroke patients. The research team did a 4-year follow-up study with 18 patients and found that EPCs are safe long-term. However, no significant neurological or functional improvement was observed (Fang et al., 2019).
In a recent study, Gabriel-Salazar et al. (2018) studied the efficacy of EPCs in stroke patients and mouse rehabilitation models following cerebral ischemia. They detected cerebral angiopoietin in both human and mouse tissues and found that EPCs derived from bone marrow were able to differentiate into ECs and replace injured endothelial cells (Gabriel-Salazar et al., 2018). In addition to this, several new studies have shown that ischemic injury leads to the release of hypoxic inducible factor-1 (HIF-1), which subsequently leads to the secretion of stromal cell-derived factor-1 (SDF-1). SDF-1 is important in stem cell migration and proliferation. SDF-1 signals through its G-protein-coupled membrane receptor, CXCR4, and mobilizes EPCs from the bone marrow (Tang and Hammond, 2015), allowing EPCs to move along the SDF-1 gradient and into the ischemic zone to promote repair of the injury (Li et al., 2018; Li et al., 2021). Dai, X. et al. (2017) proposed that SDF-1 is a survival signal that can affect cell survival under different pathological conditions. The results from these studies suggest that SDF-1 may be a therapeutic target to improve the function of EPC in brain repair after ischemic stroke.
Neural Stem Cell
Neural stem cells (NSC) have been a candidate for cell therapy to treat ischemic stroke. While essential in the development of the brain, NSCs remain quiescent in the adult stage and are mainly localized to two regions, the subventricular zone (SVZ) and the subgranular zone (SGZ). Under stroke conditions, these endogenous NSCs are triggered by chemokines and cytokines secreted from the lesion area and start migrating into the injured area and differentiate into mature neurons. Such stroke-triggered spontaneous neurogenesis, however, fails to meet the requirements for functional recovery. Therefore, one strategy to improve endogenous neurogenesis is to promote the survival rate and to increase neuronal differentiation by various stimulation methods. Another approach is to transplant exogenous NSCs into stroke brains. Such direct transplantation provides not only the replacement for dead neurons but also a more supportive microenvironment through secretion of various neurotrophic factors as well as the promotion of cell-cell communication in the local niche (Bernstock et al., 2017; Boese et al., 2018).
For these reasons, NSC transplantation has also been tested in clinical trials. In 2016, a clinical trial with 23 participants was done to treat patients suffering from chronic ischemic stroke (Kalladka et al., 2016). Though clinical trials on NSC therapy have increased over the years, only five studies using exogenous NSC have been registered in clinicaltrials.gov. This might be due to possible low survival and neuronal differentiation rate. To avoid this, several strategies were provided including NSC pre-conditioning, local niche improvements, combining with scaffolds, and genetic engineering the NSCs prior to transplantation (Bernstock et al., 2017; Othman and Tan, 2020). Though further efficacy and clinical safety still need to be examined, NSC is still a promising cell type to treat ischemic stroke.
Preclinical studies of stem cell therapy for experimental ischemic stroke
Besides the stem cells that have been translated to clinical studies, there are many ongoing studies in the preclinical stage. Among these, patient derived induced pluripotent stem cells, preconditioning of stem cells prior to translation, and gene engineered stem cells are being investigated for their potential of translation.
Induced Pluripotent Stem Cells
First uncovered by Takahashi and Yamanaka (2006) by introducing four factors (Sox2, Oct3/4, Klf4, and cMyc) into mouse fibroblasts, induced pluripotent stem cells (iPSCs) are reprogrammed cells with characteristics resembling embryonic stem cells (ESCs). Since their transformative discovery, iPSCs have been studied for disease modeling and cell therapy in regenerative medicine. In stem cell therapy, iPSCs provide fewer ethical and immunogenic concerns than allogenic ESCs, which could make them more clinically suitable. iPSCs can be propagated ex vivo and can differentiate into various human stem cell lineages. Furthermore, combining iPSCs with genome editing techniques, such as clustered regularly interspaced short palindromic repeat (CRISPR) technology, allows transplantation of healthy cell lines with desired alleles, thus providing a promising future for regenerative medicine. A main limitation of iPSCs moving from the preclinical to clinical stage is tumorigenicity and the formation of teratomas in the weeks following cell transplantation. This is due to the culture adaptation effects in high passage iPSCs resulting in genetic modifications that promote increased proliferation, growth factor independence, and clonal dominance within the niche (Lee et al., 2013). Many studies have tried to address this limitation through mechanisms such as transplantation of iPSCs with fibrin glue, which was shown to avoid formation of teratomas (Chen et al., 2010). Another study showed that treatment with small molecule inhibitors of antiapoptotic factors of stem cells induces apoptosis of the tumorigenic iPSCs whilst maintaining the functionality and survival of their differentiated cell lineage (Lee et al., 2013).
Animal model studies have shown that iPSCs are able to improve sensorimotor function, reduce lesion volume and promote neurogenesis and angiogenesis (Duan et al., 2021) in brains affected by ischemic stroke, leading to improvements in functional recovery. In many cases, these improvements rely on iPSCs differentiating into adult stem cells at the site of injury (Takagi et al., 2005; Buhnemann et al., 2006; Baker et al., 2017). In the rodent model, iPSCs were seen to migrate to the ischemic brain and differentiate into neural cells (Jiang et al., 2011) within a 4-16 day follow up period. However, a study utilizing neuronal progenitors derived from iPSCs with a longer follow-up period of 1-6 weeks, showed immune rejection after 6 weeks (Gomi et al., 2012).
Due to the relatively recent discovery of iPSCs, the evaluation of this cell type for ischemic stroke therapy is still in the preclinical stage. The matters needing to be addressed for using iPSCs in cell therapy are for the most part clinical unknowns, such as optimal time window, optimal dose, and tumorigenicity (Fernandez-Susavila et al., 2019). Furthermore, the mechanism by which therapeutic effects are observed in ischemic stroke animal models has yet to be elucidated. It is currently unclear whether this mechanism is mediated by the replacement of damaged tissue through iPSC differentiation and/or by promoting endogenous repair given that the transplanted cells disappeared a few weeks after engraftment (Dihne et al., 2011; Rosenblum et al., 2012). It seems that currently, the preferred method of overcoming such limitations relies on differentiating iPSCs into cell lineages of interest at a reduced division capacity (Fernandez-Susavila et al., 2019). However, preclinical evaluation of iPSCs is growing exponentially every year due to the speed in which human stem cells can be generated from iPSCs, thus paving the way for clinical trials involving these cells in the near future.
Preconditioning of transplanted stem cells
Preconditioning of transplanted stem cells has been shown to improve the survival of the transplanted cells as well as improve the efficacy of stem cell treatment. A recent in vitro pre-clinical study investigated the use of oxygen-glucose-deprived peripheral blood mononuclear cells (OGD-PBMCs) in ischemic stroke treatment (Hatakeyama et al., 2019). The authors identified remodeling factors secreted by OGD-PBMCs, such as VEGF and transforming growth factor-beta, which promote angiogenesis. Intra-arterial transplantation of OGD-PBMCs in a rat model 7 days after ischemic stroke onset promoted the expression of these factors in the brain parenchyma. Angiogenesis, axonal outgrowth, and functional recovery were observed, thus providing a potential novel therapy for ischemic stroke. Another recent study used ischemic-hypoxic preconditioned olfactory mucosa MSCs (IhOM-MSCs) to treat rats after middle cerebral artery occlusion (Zhuo et al., 2021). It was reported that IhOM-MSCs mediated the upregulation of the downstream target genes GRP78 and Bcl-2 by miR-181a to protect mitochondrial function and inhibit apoptosis and pyroptosis of neurons after ischemic injury.
Gene engineered stem cell therapy
Limited survival of transplanted stem cells and failure of these cells to communicate with its microenvironment results in limitations to the beneficial effects of stem cell therapy. Genetic engineering of the stem cells via a lentivirus or an adeno-associated virus (AAV) vector to induce the expression of specific proteins to improve survival or augment stem cell function, has been explored by many groups in the past twenty years.
Numerous animal model studies have explored genetically engineered MSCs as ischemic stroke therapy. In an early rat model, brain-derived neurotrophic factor (BDNF) was transfected into MSCs using an AAV vector (Kurozumi et al., 2004). BDNF transfected MSCs (MSC-BDNF) enhanced the cytokine effects of MSCs, which improved functional defects reported in MSCs after stroke in rats. Here, rats transplanted with MSC-BDNFs were seen to show significantly more functional recovery than the control rats. To combat immunogenicity, interleukin-10 (IL-10) transduced MSCs transplanted in mice have been shown to reduce the severity of GVHD after allogeneic stem cell transplantation (Min et al., 2007). IL-10 is a pro-repair cytokine that reduces proinflammatory markers while triggering alternative inflammatory markers. More recently, rats with traumatic brain injury treated with IL-10 transduced MSCs thirty-six hours after insult showed a significant improvement in motor function with a reduction in anti-inflammatory effects (Peruzzaro et al., 2019). SDF-1 gene-engineered EPCs were shown to bring synergetic effects in treating ischemic stroke (Li et al., 2018). The SDF-1 gene-engineered EPCs significantly outperformed both the gene therapy alone or the EPCs therapy alone groups with improved angiogenesis, neurogenesis, and myelin sheath protection.
A triple-gene approach has been proposed, involving the AAV mediated transduction UCB mononuclear cells with three genes, as therapy for stroke (Sokolov et al., 2018). The aforementioned genes encode VEGF, glial cell-derived neurotrophic factor (GDNF) and neural cell adhesion molecule (NCAM), and were previously shown to rescue neurons in spinal cord injury (Islamov et al., 2017). These molecules were chosen due to their biological role in promoting neuron survival to regulate neurogenesis and neuroregeneration. Sokolov et al. (2018) found stroke rats intrathecally injected with these modified UCB mononuclear cells displayed a reduction in infarct volume, attenuated neural cell death, and an increase in synaptic protein expression (Sokolov et al., 2018).
Possibly due to the relatively recent emergence of genetic engineering in human cells, there is a scarce number of clinical trials utilizing gene engineered stem cells. With the development of more precise and efficient gene editing tools such as the CRISPR, more gene-edited stem cell candidates are being developed for the treatment of ischemic stroke. In a recent review, Kimbrel and Lanza discussed the next-generation stem cell toolbox and its potential in disease treatment (Kimbrel and Lanza, 2020). Many cell types and strategies mentioned in this review could be applied to ischemic stroke treatment.
Potential mechanisms of action of stem cells therapy for stroke
Given that stem cells have the ability to differentiate into many types of cells, it was once hypothesized that the transplanted cells could replace injured cells in the brain after stroke. However, growing evidence suggest that the transplanted stem cells, either intracerebrally or intravenously, stimulate endogenous reparative processes possibly through secreting many trophic factors VEGF, BDNF, and nerve growth factor (NGF) (Singh et al., 2020). For example, it has been shown that VEGF secreted by NSCs activates the cell survival and proliferation PI3-K/Akt pathway (Bacigaluppi et al., 2016). This results in an upregulation of glutamate transporter 1 (GLT-1) in astrocytes that remove peri-ischemic extracellular glutamate, thus improving functional plasticity after ischemic stroke. For a more extensive discussion of the potential mechanisms of action, we recommend the recent review by Liu et al. (2021), which reviewed recent works that support multiple potential mechanisms of action including cell migration and neurotrophic secretion, apoptosis and inflammation inhibition, angiogenesis, and neural circuit reconstruction. It is possible that stem cell therapy functions by replacing damaged neurons and promoting synaptic formation, as well as by stimulating angiogenesis, inhibiting apoptosis, and immune-regulatory effects.
In addition to trophic factors, stem cells were also found to release extracellular vesicles that hosts an array of proteins and microRNAs (miRNAs) that could regulate a network of signaling molecules and transcription factors. miRNAs play an important role in the differentiation of ESC, somatic tissue stem cells and germline stem cells, and the self-renewal and differentiation of stem cells is mediated by different miRNAs, which are expressed in different ways depending on the cell type and of different orders (Gangaraju and Lin, 2009). Many studies have proposed a role for this miRNA in cellular senescence, aging, and cancer (Murlistyarini et al., 2021). For example, Guo et al. (2021) recently identified that GATA4-miR-206-3p signaling plays an active role in bone formation (Guo et al., 2021). For comprehensive reviews on the preclinical studies of exosomes and miRNAs in stroke, we direct readers to the works of Dehghani et al. (2021) and Kadir et al. (2020).
Challenges and Prospects
One of the biggest challenges of stem cells therapy is generating sufficient quantities of stem cells, ideally with uniform quality and of low immunogenicity. One significant advantage of using autologous stem cells is that there is no need for HLA matching, which means that autologous stem cell treatment is essentially immune rejection-free (Li and Zhong, 2009). However, this does not mean that autologous stem cell therapy is absolutely safe. In 2016, an autologous stem cell therapy using adipose cells caused blindness in some patients after the cells were transplanted into the eyes of patients. The cause of this outcome was likely that the stem cells injected into the eyes formed scar tissue that damaged the retina (Kuriyan et al., 2017). While this does not shake the fact that autologous stem cell therapy is safer than allogeneic therapy, it is important to note that there are many reasons for serious outcomes following stem cell therapy, immune rejection being only one of them. Allogeneic therapy, on the other hand, has some unique advantages over autologous therapy. For example, autologous therapy requires the patient's cells to be extracted and then cultured in vitro, which usually requires a longer production cycle. Allogeneic therapies, on the other hand, can be developed as “off-the-shelf” therapeutics. This is essential when a patient needs a large number of cells urgently for treatment. In addition to this, allogeneic stem cell therapy is also usually less costly than autologous therapy due to the uniform, larger-scale cell culture. In addition, there are different studies that indicate that there is no significant difference between autologous and allogeneic therapies in terms of efficacy (Hare et al., 2012; Colbath et al., 2020; Du et al., 2021).
Another challenge of stem cell therapy is to increase the survival of transplanted stem cells and improve efficacy. Toward this end, strategies such as pre-treatment of stem cells and gene editing of stem cells has been explored to improve the survival rate. Stem cells are being engineered to overexpress neurotrophic factors, anti- inflammatory cytokines, or angiogenic factors to facilitate the recovery after ischemic injury. Recent preclinical studies also explored using exosomes isolated from microRNA gene engineered EPCs to treat ischemic stroke (Wang et al., 2020). Such gene modified EPCs could also be used as a cargo to deliver therapeutic miRNA. For a more comprehensive review, the readers are directed to a recent work by Salehi et al. (2022), which very nicely summarize the beneficial potential of genetically modified stem cells in the treatment of stroke (Salehi et al., 2022).
Recent clinical studies support that transplantation of various stem cell types, in a variety of doses, and administered via a variety of routes could be safe and effective in treating ischemic stroke at different stages of the disease. Additional studies are warranted to further our understanding of the relationship between transplanted stem cells and the immune system, and their homing niche and microenvironment. Well-designed preclinical studies will also continue to provide insight into the mechanisms of action for stem cell therapy and promote successful translation into clinical efficacy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) project 81870921 (YW). We also thank the SJTU 2021 Summer Research Internship program for their support to ZW and KHC.
Conflict of interest statement
The authors declare no competing interest.
References
Zimeng Wu1
1Department of Natural Sciences, University College London, London, UK.
Ka Huen Cheng2
2Department of Biochemical Engineering, University College London, London, UK.
Rubing Shi3
3Med-X Research Institute and School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China.
Yongting Wang3
3Med-X Research Institute and School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China.
Corresponding author:
Yongting Wang
Email: yongting.wang@gmail.com, ytwang@sjtu.edu.cn
.png)
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7834 | 24 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA