Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Peripheral sensation and RIC inhibition: is diabetic peripheral neuropathy just the tip of the iceberg?
Time:2022-05-24
Number:7008
Jacqueline A. Epps1, Gudrun Dieberg1, Neil A Smart1
Author Affiliations


- 1School of Science and Technology & School of Rural Medicine, Trevenna Road, University of New England, Armidale, NSW, Australia 2351.
Conditioning Medicine 2022. 5(1): 11-21.
Abstract
Remote ischemic conditioning (RIC) is a therapeutic intervention, known for reducing irreversible damage caused by ischemia-reperfusion injury. Demonstrating great promise in preclinical studies, this outcome has not been consistently reproduced in larger clinical studies. The diabetic state has shown a variable response to RIC. Diabetic sensorimotor polyneuropathy (DSPN) is one of the factors limiting the protective effects of RIC; this was first identified in the 2012 Jensen study. However, the presence of neuropathy or DSPN is still very rarely included in participant characteristics, despite widely accepted evidence that DSPN inhibits the protective effects of RIC. Even if it became standard practice to report the presence and type of neuropathy in RIC studies involving patients with diabetes, much more information is required about the inhibitory effects of DSPN on RIC. The extent of its impact needs to be determined. We argue that a failure to adequately identify peripheral sensory neuropathy, especially DSPN, in study participants may be an important but overlooked confounding factor in RIC research. This review aims to identify and address numerous overlooked questions regarding DSPN, its precursors and its subtypes, and their interactions with RIC. Finally, a series of recommendations are made with respect to RIC studies likely to involve participants with DSPN or similar peripheral sensory impairments, that may assist the transition to clinical practice.
Keywords: Remote ischemic conditioning, Type 2 diabetes mellitus, Diabetic neuropathy, Small fiber neuropathy, Cryptogenic Sensory polyneuropathy, Ischemia-reperfusion injury.
Abstract
Remote ischemic conditioning (RIC) is a therapeutic intervention, known for reducing irreversible damage caused by ischemia-reperfusion injury. Demonstrating great promise in preclinical studies, this outcome has not been consistently reproduced in larger clinical studies. The diabetic state has shown a variable response to RIC. Diabetic sensorimotor polyneuropathy (DSPN) is one of the factors limiting the protective effects of RIC; this was first identified in the 2012 Jensen study. However, the presence of neuropathy or DSPN is still very rarely included in participant characteristics, despite widely accepted evidence that DSPN inhibits the protective effects of RIC. Even if it became standard practice to report the presence and type of neuropathy in RIC studies involving patients with diabetes, much more information is required about the inhibitory effects of DSPN on RIC. The extent of its impact needs to be determined. We argue that a failure to adequately identify peripheral sensory neuropathy, especially DSPN, in study participants may be an important but overlooked confounding factor in RIC research. This review aims to identify and address numerous overlooked questions regarding DSPN, its precursors and its subtypes, and their interactions with RIC. Finally, a series of recommendations are made with respect to RIC studies likely to involve participants with DSPN or similar peripheral sensory impairments, that may assist the transition to clinical practice.
Keywords: Remote ischemic conditioning, Type 2 diabetes mellitus, Diabetic neuropathy, Small fiber neuropathy, Cryptogenic Sensory polyneuropathy, Ischemia-reperfusion injury.
Introduction
Remote ischemic conditioning (RIC) has been evaluated as a therapeutic technique to protect a range of remote tissues and organs from ischemia-reperfusion injury. The technique uses alternating short doses of limb ischemia and reperfusion, typically applied by blood pressure cuff occlusion (Heusch, 2017). Although this article will focus on the acute effects of acute RIC application, RIC can also be repeated for chronic benefits (Chong et al., 2019). The conundrum of RIC and its translation into clinical practice has been extensively discussed. After decades of very promising animal model studies, results from human clinical trials have been variable (Heusch and Gersh, 2020). Numerous well-designed mechanistic studies have strived to improve the understanding of the underlying complex neurohumoral and physiological pathways involved in acute RIC. However, this research has lagged behind clinical studies, which could partially explain the variable results of trials (Heusch, 2017; Kleinbongard et al., 2017). Over the last 30 years, research on acute RIC has predominantly focused on humoral and immune responses, compared to neural pathways and mechanisms. Mechanistic studies investigating chronic RIC use are even more limited in number. Without a firm understanding of all the mechanisms involved in RIC, it is very likely that some confounding variables have not been identified or addressed, resulting in misleading conclusions being drawn.
People with diabetes are particularly susceptible to ischemia-reperfusion injury (Whittington et al., 2012; Ferdinandy et al., 2014; Penna et al., 2020), and researchers had hoped to uncover beneficial effects of RIC in this group. However, in type 1 and type 2 diabetes mellitus (T2DM) local and remote ischemic conditioning is frequently inhibited (Ferdinandy et al., 2014; Sloth et al., 2015; Epps and Smart, 2016; Tyagi et al., 2019; Penna et al., 2020). This inhibition appears to occur whether the conditioning is applied before, during, or after the onset of reperfusion (Tyagi et al., 2019) predominantly in animal models and translational studies, as prospective clinical trials specifically assessing RIC cardioprotection in patients with diabetes are still lacking (Penna et al., 2020). Multiple important factors responsible for this inhibition including pharmacotherapies (e.g. anti-hyperglycaemic agents), comorbidity, hyperglycemia, cardiomyocyte changes including altered O-linked β-N-acetylglucosamine signaling, exosome-associated signaling changes, and neuropathy have been implicated (Jensen et al., 2012; Jensen et al., 2013; Ferdinandy et al., 2014; Wider and Przyklenk, 2014; Epps and Smart, 2016; Lejay et al., 2016; Wider et al., 2018; Tyagi et al., 2019; Penna et al., 2020). Subsequent research has generally focused on investigating the impact of the first five mentioned factors on RIC efficacy in diabetes mellitus. However, the impact of neuropathy remains largely under-appreciated and unexplored, although there is strong evidence that neurological pathways have an important mechanistic role in acute RIC (Jensen et al., 2012; Gourine and Gourine, 2014; Basalay et al., 2016; Mastitskaya et al., 2016; Pickard et al., 2016; Pickard et al., 2017; Basalay et al., 2018; Hausenloy et al., 2019a). The Jensen RIC cardioprotection study found that diabetic sensorimotor polyneuropathy (DSPN) in human T2DM completely abolishes the cardioprotective effects of RIC (Jensen et al., 2012). Although the Jensen study findings have been known for nearly a decade, the unanswered questions raised by it remain just as relevant today. The American Diabetes Association defines DSPN as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” (p 138 Pop-Busui et al., 2017). Given the increasing prevalence of diabetes both worldwide and amongst the populations being studied in clinical RIC trials, with DSPN being a frequent complication of diabetes, this lack of knowledge needs to be addressed.
This review aims to highlight key knowledge gaps regarding the effects of peripheral sensory abnormalities on neural pathways in the limb of the RIC ischemic stimulus. The need for further RIC research investigating the impact of peripheral sensory neuropathy in people with diabetes and abnormal glucose metabolism is also emphasised. We discuss the diagnostic methods used and threshold of significance for peripheral sensory neuropathy in RIC studies. Finally, we draw a contrast between small and large fiber sensory neuropathy and outline why the former may be of particular importance in RIC studies, both in people with DSPN and for small fiber neuropathy in non-diabetic patients.
Literature searches using PubMed and Scopus databases were performed until July 2021, using different combinations of key search terms including “diabetes and remote ischemic conditioning”, “remote ischemic conditioning and mechanism”, “neural mechanism”, “diabetic neuropathy”, “impaired glucose tolerance”, “cryptogenic sensory polyneuropathy” and “small fiber neuropathy”. Cross referencing from these papers was performed to further identify suitable literature.
Neural pathways in Remote Ischemic Conditioning
Many eloquent studies have attempted to map out RIC’s complex neurohumoral mechanisms of action and its signal transduction. Research convincingly demonstrates that the release of circulating, transferable substances (including proteins, microRNAs, and exosomes) are involved, and this is referred to as the humoral mechanism (reviewed in Kleinbongard et al., 2017). The exact source of these humoral effector(s) is unknown. There is equally strong evidence of a neural pathway, extending from sensory nerve fibers in the remote stimulus organ that are relayed via the spinal cord, nuclei of the brain stem, and efferent autonomic nerves (particularly vagal) with involvement of the autonomic ganglia in the target organ (particularly the heart) (reviewed in Basalay et al., 2018; Hausenloy et al., 2019a). Intriguingly, it would appear these humoral and neural mechanisms are co-dependent, interactive and integrally linked. Abnormalities of peripheral sensory nerves (Jensen et al., 2012) and vagotomy (Basalay et al., 2016) in donors, prior to receiving the RIC intervention, abrogated the transfer of RIC protective humoral factors to naïve recipient hearts in animal studies. Likely involvement of the systemic immune system has also been identified (Konstantinov et al., 2004; Shimizu et al., 2010; Liu et al., 2015; Chen et al., 2018a; Liu et al., 2019). To further complicate the picture, the exact mechanisms appear to differ slightly depending on whether the ischemic stimulus is applied before, during or after the ischemia-reperfusion injury (termed remote ischemic pre-, per- and post- conditioning respectively), and potentially the target organ involved (Hess et al., 2015; Heusch, 2015; Zarbock and Kellum, 2016; Chen et al., 2018b).
Peripheral neural pathways of remote ischemic preconditioning require intact capsaicin-sensitive C-and Aδ-sensory fibers, termed nociceptors, in the stimulus organ. This has been confirmed by the discovery that transection or blockade of sensory nerves of the stimulus limb, and more specifically of the afferent sensory C-fibers, inhibit the release of circulating cardioprotective substances and remote ischemic preconditioning effects in rat studies (Lim et al., 2010; Steensrud et al., 2010; Basalay et al., 2012; Redington et al., 2012; reviewed in Gourine and Gourine, 2014). Capsaicin-sensitive afferent C- and Aδ-sensory fibers are typically activated by trauma and ischemia (Gourine and Gourine, 2014). Specific limb afferent C-fiber blockade inhibited remote ischemic preconditioning cardioprotection in a rat study (Basalay et al., 2012). Direct stimulation of the afferent sensory C-fibers of the skin with capsaicin or by trauma from a surgical skin incision also mimic the effects of RIC in animal studies (Redington et al., 2012; Ren et al., 2019). Blockade of the release of calcitonin gene-related peptide (CGRP) prior to remote ischemic preconditioning abolished cardioprotective effects in rats (Singh et al., 2017). CGRP is a neuropeptide released by nociceptive sensory C- and Aδ-sensory fibers in response to ischemia, capsaicin, trauma or other noxious stimuli (Russell et al., 2014; Singh et al., 2017). It has been found in perivascular nerves, from where it is thought to be released into plasma (Russell et al., 2014), and has been identified as a possible cardioprotective humoral mediator of limb (Singh et al., 2017) and mesenteric RIC (Wolfrum et al., 2005). Of note, DSPN is known to diminish the release of CGRP in response to a noxious stimulus (Mangialardi and Madeddu, 2016). The key study by Jensen et al. (2012) demonstrated that immediately after an acute bout of RIC, serum dialysate collected from human donors with T2DM without DSPN, could transfer significant cardioprotection against subsequent myocardial infarction to isolated rabbit hearts. For a separate group of participants in the study with both T2DM and DSPN that transfer of protection was completely lost (Jensen et al., 2012). This study highlights that both neural and humoral pathways are involved in the RIC mechanism, but also that the loss of peripheral sensation alone is sufficient to abrogate transfer of RIC protection from the donor.
Studies have also identified an essential role for the autonomic nervous system in RIC mechanisms, again integrally linked to humoral pathways, through procedures such as surgical ligation of nerves, or by utilizing pharmacological agents blocking these pathways (Mastitskaya et al., 2012; Basalay et al., 2016; Mastitskaya et al., 2016; Pickard et al., 2016; Pickard et al., 2017; reviewed in Hausenloy et al., 2019a).
A description of the neural pathways involved in RIC is comprehensively reviewed in Basalay et al. (2018) and Hausenloy et al. (2019a).
Diabetic sensorimotor polyneuropathy
The causes of DSPN are likely to be multifactorial, including hyperglycemia, inflammation, oxidative stress, dyslipidaemia, and pathology of the microvasculature (Tesfaye et al., 2010; Pop-Busui et al., 2017; Shillo et al., 2019). Initially these abnormalities commonly result in damage to smaller and more vulnerable sensory unmyelinated C- and myelinated Aδ- fibers, which are both responsible for the perception of noxious stimuli and pain. Larger sensory fibers are affected as DSPN progresses, leading to abnormal light touch sensation, reduced vibration perception, tingling in limbs or loss of proprioception (Pop-Busui et al., 2017; Shillo et al., 2019; Sierra-Silvestre et al., 2020). Around 50% of people with diabetes ultimately develop chronic DSPN, typically only diagnosed once some sensation is lost. (Shillo et al., 2019; Tesfaye and Sloan, 2020). The abnormalities are length-dependent, with longer sensory nerves in the toes becoming symptomatic before the feet, lower legs and upper limbs (Tesfaye et al., 2010; Sierra-Silvestre et al., 2020).
DSPN appears to abrogate cardioprotection from RIC (Jensen et al., 2012). Despite this important discovery, no further research has explored these findings, including use of a larger sample size, or investigated the effects of DSPN on RIC protection in other target organs. In the only available post-hoc analysis assessing cardioprotection of RIC in 71 patients with ST-elevation myocardial infarction (STEMI) in addition to primary percutaneous coronary intervention (PPCI), the presence or absence of DSPN or other neuropathies in the 14 patients with diabetes included in the RIC group was not documented (Sloth et al., 2015). The importance of detecting and reporting the presence or absence of DSPN in RIC clinical studies has been highlighted (Epps and Smart, 2016), yet is frequently omitted from study protocols, including studies specifically designed to involve patients with diabetes.
Cardiac autonomic neuropathy
This review focuses on DSPN and RIC; however, it is worth mentioning at this point that another common form of diabetic neuropathy, cardiac autonomic neuropathy (CAN), is a further potential factor that reduces the protective effects of RIC in diabetes. A systematic review identified that this form of neuropathy can also be present in prediabetes, with a prevalence of up to 25%, and furthermore is associated with obesity, hyperlipidemia and hypertension (Eleftheriadou et al., 2021). Despite the high prevalence of CAN and the importance of the autonomic nervous system as part of the RIC mechanism, very few studies have investigated the role of CAN in acute human RIC studies. In a notable exception, Hansen and colleagues (2019) commendably included identification of both CAN and DSPN as part of their long-term repeated RIC study in T2DM, with comprehensive baseline and post-intervention assessments (Hansen et al., 2019).
The presence of CAN is very rarely reported in RIC studies, including studies of participants with diabetes. Although CAN is often present in non-diabetics, particularly in patients at higher cardiovascular risk (hence likely to be found in many RIC clinical study groups) (Eleftheriadou et al., 2021), diagnostic assessment has rarely been part of baseline assessments in RIC study protocols, other than in the Hansen et al. study (2019). CAN frequently remains undetected or subclinical, despite its prevalence of up to 25% in prediabetes (Eleftheriadou et al., 2021) and as high as 65% in patients with long-term diabetes (Ang et al., 2020). Potential negative effects of diabetic autonomic neuropathy and CAN on RIC have previously been discussed in depth (Epps and Smart, 2016).
RIC and DSPN: Unaddressed questions
A number of questions directly arise from the Jensen et al. (2012) DSPN study and from existing knowledge of the neural pathways involved in RIC. These questions broaden the discussion on what effect peripheral sensory neuropathy may have on RIC efficacy. In particular, they focus attention on the methods used in baseline assessments of participants involved in RIC studies with T2DM and its precursors.
Is location of the neuropathy important?
The assessment for presence of DSPN in Jensen’s study involved biothesiometry performed by a physician, although the body regions assessed were not listed in the published methodology (Jensen et al., 2012). We have previously queried this absence of a small, but important detail (Epps and Smart, 2016). It is crucial to the study’s conclusions to know if inclusion in the T2DM neuropathy group was defined by abnormal biothesiometry in the upper limb (where the RIC stimulus was applied), lower limb, or both. In the Jensen study, participants with diabetes did not have the site of their abnormal biothesiometry readings recorded in the data collection. Only the presence or absence of peripheral neuropathy was reported based on recent medical records from annual diabetic clinics attended. Assessment of patients with diabetes in endocrinology clinics in their region in 2012 routinely involved biothesiometry of both upper and lower limbs according to the authors (R. V. Jensen, personal communication). From a methodological perspective, it would be very insightful to establish whether RIC applied to the upper arm of a participant with diabetes with normal sensation in that limb could still bestow benefit from RIC when DSPN is only present in the lower limbs, or if the benefit was lost despite normal sensation in the stimulus organ. For participants with only lower limb DSPN, the difference in cardioprotective effects between using lower or upper limb for the RIC application is important to determine. It is plausible that patients with DSPN only affecting the lower limb may benefit more from upper limb RIC, given that DSPN causes abnormalities in longer neurons in the lower limbs well before upper limb sensory changes develop (Tesfaye et al., 2010; Sierra-Silvestre et al., 2020).
It is also possible that more participants in the DSPN group of the Jensen et al. study (2012) had co-existent CAN compared to those in the non-DSPN and non-diabetic groups. Testing for or reporting the presence of CAN was not part of the Jensen et al. (2012) study protocol, and CAN and DSPN commonly co-exist (Tentolouris et al., 2001). The findings in the Jensen et al. (2012) study certainly point to DSPN as an inhibitor of the cardioprotective effects of RIC, but CAN may have contributed as well. The potential impact of CAN and associated cardiac sensory neuropathy on RIC efficacy has been reviewed in detail, in addition to the co-occurrence of CAN with DSPN and other causes of sensory neuropathy (Bencsik et al. 2020). CAN may be an important but often unidentified reason for the lack of a cardioprotective response in RIC studies, noting that routine screening for CAN is rarely performed or listed in baseline characteristics in most translational or clinical studies. Similarly, further investigation needs to ensure the apparent inhibitory effects of DSPN on RIC were not simply due to an association with other known or unknown inhibitory factors, rather than a definite causal factor. With so many known confounding variables in diabetic population groups due to comorbidity, co-medication, diabetic complications, and duration of diabetes, larger sample sizes are necessary to allow for adequate mediation analyses.
Does the testing for diabetic sensorimotor polyneuropathy have sufficient sensitivity?
Many publications and guidelines list recommended criteria for the diagnosis of DPSN, ranging from patient questionnaires and basic physical examination to more sensitive methods such as vibration perception threshold (VPT) used in biothesiometry, nerve conduction studies (NCS), and skin biopsy. VPT testing and NCS have the disadvantage of not being sufficiently sensitive to detect purely small fiber neuropathy (Papanas et al., 2011; Hoeijmakers et al., 2012; Backonja et al., 2013; Stino and Smith, 2017). DSPN can affect either small, large, or both types of fibers and it is unclear which of these neuropathy types has the inhibitory effect on RIC. However, as referred to above, research has confirmed that it is the small nociceptor fibers (C-fibers and Aδ-fibers) in the stimulus organ that are important for the RIC mechanism (Gourine and Gourine, 2014; Pickard et al., 2015). It is therefore logical that the testing utilized for identifying neuropathy in RIC studies needs to have a high sensitivity for detecting abnormalities specifically in these small fibers.
Relying on VPT, as was the case in the Jensen study, (Jensen et al., 2012) and/or NCS will miss isolated small fiber neuropathy. Abnormalities of the small fibers are likely to be the first affected in diabetic peripheral neuropathy (Papanas et al., 2011; Stino and Smith, 2017; Sierra-Silvestre et al., 2020; Kazamel et al., 2021). Quantitative Sensory Testing (QST) has a reasonable sensitivity for assessing small fiber function (Sierra-Silvestre et al., 2020), though it is prone to bias as a psychophysical and semiobjective test (Terkelsen et al., 2017). Skin or nerve biopsies are considered the gold standard in identifying early changes and intraepidermal nerve fiber density evaluation, including small fiber neuropathy, though they are more invasive, costly, and only assess histopathological appearance rather than function (Devigili et al., 2020). A new and highly promising alternative method is corneal confocal microscopy (CCFM), which has the advantage of being less invasive than biopsies (Papanas and Ziegler, 2015; Moulton and Borsook, 2019). Another modality that instead assesses C-fiber function is axon reflex-elicited flare area or Laser Doppler Imaging Flare measurement (LDIflare). After applying heat to the skin, LDIflare measures the resultant neurogenic vasodilatation. This method appears to have a high sensitivity for small fiber function, though is yet to be widely used (Green et al., 2010; Stirban, 2014; Sharma et al., 2018). Although this may not be of great practical use in the clinical setting for diagnosis of DSPN, a method with high sensitivity and ability to quantifiably assess nociceptor C-fiber function for RIC studies in relevant population groups could prove worthy of further research.
Research investigating (or controlling for) the effects of DSPN in RIC has typically assessed participants for neuropathy by physical examination and/or biothesiometry using established clinical thresholds. What is not known, is the threshold at which neuropathy changes begin to abrogate RIC protection. It is plausible that changes occurring prior to reaching a clinical diagnostic threshold may also affect C-fiber function, and hence RIC study outcomes. A recent meta-analysis supports this hypothesis, confirming the loss of small fiber function before the onset of DSPN symptoms in patients with Type 1 and 2 diabetes (Sierra-Silvestre et al., 2020). Skin biopsies demonstrate subclinical DSPN changes on C-fiber histopathological examination, such as axonal swelling, before abnormalities are detected through medical history and physical examination (Terkelsen et al., 2017; Devigili et al., 2020). Similarly, CCFM has a higher sensitivity than standard methods for evaluating early DSPN, and frequently detects subclinical abnormalities in nerve fibers even at diagnosis of T2DM (Papanas and Ziegler, 2014; Papanas and Ziegler, 2015). A question of interest is whether these subclinical changes are significant in abrogating the beneficial effects of RIC?
As is current practice for measures such as HbA1c and age, we anticipate that DSPN potentially needs to be viewed more as a continuum or spectrum, rather than merely clinically dichotomous data, if this is to be used as a study group allocation criterion. Until a threshold has been determined for the degree of DSPN likely to inhibit RIC, it may become necessary to screen all study participants with diabetes with standardized and more sensitive detection methods than those used in routine clinical practice and in RIC studies to date. Given that technology is now available to more sensitively assess C-fibers and early DSPN changes, this technology should be utilized in research. Listing these results in a quantifiable format for DSPN should ideally be included alongside many other routinely reported baseline characteristics, such as HbA1c, age, duration of diabetes, and lipid profile. Once the threshold for the onset of inhibitory effects of DSPN on RIC is determined, the relationship beyond that threshold with increasing neuropathy severity needs to be defined. Using highly sensitive quantitative methods to assess the extent of DSPN and correlating results with various RIC protection outcomes would allow the exact relationship between DSPN and RIC efficacy to be defined. Such information would be considerably more valuable than documenting mere presence or absence of DSPN based on clinical examination or accepted clinical thresholds for NCS and VPT diagnosis.
The likelihood of developing clinical DSPN is estimated to be up to 50% within 10 years of T2DM diagnosis (Tesfaye et al., 2010; Hoeijmakers et al., 2012; Stino and Smith, 2017; Feldman et al., 2019). This prevalence rate is high enough to affect a sizeable impact on RIC clinical study outcomes. If the threshold of significance for RIC research were lower than for clinical diagnosis, this rate would be higher still.
Lastly, it is also unknown how widespread the distribution of DSPN in a limb needs to be to inhibit RIC effects. Does small patchy sensory loss confined to one limb have the same degree of effect as widespread, uniform loss in both upper and lower limbs? Is it the function of more superficial nociceptors in the skin that is crucial, deeper nociceptors (that are much harder to assess clinically), or both, when detecting and relaying the signal from the ischemic stimulus organ?
Subtypes of neuropathic sensory symptoms
An estimated 50% of patients with DSPN develop painful neuropathy (Tesfaye and Sloan, 2020). These pain symptoms are often termed “positive” neuropathic sensory symptoms, which can be a feature of both small and large fiber DSPN (Hoeijmakers et al., 2012; Dyck et al., 2013). It would be worth establishing if such patients have their response to RIC relatively preserved compared to those with DSPN causing predominantly decreased pain sensation, or “negative” neuropathic symptoms (Hoeijmakers et al., 2012; Dyck et al., 2013). Another possible hypothesis could be that some people with diabetes may already be receiving frequent nociceptive stimuli from their painful neuropathy compared to those who only experience decreased sensation. In this case, additional conditioning stimuli, also utilizing nociceptor pathways, may not yield further benefit. To our knowledge, this concept has not yet been explored in RIC studies including participants with diabetes, nor in patient groups with other forms of painful sensory neuropathy. A meta-analysis showed that DSPN with painful neuropathy was not associated with improved small fiber function (Sierra-Silvestre et al., 2020). Interestingly, this was in direct contrast to other forms of painful neuropathy, such as commonly diagnosed radiculopathy, which have an increased small nerve fiber function (Sierra-Silvestre et al., 2020). This concept raises yet more questions for the efficacy of RIC in non-diabetic patient groups with neuropathic pain, and perhaps partly explains why chronic neuropathic pain has been found to be cardioprotective in a murine model (Cheng et al., 2017; Cheng and Chen, 2018).
Who should be tested for peripheral sensory neuropathy?
By definition, DSPN occurs only in people with diabetes, with up to 20% of T2DM patients having DSPN at diagnosis (Stino and Smith, 2017). It is increasingly apparent that the onset of DSPN is much earlier than once thought. Early signs of DSPN have also been found in prediabetes, particularly in patients with impaired glucose tolerance (IGT) rather than impaired fasting glycemia (Papanas et al., 2011). These peripheral sensory abnormalities are not being assessed for in participants with IGT for RIC studies. Sensory changes in IGT are often termed peripheral neuropathy or cryptogenic sensory polyneuropathy (CSPN), which appears to be a similar pathophysiological process to early DSPN (Stino and Smith, 2017). They may well in fact be the same disease, simply commencing at an earlier point in the development of T2DM than previously thought (Stino and Smith, 2017). It has been reported that up to 25% of patients with pre-diabetes have peripheral neuropathy (or CSPN) meeting clinical diagnostic criteria (Papanas et al., 2011).
A study by Green et al. (2010) was performed on patients with no clinically evident peripheral neuropathy as established using normal VPT, neuropathy disability scale and quantitative sensory testing. Those in the IGT group (but not those with type 1 diabetes) had significantly reduced LDIflare area results than controls. This indicated small fiber dysfunction even in patients with no peripheral neuropathy detected using other accepted clinical diagnostic methods, and before the onset of T2DM (Green et al., 2010). In another study of participants with IGT, CCFM identified significant small fiber abnormalities consistent with neuropathy in 40% of this group (Asghar et al., 2014). Such results would suggest that, beyond the approximate 25% of patients with IGT and peripheral neuropathy/ CSPN (Papanas et al., 2011), there are even more patients with likely sensory C-fiber functional abnormalities, despite falling short of a clinical diagnostic threshold of peripheral neuropathy. In a 2003 study of patients with both CSPN and IGT, a significantly higher proportion of the IGT group had small fiber sensory neuropathy compared to large fiber, following analysis of NCS and skin biopsies (Sumner et al., 2003). This could easily be overlooked in RIC studies, due to a lack of screening in non-diabetic patients for peripheral neuropathy in the first place, and/or by using methods that are more sensitive in detecting large rather than small fiber peripheral neuropathy. As a consequence, many patients with abnormal sensory C-fiber function are likely to have been missed in multiple RIC studies, thus potentially skewing results.
Considering these findings, it is evident that small fiber neuropathy ideally needs to be considered and adequately screened for in RIC studies expecting to involve patients with IGT. Globally, the prevalence of IGT is high and increasing (Eleftheriadou et al., 2021), with approximately 36% of adults in China and the USA having IGT and substantially elevated cardiovascular risk (Cai et al., 2020). Consequently, it is inevitable that patients with IGT (in addition to diabetes) are over-represented in many RIC studies, such as those investigating its use in major adverse cardiac events, stroke, vascular disease, and other conditions associated with a risk of ischemia-reperfusion injury. With the high prevalence of sensory abnormalities in the IGT population and the likely high numbers of IGT patients in many RIC studies, IGT has the potential to be an important and under-recognized confounding variable, yet this is not reported in baseline characteristics. Casting the net yet further, patients with other conditions falling short of IGT and T2DM are also at increased risk of CSPN. Obesity, hypertriglyceridemia, and metabolic syndrome are known to be associated with peripheral neuropathy (Papanas et al., 2011; Hanewinckel et al., 2016; Stino and Smith, 2017; Terkelsen et al., 2017; Kazamel et al., 2021), particularly small fiber neuropathy (Stino and Smith, 2017; Terkelsen et al., 2017) with an odds ratio of 2.84 for elevated waist circumference and 2.01 for hypertriglyceridemia. The odds ratio for metabolic syndrome increases with the number of criteria present (Hanewinckel et al., 2016).
When reviewing participant characteristics in RIC studies, particularly clinical trials, it becomes obvious that the majority of patients have at least one or more of these risk factors for small fiber neuropathy. Examples of this can be found in recent randomized phase III trials assessing cardioprotection from RIC – CONDI-2 / ERIC-PPCI (Hausenloy et al., 2019b), RIC-STEMI (Gaspar et al., 2018), ERICCA (Hausenloy et al., 2015), and RIPHeart (Meybohm et al., 2015). Baseline characteristics for the RIC groups included 11.9 - 26% participants with diabetes, 43.7 - 83% with hypertension, and 28 - 72.2% with hypercholesterolemia. None of these trials included the percentage of participants with obesity, IGT, hypertriglyceridemia, metabolic syndrome, or diabetic neuropathy. Only two trials reported participants’ body mass index, and the RIPHeart trial omitted hypercholesterolemia, although 62.7% of participants were taking cholesterol-lowering drugs (Hausenloy et al., 2015; Meybohm et al., 2015; Gaspar et al., 2018; Hausenloy et al., 2019b).
The potential importance of these conditions associated with DSPN or CSPN in RIC studies is further emphasized by their prevalence in patients presenting with acute myocardial infarction. For example, a study involving data from 1000 hospitals in the USA from 2003 to 2013 calculated the prevalence of cardiovascular risk factors in patients admitted with a STEMI. In 2013, 29% had diabetes, 65.3% hypertension, 63.6% hyperlipidemia, and 14.9% obesity. The prevalence of each of these risk factors was also 5-10% higher for patients with non-STEMI (Agarwal et al., 2017). In a 2004 European analysis of patients presenting with acute coronary artery disease, 32% of patients had known diabetes. Of the patients without known diabetes, 32% met diagnostic criteria for IGT and a further 22% had undiagnosed diabetes. (Bartnik et al., 2004). In separate studies, 37.9-44.4% of patients with STEMI had metabolic syndrome, depending on the classification used (Lovic et al., 2018) and approximately 50% of the USA population met diagnostic criteria for metabolic syndrome in 2016 (Hanewinckel et al., 2016).
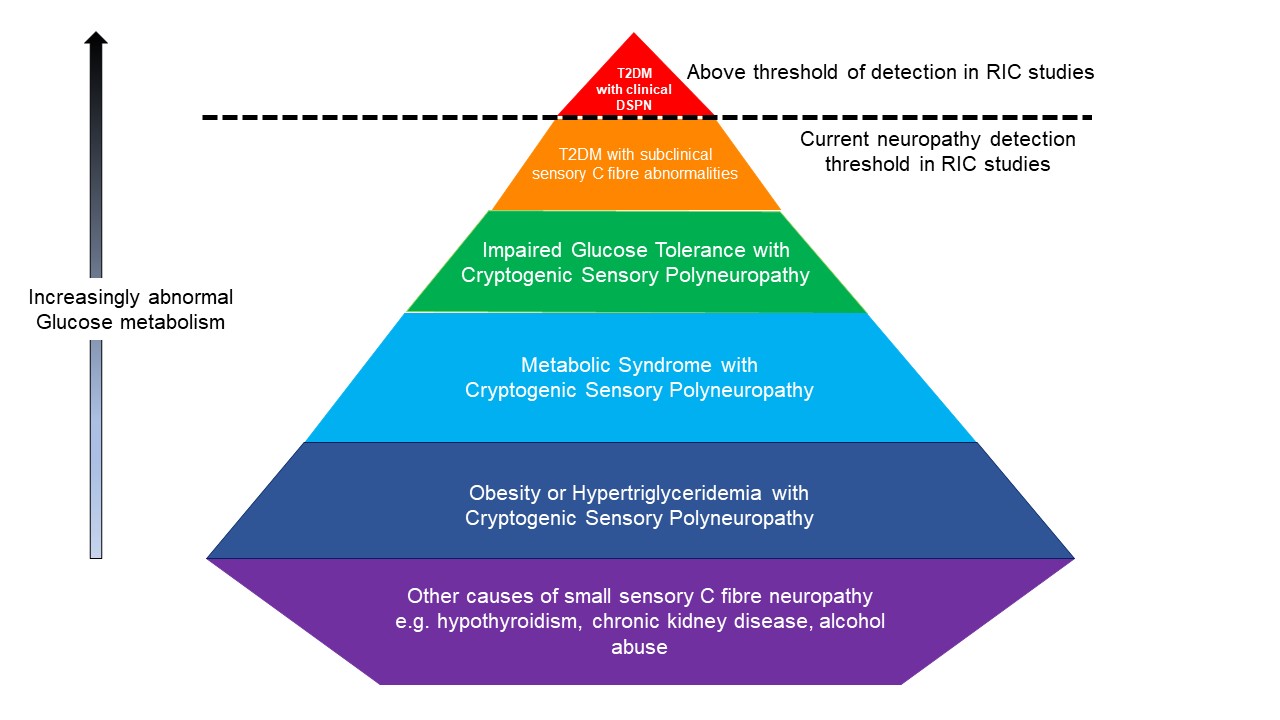
To our knowledge, the role of coexisting peripheral sensory neuropathy has not been considered in RIC study participants with IGT, metabolic syndrome, hypertriglyceridemia, and obesity. We therefore conclude that screening only patients with diabetes for peripheral sensory abnormalities in RIC cardioprotection studies would simply be the tip of the iceberg, given the frequency of conditions known to cause small fiber neuropathy in these population groups (Figure 1).
In a new window | Download PPT
Figure 1: This pyramid highlights the groups of participants at risk of sensory C fiber abnormalities that may be undetected in RIC studies. Red- T2DM with clinical DSPN; orange- T2DM with subclinical sensory C fiber abnormalities; green- Impaired Glucose Tolerance with Cryoptogenic Sensory Polyneuropathy; light blue- Metabolic Syndrome with Cryoptogenic Sensory Polyneuropathy; dark blue- Obesity or Hypertriglyceridemia with Cryoptogenic Sensory Polyneuropathy; purple- other causes of small sensory C fiber neuropathy e.g. hypothyroidism, chronic kidney disease, alcohol abuse. Note, figure is conceptual only and not drawn to scale
T2DM = Type 2 Diabetes Mellitus DSPN = Diabetic Sensorimotor Polyneuropathy.
Neuropathy beyond diabetes mellitus and impaired glucose tolerance
Although diabetes and IGT are some of the most common causes of neuropathy, sensory neuropathies occur in many other conditions. These may be subclinical or overt, involve purely small fibers or a combination of large and small fibers. Similarly to DSPN, it is logical that other forms of sensory neuropathy affecting small sensory C-fibers need to be assessed for their impact on RIC protection. Small fiber neuropathies have a wide range of causes, and to our knowledge their impact on RIC efficacy has not been studied. For small fiber neuropathy, causes include hypothyroidism, inflammatory bowel disease, vitamin B12 deficiency, celiac disease, alcohol abuse, systemic lupus erythematosus, HIV, Guillain-Barré syndrome, chemotherapy, a variety of medications, hemochromatosis, sarcoidosis, Sjögren syndrome, and chronic kidney disease, in addition to hypertriglyceridemia, IGT and obesity already discussed (Hoeijmakers et al., 2012; Terkelsen et al., 2017; de Greef et al., 2018; Sierra-Silvestre et al., 2020). Conditions such as hypothyroidism, chronic kidney disease and alcohol abuse are very common. Routine examination for sensory neuropathy is not typically undertaken in many of these causes of small fiber neuropathy in clinical practice and especially not in RIC studies.
Beyond cardioprotection
Earliest RIC studies focused on cardioprotection, but many target organ have now been researched (reviewed in Veighey and Macallister, 2012; Candilio et al., 2013; Epps and Smart, 2016; Zhou et al., 2018; Sprick et al., 2019). Neuroprotection, renoprotection, and reduction of adverse outcomes following a wide variety of surgical procedures have been studied, with neuroprotection perhaps showing the most promise (Basalay et al., 2018). Research into the effects of diabetes and diabetic neuropathy (such as the Jensen study) on RIC have predominantly involved studies assessing cardioprotection. The effect of DPSN on RIC-induced protection of other organs is largely unknown despite early evidence of neural pathways’ involvement in RIC-induced neuroprotection (Basalay et al., 2018) and renoprotection (Sedaghat et al., 2017). The effectors of RIC protection may well involve different pathways depending on the target organ in question. Unfortunately, little mechanistic research has been undertaken beyond cardioprotective studies (Epps and Smart, 2016; Basalay et al., 2018). Therefore, factors that are known to enhance or abrogate the cardioprotective response to RIC cannot necessarily be extrapolated to differing target organs.
Impact of pharmacotherapies on sensory C-fibers
Prescription for medications to manage neuropathic pain has rapidly increased in the past decade, particularly gabapentinoids (McAnally et al., 2020). It is generally agreed that gabapentinoids downregulate the quantity of substance P and CGRP released from nociceptors in the dorsal root ganglion in response to a noxious stimulus (Verma et al., 2014; Bannister et al., 2017; McAnally et al., 2020). DSPN has a similar effect of inhibiting nociceptor release of CGRP and substance P (Mangialardi and Madeddu, 2016). This neurotransmitter release has been identified as being mechanistically important in response to the ischemic limb stimulus of RIC (Wolfrum et al., 2005; Gao et al., 2015; Randhawa and Jaggi, 2017). Gabapentinoids are widely used to treat painful DSPN (Tesfaye and Sloan, 2020), in addition to being heavily prescribed for common causes of radicular pain, such as sciatica. To our knowledge, translational studies assessing the impact of gabapentinoids on RIC efficacy have not yet been undertaken, but if the effects prove to be negative, an investigation into their safety may be indicated.
Many oral anti-hyperglycemic agents are established inhibitors of RIC-induced cardioprotection, although importantly the mechanisms are not known to be directly due to drug-specific effects on peripheral sensory nerves (Ferdinandy et al., 2014; Penna et al., 2020). An exception to this is the condition of “insulin neuritis”, or more correctly, treatment-induced neuropathy of diabetes. A sudden and dramatic improvement in glycemic control precipitated by dietary change, oral hypoglycemics, or insulin can be the underlying cause of painful peripheral sensory neuropathy in up to 10% of DSPN cases (Gibbons, 2017). Metformin therapy is also known to be a risk factor for developing vitamin B12 deficiency (Chapman et al., 2016), which in turn can lead to small fiber neuropathy (Terkelsen et al., 2017; de Greef et al., 2018).
As referred to in section 4, many medications prescribed for other conditions can lead to small fiber neuropathy (Hoeijmakers et al., 2012; Terkelsen et al., 2017). Ideally these would be further investigated in RIC studies for their potential impact.
Future Research
Based on the discussion above, we would like to make some recommendations on the assessment of study participants, neuropathies, and study design, which we hope will generate future research into RIC and ultimately improved translation into the clinical setting (Box 1 and 2). Once most factors that abrogate protection from RIC in patients with diabetes can be adequately identified, together with the target organs these factors apply to, there may yet be many patient groups with diabetes that can benefit from RIC. Previous trials may need to be re-evaluated or repeated with an improved methodology, as it is plausible that outcomes may be different and more clinically relevant when overlooked factors and confounding variables are taken into consideration.
Box 1

Box 2

Conclusion
The causes of variable results in RIC studies are multifactorial. Peripheral sensory abnormalities are very unlikely to account for disappointing RIC study outcomes alone, however they do warrant further consideration and investigation as a confounder. Despite DSPN being an accepted and frequently cited cause of RIC inhibition, many unknowns remain in establishing the impact of neuropathy on RIC. It is important to clarify the extent and subtypes of DSPN and potentially other sensory neuropathies that inhibit RIC in order to ensure that the screening for sensory neuropathy in RIC participants is sufficiently broad and sensitive. There are many issues (see our recommendations) identified in this review that, if addressed, may assist this promising therapy to be successfully translated into clinical practice. The knowledge gained will hopefully provide an improved understanding of the puzzle of signal transduction and mechanistic pathways of RIC and provide insight into which patients (and target organs), if any, are likely to benefit from RIC. Finally, if DSPN is abrogating cardioprotective effects of RIC, the authors suggest that this merits investigation as an independent cardiovascular risk factor for poorer outcomes in ischemia.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Acknowledgements
J Epps is the recipient of an Australian Government Research Training Program (RTP) Stipend Scholarship to support her PhD research at the University of New England.
References
Jacqueline A. Epps1
1School of Science and Technology & School of Rural Medicine, Trevenna Road, University of New England, Armidale, NSW, Australia 2351.
Gudrun Dieberg1
1School of Science and Technology & School of Rural Medicine, Trevenna Road, University of New England, Armidale, NSW, Australia 2351.
Neil A Smart1
1School of Science and Technology & School of Rural Medicine, Trevenna Road, University of New England, Armidale, NSW, Australia 2351.
Corresponding author:
Jacqueline A. Epps
Email: jepps@une.edu.au

In a new window | Download PPT
Figure 1: This pyramid highlights the groups of participants at risk of sensory C fiber abnormalities that may be undetected in RIC studies. Red- T2DM with clinical DSPN; orange- T2DM with subclinical sensory C fiber abnormalities; green- Impaired Glucose Tolerance with Cryoptogenic Sensory Polyneuropathy; light blue- Metabolic Syndrome with Cryoptogenic Sensory Polyneuropathy; dark blue- Obesity or Hypertriglyceridemia with Cryoptogenic Sensory Polyneuropathy; purple- other causes of small sensory C fiber neuropathy e.g. hypothyroidism, chronic kidney disease, alcohol abuse. Note, figure is conceptual only and not drawn to scale
T2DM = Type 2 Diabetes Mellitus DSPN = Diabetic Sensorimotor Polyneuropathy.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7008 | 23 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA