Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
A shift of voltage-gated Na+-channel isoforms can contribute to age-dependent remodeling in the mammalian heart
Time:2022-08-01
Number:8735
Deniz Billur1, Belma Turan2
Author Affiliations


- 1Department of Histology & Embriyology, Ankara University Faculty of Medicine, Ankara, Turkey.
- 2Department of Biophysics, Lokman Hekim University Faculty of Medicine, Ankara, Turkey.
Conditioning Medicine 2022. 5(2):75-84.
Abstract
The prevalence of cardiovascular diseases increases dramatically as the population grow older worldwide. To improve the life of the elderly and prevent age-related cardiac diseases, novel strategies should be undertaken, which include new analysis of processes leading to cardiac aging. The disturbances and abnormalities in cardiac aging can be summarized as functional changes (including remodeling in both the electrical conduction system and vessel system characterized by high blood pressures as well as depressed contraction), structural changes (such as hypertrophy, cardiac lipotoxicity, fibrosis, and abnormal collagen deposition), cellular changes (including loss of several ionic channels, alterations in function of several transporters, exchangers, pumps, increases in the numbers of hypertrophic cardiomyocytes, loss of healthy cardiomyocytes, and senescence cardiomyocytes), molecular changes (including alterations in function of sarcoplasmic reticulum and mitochondria, increase in reactive oxygen species production, and abnormalities in Ca2+-homeostasis). Moreover, since NaV1.5, the major cardiac voltage-gated Na+-channels, plays a central role in the generation of the cardiomyocyte action potential and the propagation of electrical impulses in the heart, here, we document its major role in the aging heart and the contribution of a shift of its isoforms to age-dependent remodeling in the mammalian heart, in addition other ionic currents. From this perspective, the above-mentioned steps are common processes affecting the development of aging cardiac pathophysiology. However, each of these abnormalities alone does not lead to heart dysfunction, hence comorbid chronic conditions are the main factors of an aging heart leading to heart dysfunction. To improve patient care and prevention of age-related cardiac diseases, novel additional insight, including the changes in voltage-gated Na+-channel isoforms, should be gained from the analysis of processes involved and leading to cardiac aging.
Keywords: Sodium channel, cardiovascular, elderly, therapeutics, ion channel
Abstract
The prevalence of cardiovascular diseases increases dramatically as the population grow older worldwide. To improve the life of the elderly and prevent age-related cardiac diseases, novel strategies should be undertaken, which include new analysis of processes leading to cardiac aging. The disturbances and abnormalities in cardiac aging can be summarized as functional changes (including remodeling in both the electrical conduction system and vessel system characterized by high blood pressures as well as depressed contraction), structural changes (such as hypertrophy, cardiac lipotoxicity, fibrosis, and abnormal collagen deposition), cellular changes (including loss of several ionic channels, alterations in function of several transporters, exchangers, pumps, increases in the numbers of hypertrophic cardiomyocytes, loss of healthy cardiomyocytes, and senescence cardiomyocytes), molecular changes (including alterations in function of sarcoplasmic reticulum and mitochondria, increase in reactive oxygen species production, and abnormalities in Ca2+-homeostasis). Moreover, since NaV1.5, the major cardiac voltage-gated Na+-channels, plays a central role in the generation of the cardiomyocyte action potential and the propagation of electrical impulses in the heart, here, we document its major role in the aging heart and the contribution of a shift of its isoforms to age-dependent remodeling in the mammalian heart, in addition other ionic currents. From this perspective, the above-mentioned steps are common processes affecting the development of aging cardiac pathophysiology. However, each of these abnormalities alone does not lead to heart dysfunction, hence comorbid chronic conditions are the main factors of an aging heart leading to heart dysfunction. To improve patient care and prevention of age-related cardiac diseases, novel additional insight, including the changes in voltage-gated Na+-channel isoforms, should be gained from the analysis of processes involved and leading to cardiac aging.
Keywords: Sodium channel, cardiovascular, elderly, therapeutics, ion channel
Introduction
The identification of several ion channels that generate and contribute to the action potential in cardiomyocytes provided important information about heart function, particularly under pathophysiological conditions. Several ion channels expressed in the heart may be important therapeutic targets under pathological conditions. However, off-target effects of some therapeutics may lead to side effects. The major cardiac voltage-gated Na+-channel, NaV1.5 encoded by the gene SCN5A, plays an essential role in the generation of the action potential in cardiomyocytes and the propagation of electrical impulses in the heart. Their importance for the pathological heart has been well-documented by data from numerous mutations found in the gene SCN5A of cardiac patients (Zumhagen et al., 2013; Veerman et al., 2015; Zaklyazminskaya and Dzemeshkevich 2016; Li et al., 2018; Wilde and Amin 2018). As documented in these articles, there are genetic variants of SCN5A, which are involved in many inherited cardiac channelopathies including long-QT syndrome, cardiac dysfunction associated with abnormalities in the electrical conduction system and contractile activity, further leading to dilated cardiomyopathy and heart failure. Furthermore, various studies have documented abnormalities in potential ionic mechanisms underlying age-associated heart dysfunction and/or insufficiencies. These studies demonstrated the considerable changes in gene expression with up-regulation and/or down-regulation of mRNAs in ion channels responsible for cardiomyocytes, indicating important age-related ionic and molecular remodeling patterns in the heart (Ahmad et al., 2018; Alghamdi et al., 2020; Chambers et al., 2010; Strait and Lakatta, 2012; van den Boogard et al., 2014; Signore et al., 2015; Song and Belardinelli, 2018).
The current analysis of the molecular basis of heart dysfunction, provided by both experimental and clinical studies have determined the importance of ion channels through their critical roles in the generation of cardiomyocyte action potentials. Many ion channels function as part of macromolecular complexes, in which many components are assembled at specific sites within the membrane, with NaV1.5 being part of dynamic multiprotein complexes located in the cardiomyocyte membrane. Although studies demonstrated the expression of the voltage-gated Na+-channels in mammalian hearts, only a small amount of transcripts for tetrodotoxin (TTX)-sensitive Na+-channels were detectable in human hearts (Haufe et al., 2007; Blechschmidt et al., 2008; Zimmer, 2010). Therefore the results of these studies do not suggest the involvement of these channels in cardiac excitation. These results may also indicate less contribution of mutations in TTX-sensitive Na+-channels to a pathological cardiac phenotype. Recent genome-wide association studies identified SCN10A, the gene encoding NaV1.8, as a determinant of cardiac conduction parameters, and mutations in SCN10A have been associated with abnormalities in the parameters of surface electrocardiogram (ECG), including long-QT interval (Chambers et al., 2010; Pfeufer et al., 2010; Sotoodehnia et al., 2010). More importantly, various TTX-sensitive NaV channels, including NaV1.6 are also shown to be present in cardiac tissue, particularly in nodal tissue (Valdivia et al., 2005; Haufe et al., 2007; Valdivia et al., 2010). Under pathological stimuli, there can be various perturbations in NaV1.5 leading to changes in their structure and function, which are associated downstream with a wide variety of pathologies in the heart, including arrhythmias. There are several factors affecting cardiac aging besides continuous physiological progression (biological aging) in humans. Indeed, aging and longevity are determined by a complex combination of genetic, nongenetic, and environmental factors (Figure 1). Furthermore, some co-occuring factors such as diabetes, exposure to over oxidants (high oxidative stress), insulin resistance via obesity, and/or metabolic syndrome, can accelerate the biological aging in the heart. At the cellular level, the alterations in the structure and function of mitochondria further lead to severe deleterious changes in the heart (Rao et al., 2016; Khan et al., 2017; Paneni et al., 2017; Ullah and Sun, 2018). From the environmental perspective, the daily diet and dietary restrictions can importantly affect lifespan, including delay and/or prevention of many aging-related insufficiencies/disorders (Mirzaei et al., 2016). Besides environmental factors, there are important contributions of genetic background to the process of aging in humans (Rubinek and Modan-Moses, 2016). In addition, we and others have demonstrated that increases in mitochondrial oxidative stress, parallel to mitochondrial dysfunction, play a central role in cardiovascular disorders in elderly individuals, and seem to be plausible mechanisms underlying the progression toward heart failure in the associated aging hearts (Dai et al., 2012; Lesnefsky et al., 2016; Olgar et al., 2018; Olgar et al., 2019; Olgar et al., 2020a; Olgar et al., 2020b; Olgar et al., 2021). In different animal model studies on the aged heart as well as aged heart cardiomyocytes, we demonstrated marked increases in both systemic and cellular levels of oxidative stress, clear insulin resistance, hypertrophy, and significant prolongations in RR- and QT-intervals in ECGs of aged rats. Our data also emphasized the presence of marked increases in heart rate and mean arterial pressure and decreases in the ejection fraction and preload-recruitable stroke-work (Olgar et al., 2018). In addition, we have shown that a direct mitochondria-targeting antioxidant treatment could be an effective therapeutic strategy during aging of the heart by controlling intracellular ion levels such as Ca2+ and Zn2+ in isolated ventricular cardiomyocytes, in part, via regulation of the sodium-glucose co-transporter 2 (SGLT2) (Olgar et al., 2019; Olgar et al., 2020a; Olgar et al., 2020b). Moreover, ultrastructural and biochemical changes in cardiomyocytes from aged rat hearts (such as interfibrillar mitochondria and impaired mitochondrial oxidative phosphorylation) appear to play key roles in heart dysfunction in aged individuals.
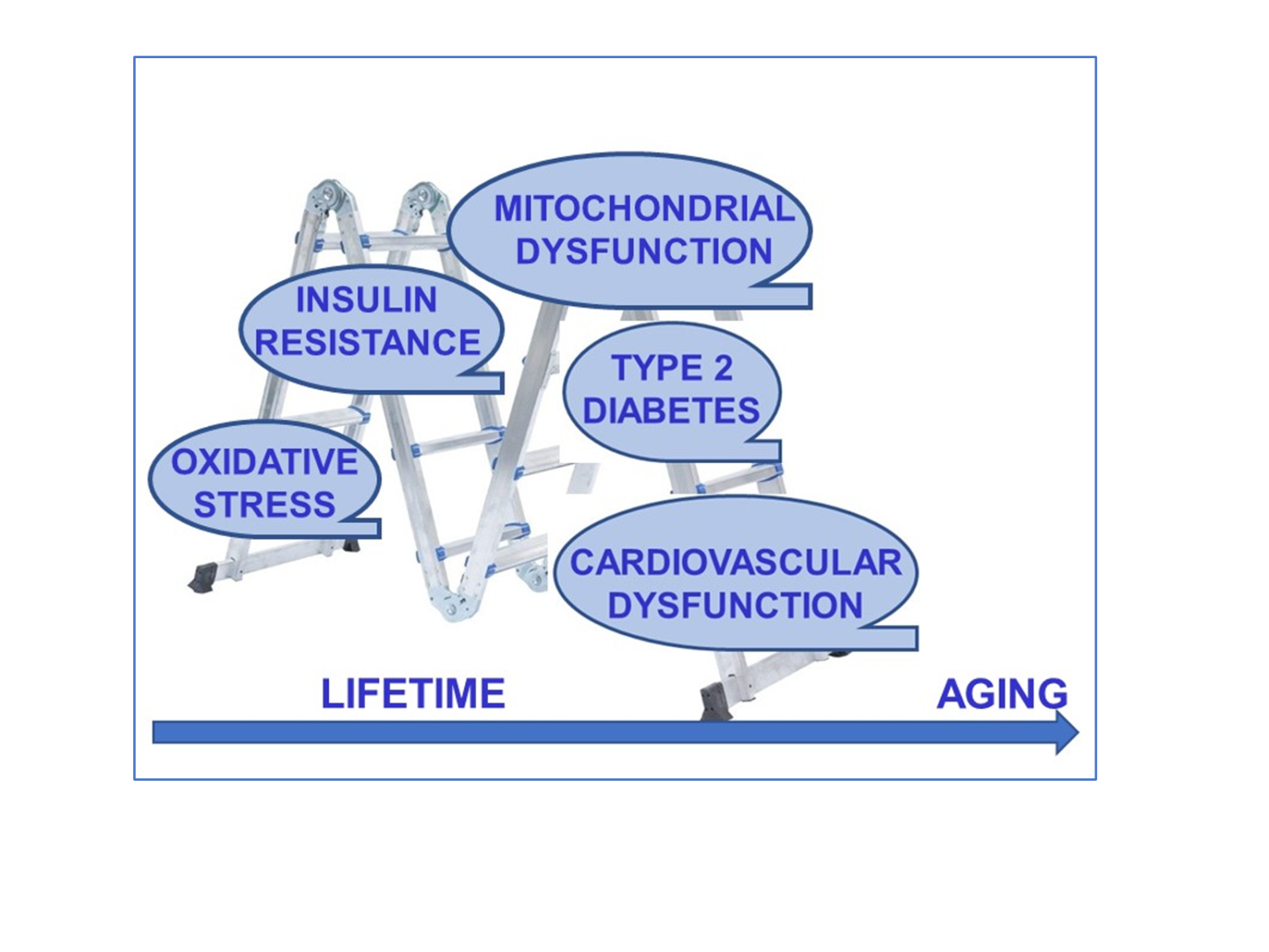
In a new window | Download PPT
Figure 1: The continuum of the aging progress and age-related contributors to heart function in the elderly. During physiological aging, external contributors and diseases can promote the development of cardiovascular diseases in humans. Studies have shown that the prevalence of diabetes and impaired glucose tolerance (including insulin resistance) in individuals is increasing with advanced age. Cellular and organelle level changes in the heart (cardiometabolic disturbances) promote the incidence of cardiovascular disorders in physiological/biological aging in humans.
Recent studies have shown a close relation between artial fibrillation, the most common cardiac arrythmia, and altered expression levels of voltage-gated Na+-channels, particularly shifts in their isoforms, in the development of age-associated changes in heart function (Cooper and Jones, 2015; Isaac et al., 2020; Nowak et al., 2021; Horváth et al., 2022). In these studies, the authors mentioned how voltage-gated Na+-channels may predispose the elderly heart to damage. Here, in this review, we focus on the role a shift in voltage-gated Na+-channel isoforms play in age-dependent remodeling in the mammalian heart, including structural distribution of NaV1.5 in the ventricle of the heart from insulin-resistant aged rats.
Role of sex differences on the aging heart
A large body of clinical evidence supports the concept that there is a close relationship between sex-related physiological differences and propensity for some diseases in mammalians. However, there are contradicting data regarding the increase in the female risk for cardiovascular disease not only in after menopause but also with advanced age (Kannel et al., 1976; Kalin and Zumoff, 1990; Barrett-Connor, 1997; Atsma et al., 2006; Mikkola et al., 2013). Therefore, age is the most significant determinant for cardiovascular diseases in both mammalian sexes, but further data are needed to clarify the underlying mechanisms for these differences. However, Merz and Cheng (2016) widely documented why studies examining sexes-dependent differences are limited in terms of prevalence and effectiveness of cardiovascular disease even though there has been recent progress in identifying and narrowing the gaps in cardiovascular outcomes between men and women (Merz and Cheng, 2016). Over our life course, our hearts continually undergo progressive remodeling, and the the remodeling patterns differ between the sexes. For instance, there are both hormonal (i.e. sex hormones, estrogen, etc.) and non-hormonal factors (i.e. increase in left ventricular wall thickness, decrease in left ventricular dimensions, increase in concentricity, etc.) underlining the sex-dependent differences in cardiovascular aging (Hulley et al., 1998; Cheng et al., 2009; Keller and Howlett, 2016; Asatryan et al., 2021; Yusifov et al., 2022). However, there are contradictory data on how these sex-related differences affect cardiovascular outcomes, clinically. There are even sex-dependent differences in the electrical activity of the heart, including several parameters of ECGs. As demonstrated in many articles, although the QT-interval calculated from surface ECGs is similar in boys and girls at birth and during childhood, the onset of puberty brings sex-related differences such as shortening in QT-interval in males and prolongation in the QT-interval in female patients. These differences diminish after menopause, indicating an effect of sex hormones on cardiac function (Rautaharju et al., 1992; Bidoggia et al., 2000; Odening and Koren 2014). Supporting the above statements, the long QT syndrome (LQTS) is higher in women than in men (Locati et al., 1998). Furthermore, adult women with LQTS are generally at increased risk of Torsades de Pointes compared with male individuals and prepubescent girls (Salama and Bett 2014). On the other hand, there are marked differences among male and female patients with short QT syndrome while its predominance is about 70% higher in men than women, at least with regards to the longer resting QT-interval in women than in men (El-Battrawy et al., 2020).
The effects of sex hormones on cardiac electrophysiology and risk for sudden cardiac death are mainly due to the effects of hormones (such as estrogen, progesterone, and testosterone) through mechanisms similar to their physiological effects on ionic currents (L-type Ca2+ current, inward rectifier K+ current, rapid and slow components of the delayed rectifier K+ current, and transient outward K+ current, etc.). Correspondingly, it has been demonstrated that estrogen increased the duration of the QT interval, whereas endogenous progesterone and testosterone shortened the QT interval (Odening and Koren 2014). Overall, it is obvious that sex-dependent differences in cardiac aging likely contribute to different age-related cardiac pathogenesis observed in clinical populations (Cheng et al., 2009; Hulley et al., 1998; Asatryan et al., 2021; Yusifov et al., 2022).
Structure and function of cardiac voltage-gated Na+-channels
Voltage-gated Na+-channels are transmembrane proteins responsible for the rapid upstroke changes in membrane potential during electrical stimulation of excitable cells. The rapid changes following the threshold voltage in the membrane and also rapid impulse conduction support the general roles of the voltage-gated Na+-channels in cardiac function and thereby their central roles in the generation of cardiac arrhythmias (Li et al., 2018). This initial Na+-influx provides the depolarization trigger for voltage-gated Ca2+-channel activation, leading to Ca2+-dependent Ca2+-release from the sarcoplasmic reticulum (SR), followed by a contraction in ventricular cardiomyocytes. In addition, Na+-channels also behave as the driver of cardiac conduction. Consequently, the sum of the individual action potential of each cardiomyocyte underlies the ventricular depolarization wave responsible for the QRS-complex in the surface ECGs (Balser 1999; DeMarco and Clancy 2016; Rook et al., 2012).
A typical structure of the voltage-gated Na+-channels consists of a protein complex of a large α-subunit with four homologous domains, each of them containing six transmembrane segments (Noda et al., 1986). There are also one or two β-subunits. While the accessory β-subunits are not required to form functional channels, they do exert some effects on the biophysical characteristics of the channel (Rook et al., 2012). In mammalian myocardium, the SCN5A gene-encoded NaV1.5 is the most prominent Na+-channel determining cardiac conduction. There are also other subtypes of Na+-channels in the heart such as NaV1.8 encoded by the SCN10A gene (Verkerk et al., 2007). Consequently, the main voltage-gated Na+-channel subtypes found in the heart are the TTX-insensitive subtypes NaV1.5 and NaV1.8. Furthermore, the expression of TTX-sensitive voltage-gated Na+-channels such as NaV1.6 has been described in the heart (Lopreato GF et al., 2001; Meisler MH et al., 2004; Zakon HH et al., 2011), and its possible role in cardiac arrhythmias in mice has been proposed although its expression is low in isolated cardiomyocytes (Noujaim et al., 2012). The cardiovascular effects of the NaV1.6 channel need further studies to be clarified.
Cardiac ion channels are critical for all aspects of cardiac function, including rhythmicity and contractility, as they are key targets for therapeutics in cardiac pathophysiologies (Priest and McDermott, 2015). For instance, NaV1.5 is the target of many common antiarrhythmic therapies. Similar to NaV1.5, NaV1.8 is a member of the TTX-resistant voltage-gated Na+-channel family (Balser, 1999; Rook et al., 2012; DeMarco and Clancy 2016). To identify genetic factors influencing cardiac conduction, Chambers and co-workers (2010) carried out a genome-wide association study of ECG time intervals in over 5,000 people to verify the effect of genetic variation in SCN10A on cardiac conduction. Their analyses on the association between SCN10A expression levels in SCN10A(-/-) mice in comparison to wild-type mice and the shorter PR interval generated new insight into the pathogenesis of cardiac conduction, heart block, and ventricular fibrillation. Subsequently, NaV1.8 expression was observed in mouse and human cardiac myocytes and intracardiac neurons (Facer et al., 2011). In addition, further studies emphasized the role of NaV1.8 in cardiomyocyte in heart function, as its blockage decreases the late Na+-current and shortens the action potential duration (Yang et al., 2012). Blockade of NAV1.8 in intracardiac neurons decreases the action potential frequency (Verkerk et al., 2012). How these observations relate to cardiac conduction is not entirely clear. Indeed, human polymorphisms occur in non-coding regions of SCN10A and their functional consequences are not known. However, there are some indications the effects of at least one of these polymorphisms influences the transcriptional regulation of SCN5A and/or SCN10A (van den Boogaard et al., 2014). Therefore, it appears some mutations in NaVs can be linked to many severe conditions in humans, such as cardiac arrhythmias, thereby, related NaVs ion channels may be the most promising drug targets for heart diseases. Ion channels also play critical roles in all aspects of cardiac function, including contractile activity. Consequently, most ion channels are possible key targets for therapeutics aimed at cardiac pathophysiologies (Chambers et al., 2010; Wilde and Brugada, 2011; Verkerk et al., 2012; Kaufmann et al., 2013; van den Boogaard et al., 2014; Zimmer, 2014; Priest and McDermott, 2015; Veeraraghavan et al., 2017; Li, et al., 2018; Horváth et al., 2020; Ton et al., 2021).
Correspondingly, there are some clinical findings examining changes in NaVs induced currents, the Na+-channel currents (INa). As emphasized by several authors, the time and voltage dependence of NaV channel transitions between discrete channel states is strongly dependent upon a complex interplay of various structural, chemical, genetic, and electrical factors (Chen-Izu et al., 2015; DeMarco and Clancy 2016; Herren et al., 2013; (Marionneau and Abriel, 2015; Rook et al., 2012). The above clinical and experimental results are supported by computational modeling findings. For instance, a reduced INa via a depolarizing shift in NaV1.5 activation could underlie a marked right ventricular conduction delay and a transmural activation gradient (Tan et al., 2001). As another example, it has been demonstrated that an increased late Na+-current (INaL) plays an important role in various cardiac diseases, including rhythm disorders (i.e. cardiac arrhythmias) and changes in contractile activity (Coppini et al., 2013; Maier and Sossalla, 2013; Mitsuiye and Noma, 2002; Pourrier et al., 2014). More importantly, it has been well-documented that an upregulated INaL can play a role in preventing repolarization, providing an increase in intracellular Na+-level via a large amount of Na+-entry, further leading to a larger intracellular Ca2+-level. All these alterations can cause contractile dysfunction (Antzelevitch et al., 2014; Liu and O'Rourke , 2008; Sossalla et al., 2011).
Numerous studies have emphasized the role of mutations in Na+-channels in the development of heart diseases. For instance, naturally occurring mutations in Na+-channels can cause delayed NaV1.5 activation, while channelopathies can arise from altered fast inactivation ( Zimmer, 2014; DeMarco and Clancy 2016; Veeraraghavan et al., 2017). In addition, early theoretical approaches hypothesized a contribution and/or induction of prolonged repolarization in cardiomyocyte action potential leads to the long-QT syndrome in humans through functional changes in NaV1.5 (Clancy et al., 2003). There are several functional modulators of NaV1.5 associated with posttranslational modification of this channel, including phosphorylation by Ca2+/calmodulin-dependent protein kinase II (CaMKII; inducing changes into macroscopic NaV1.5 gating), resulting from a reduction in channel availability (Wagner et al., 2006), and increased intermediate inhibition of the phosphatidylinositol kinase (PI3K) promoting INaL (Lu et al., 2012). In addition, the methylation and ubiquitylation as well as redox modifications can induce multiple functional changes in NaV1.5 through various signaling mechanisms, both direct and indirect (Beltran-Alvarez et al., 2014; Marionneau and Abriel, 2015).
Recent and early findings point out that channelopathies are an increasingly recognized category of not only inherited but also other types of human heart diseases (Priori et al., 2004; Sauer et al., 2007; Baltogiannis et al., 2020; Pappone et al., 2020; Asatryan et al., 2021; Gnecchi et al., 2021; Mellor and Behr, 2021). The channelopathies are responsible for sudden cardiac death including pathologies such as long-QT syndrome, short-QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia syndrome, and early repolarization syndrome. Therefore, the authors emphasize that clinicians and researchers should take these consideration as diagnostic and contemporary management tools.
Status of cardiac Na+-channels in elderly mammalians
The percentage of the global elderly population is expected to more than double to 1.5 billion for those older than 65 by 2050, according to the United Nations documentation, and therefore, we must be committed to extending health in older age. In the current century, people are living longer than ever before, and older people need a good quality of life. In this regard, for over a decade, researchers have been discussing aging-associated cardiovascular changes and their relationship to heart failure, which is typically detected with high frequency in the elderly.
Aging-associated cardiovascular diseases mostly include left ventricular hypertrophy, ischemic heart disease, and heart failure. There are also a close correlations among long-QT with increasing age, increases in the risk of ventricular arrhythmias, and cardiac mortality in elderly individuals (Lakatta, 2002; Lakatta and Levy, 2003). More importantly, in the last two decades, the relationship between aging and insulin resistance has gained the attention of researchers and clinicians (Ryan 2000; van Noord et al., 2010; Boudina, 2013; Shou et al., 2020; Olgar et al., 2020a; Olgar et al., 2022). Although differences in cardiovascular function between adult and aged individuals have been extensively described in the literature, the underlying mechanisms are yet completely understood. However, there may be several different factors affecting aging-associated heart dysfunction such as lifestyle in adition to genetic component.
During advanced age, remodeling is a continuous process in the heart to compensate for structural, metabolic, and functional changes. The electrical changes, through alterations in ionic mechanisms, are important factors that contribute to the remodeling of the heart during the aging period (Xie et al., 2013; Signore et al., 2015). The increased prevalence of cardiac remodeling with age advance is partially due to ventricular remodeling with or without hypertrophy (Lavie et al., 2006). Studies have shown that cardiac pacemaker dysfunction can arise from some ion channel remodeling (i.e. some Ca2+-channels such as CaV1.2, CaV1.3, and some K+-channels such as KVLQT1, KV4.2), as well as in some Ca2+-handling proteins (i.e. SR Ca2+-pumps, Na+/Ca2+-exchanger, and SR Ryanodine receptors) in the aging rat heart (Rao et al., 2016; Sanchez-Alonso et al., 2018; Alghamdi et al., 2020). Furthermore, there are also clinical data that demonstrated a relationship between alterations in some ion channels, mitochondrial dysfunction, and aging heart dysfunction (Strickland et al., 2019). Consequently, one can thus posit that changes in ionic channels are associated with alteration in metabolic and immune system signaling pathways than can contribute to aging-associated heart dysfunction in mammalians. Therefore, age-associated functional changes in ion channels can lead to further clinical phenotypes, which are named channelopathies in the aging heart. In this context, Signore and co-workers (2015) observed an increase in the INaL in aging cardiomyocytes, which was contributing to prolonged action potential duration and affected the temporal kinetics of Ca2+-cycling and contractility in C57Bl/6 mice in an age-dependent manner, while the inhibition of INaL provided significant recovery in defective heart performance. In these regards, Isaac and co-workers (2020) discussed the role of NaV1.8 (an isoform of NaV1.5) in the development of arrhythmias in the diseased heart. They demonstrated how a reduction in NaV1.5 and an increase in NaV1.8 contributes to both heart failure and ventricular hypertrophy (Ahmad et al., 2018). The above data strongly suggests a critical role of voltage-gated Na+-channel isoforms in myocardial aging in elderly mammalians. Recently, there has been increased investigation in to how age-associated changes in voltage-gated Na+-channel isoforms in aged mammalians contributes to severe cardiac dysfunction. In earlier studies, a close link between a single channel mutation in NaV1.5 (i.e. characterized by a depolarizing shift of the activation curve of the Na+-channels) and serious conduction system disease in humans was demonstrated (Tan et al., 2001; Grant et al., 2002; Chadda et al., 2017; Song and Belardinelli, 2018). In addition, since inactivation of NaV1.5 is critically affects the duration and frequency of action potentials in the heart, one can surmise that inactivation of either fast, slow, or both of these channels could lead to the development of heart dysfunction in elderly individuals. It has been documented that aging promotes structural and functional remodeling of the heart, even in the absence of external factors. There is growing clinical and experimental evidence supporting the existence of sex-specific patterns of cardiac aging that are partially dependent on differential responses to cardiac stresses such as adrenergic activation and oxidative stress. Indeed, in hyperglycemic animals, significantly less phosphorylation in RyR2 and a smaller decrease in the protein level of FK506 binding protein 12.6 concurrent with lower levels of oxidative stress markers in female cardiomyocytes could provide strong support for why there were fewer decreases in the contractile activity of female rat heart than the age-matched male rats (Yaras et al., 2007).
There are also important alterations in intracellular Na+-homeostasis associated with increased Na+-influx by either increased INa activation by both Na+/glucose co-transporter 2 and Na+/Ca2+ exchanger, or all of them in aged rat ventricular cardiomyocytes with insulin resistance (Durak et al., 2018; Olgar et al., 2020a; Olgar et al., 2021). Further support is that the protein expression level was found to be increased in insulin-resistant aged rats. Immunohistochemical analysis of the left ventricular tissue showed markedly high levels of NaV1.5 immunostaining in both aged or insulin-resistant metabolic syndrome rats compared to the normal rats (Figure 2). The present and previously published data strongly imply that the expression level of voltage-gated Na+-channels plays a crucial role in the development of arrhythmias in the diseased heart. Therefore, a novel therapeutic approach that directly targets the aberrant Na+-currents may protect the aging heart against insufficiencies/disorders in elderly individuals. Consequently, it seems that voltage-gated Na+-channel blockers can be novel therapeutic targets for the aging heart.
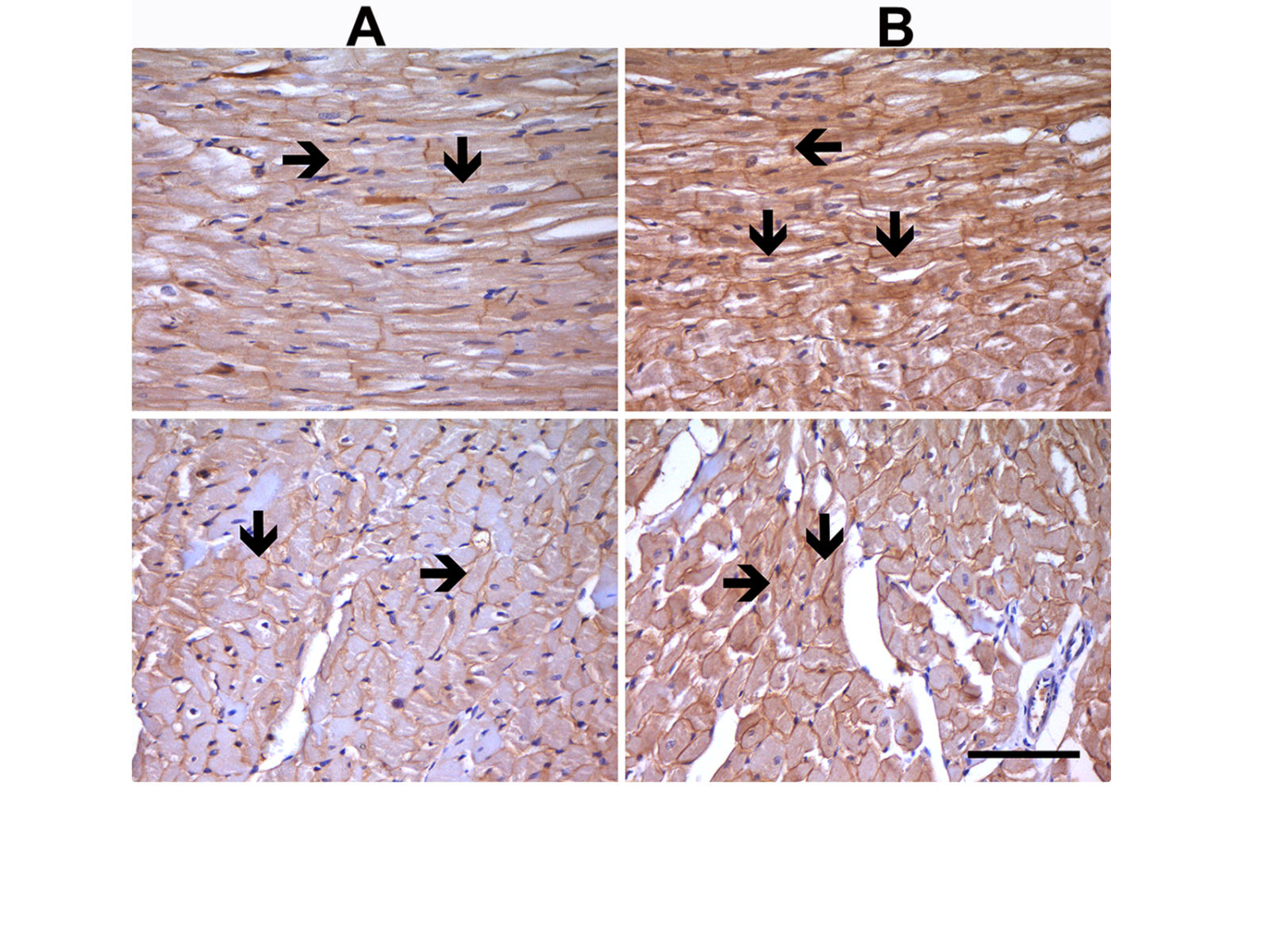
In a new window | Download PPT
Figure 2: Expression levels for Nav1.5 at the sarcolemma of left ventricular cardiomyocytes. Nav1.5 immunostaining analysis in adult (8-month-old) male Wistar rat heart tissue with normal staining level (A) and in insulin-resistant aged rat heart (24-month-old) with high-density staining (black arrow) (B). Immunocytochemical staining of longitudinal sections and transverse sections of heart tissues are given in the upper and lower parts of the figures, respectively. Magnifications: x400, and scale bar =100 µm.
In summary, during biological aging functional remodeling of the heart is influenced by various unexpected factors, including ionic changes associated with electrical remodeling, structural remodeling, and metabolic remodeling. More importantly, these remodelings can in turn lead to positive feedback signaling, promoting further worsening of the abnormalities during each individual remodelings.
Future perspectives
During aging the heart exhibits a markedly depressed contractile activity through both electrical and structural remodelings. The most of the data from both experimental and clinical studies have shown that the voltage-gated Na+-channels and their regulatory subunits play important roles in cardiac arrhythmia and left ventricular repolarization abnormalities, which can further lead to heart failure in elderly people.
In the aging heart, there are several alterations in the levels of both cardiomyocytes and their organelles, which underlie aging-associated cardiac remodeling. Among them, we previously demonstrated that there are significant increases in the INa, Na+/K+-pump currents (INaK), and Na+/Ca2+-exchanges currents (INCX) without changes in the L-type voltage-gated Ca2+-channel currents (ICaL) (Durak et al., 2018; Olgar et al., 2020a; Olgar et al., 2021; Durak et al., 2022; Olgar et al., 2022). In addition, abnormal function of the SR and mitochondria induced significant increases in intracellular and mitochondrial levels of free Ca2+ and Zn2+, as well as high-level ROS production and less ATP production in left ventricular cardiomyocytes. More importantly, most of these alterations seem to be associated with post-transcriptional modifications in the associated proteins (Drazic et al., 2016; Lugenbiel et al., 2018; Dong et al., 2020; Okada et al., 2021). Post-translational modifications in proteins of ion channels, transporters, and pumps are mostly associated with various changes in different reactions such as glycosylation, phosphorylation, methylation, acetylation, and oxidation. Here, K-acetylation and thiol-oxidation in ion channel proteins and phosphorylation in Ca2+-handling proteins are very common protein modifications in cardiomyocytes under hyperglycemic and hyperinsulinemic conditions (Okatan et al., 2013; Tuncay et al., 2014; Okatan et al., 2016; Durak et al., 2018; Olgar et al., 2020a; Olgar et al., 2021; Durak et al., 2022; Olgar et al., 2022).
In the line with these above statements, there are important studies that emphasize the importance of the life cycle of NaV1.5 starting from birth, including transcription and translocation of its gene SCN5A. Butler and co-workers (2015) investigated a potential role for lysine acetylation in epithelial Na+-channel regulation and demonstrated its regulation is via acetylation-stimulated reduction in its ubiquitination and degradation. Also, other studies demonstrated why deacetylation of the cardiac Na+-channels has important benefits for the regulation of cardiac electrical activity under pathological conditions such as various arrhythmia phenotypes (Vikram et al., 2017). Moreover, the authors, taking into consideration that post-translational modification in dysfunctional NaV1.5 either decreases peak INa or increases INaL leading to sudden cardiac death, performed experiments to demonstrate the role of suppression of mitochondrial ROS-mediated NaV1.5 phosphorylation in cardiac performance under arrhythmic stimuli (Matasic et al., 2020). In a cell culture study, it has also been shown that inhibition of histone deacetylases could induce voltage-gated K+-channel remodeling and action potential prolongation in HL-1 atrial cardiomyocytes (Lugenbiel et al., 2018).
Age-related cardiac insufficiency/dysfunction, including cardiomyopathy, accounts for several alterations at the molecular, cellular, organ, and system levels in the elderly. As mentioned in the previous section, during the physiological/biological aging process, proteins can go through K-acetylation, thiol-oxidation, and phosphorylation, which further leads to the loss of several ionic channels and organelle level changes, as well as increases in the number of hypertrophic and senescence cardiomyocytes with loss of the number of healthy cardiomyocytes. These alterations, individually and/or their combination induce significant changes in electrical and structural changes in left ventricular cardiomyocytes, and these changes further underlie the long QT-interval in ECG and depressed contractile activity in the heart. In addition, an increase in elastic and collagenous tissue in all parts of the conduction system increases the extracellular matrix, and increases in arrhythmia prevalence, further promoting heart dysfunction and heart failure in the elderly.
The putative pathways involved in exacerbating myocardial dysfunction in the aging period are provided in Figure 3 (Tuncay et al., 2014; Okatan et al., 2016; Durak et al., 2018; Okatan et al., 2021; Olgar et al., 2020a; Olgar et al., 2021; Olgar et al., 2022; Durak et al., 2022). The mechanisms of cellular excitability and propagation of electrical signals in cardiac muscle are very important functionally and pathologically, although further studies are needed to clarify the underlining mechanisms. Nonetheless, we put forth regulating shifts in cardiac voltage-gated Na+-channel isoforms as a novel therapeutic target in the aging heart.
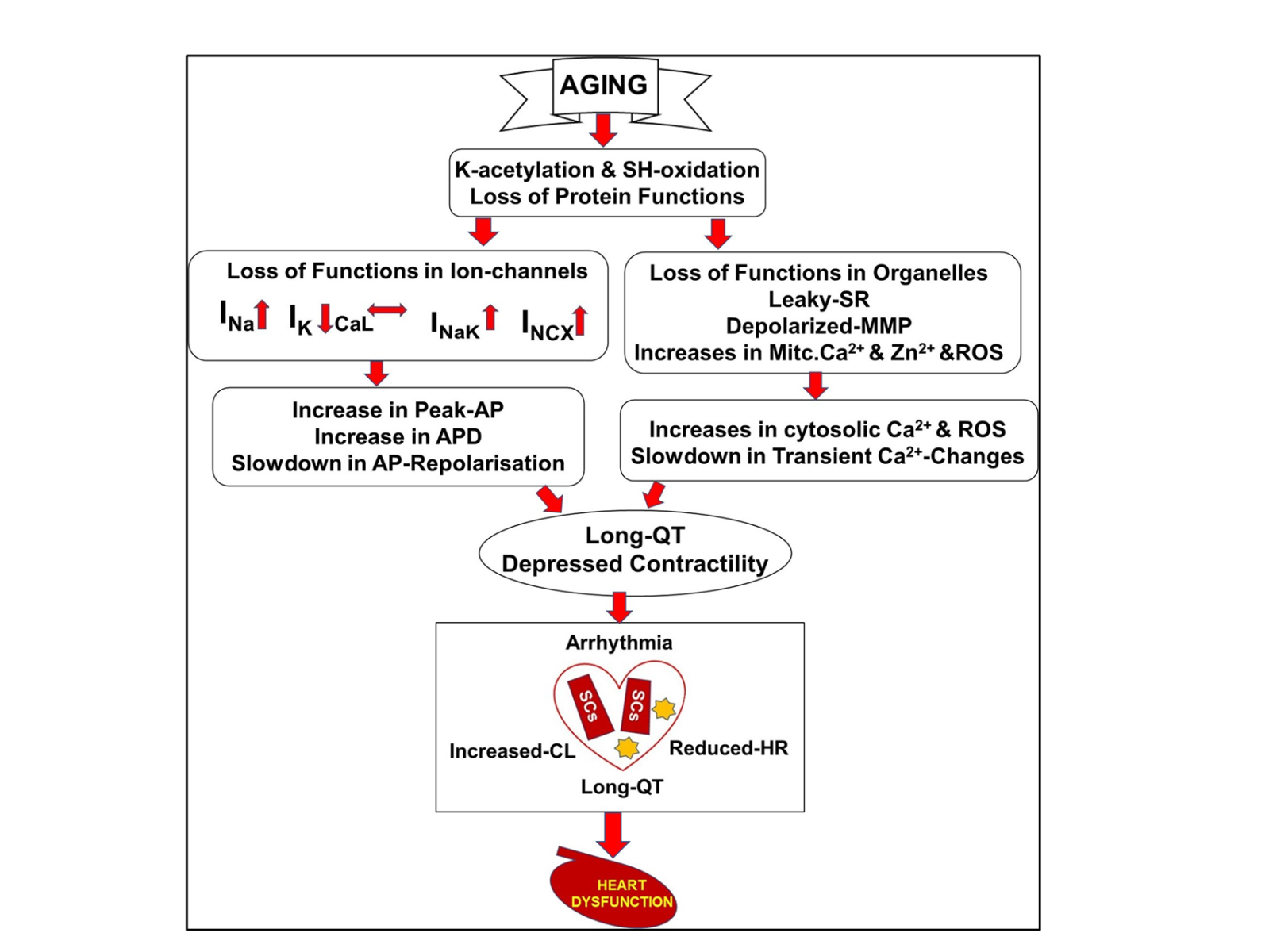
In a new window | Download PPT
Figure 3: Putative pathways involved in exacerbating myocardial abnormalities that further lead to heart failure in insulin-resistant aged rats. Aging-associated cardiac remodeling from the molecular level to organ function. Abbreviations: SR, sarcoplasmic reticulum; MMP, mitochondrial membrane potential; Mitc. Ca2+ and Zn2+, mitochondrial levels of calcium and zinc ions; ROS, reactive oxygen species; voltage-gated Na+-channel currents (INa); L-type voltage-gated Ca2+-channel currents (ICaL); voltage-gated K+-channel currents (IK); Na+/K+-pump currents (INaK); Na+/Ca2+-exchanges currents (INCX); action potential, AP; action potential duration, APD; cardiac lipotoxicity, CL; lipid droplets, stars; senescence cells, SCs; heart rate, HR ( Okatan et al., 2013; Tuncay et al., 2014; Okatan et al., 2016; Durak et al., 2018; Durak et al., 2022; Olgar et al., 2020; Olgar et al., 2021; Olgar et al., 2022).
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Turkey (SBAG-119S661) to Belma Turan.
Conflict of interests
The author declares that there is no conflict of interest.
Ethical decleration
The experimental protocol and handling of animals during the duration of the experiments were approved by the Ankara University ethics committee (No: 2015-12-137 & No: 2016-18-165).
References
Deniz Billur1
1Department of Histology & Embriyology, Ankara University Faculty of Medicine, Ankara, Turkey.
Belma Turan2
2Department of Biophysics, Lokman Hekim University Faculty of Medicine, Ankara, Turkey.
Corresponding author:
Belma Turan
Email: belma.turan@medicine.ankara.edu.tr; belma.turan@lokmanhekim.edu.tr

In a new window | Download PPT
Figure 1: The continuum of the aging progress and age-related contributors to heart function in the elderly. During physiological aging, external contributors and diseases can promote the development of cardiovascular diseases in humans. Studies have shown that the prevalence of diabetes and impaired glucose tolerance (including insulin resistance) in individuals is increasing with advanced age. Cellular and organelle level changes in the heart (cardiometabolic disturbances) promote the incidence of cardiovascular disorders in physiological/biological aging in humans.

In a new window | Download PPT
Figure 2: Expression levels for Nav1.5 at the sarcolemma of left ventricular cardiomyocytes. Nav1.5 immunostaining analysis in adult (8-month-old) male Wistar rat heart tissue with normal staining level (A) and in insulin-resistant aged rat heart (24-month-old) with high-density staining (black arrow) (B). Immunocytochemical staining of longitudinal sections and transverse sections of heart tissues are given in the upper and lower parts of the figures, respectively. Magnifications: x400, and scale bar =100 µm.

In a new window | Download PPT
Figure 3: Putative pathways involved in exacerbating myocardial abnormalities that further lead to heart failure in insulin-resistant aged rats. Aging-associated cardiac remodeling from the molecular level to organ function. Abbreviations: SR, sarcoplasmic reticulum; MMP, mitochondrial membrane potential; Mitc. Ca2+ and Zn2+, mitochondrial levels of calcium and zinc ions; ROS, reactive oxygen species; voltage-gated Na+-channel currents (INa); L-type voltage-gated Ca2+-channel currents (ICaL); voltage-gated K+-channel currents (IK); Na+/K+-pump currents (INaK); Na+/Ca2+-exchanges currents (INCX); action potential, AP; action potential duration, APD; cardiac lipotoxicity, CL; lipid droplets, stars; senescence cells, SCs; heart rate, HR ( Okatan et al., 2013; Tuncay et al., 2014; Okatan et al., 2016; Durak et al., 2018; Durak et al., 2022; Olgar et al., 2020; Olgar et al., 2021; Olgar et al., 2022).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8735 | 9 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA