International bi-monthly journal of cell signaling, tissue protection, and translational research.
Efficacy of cardioprotective interventions depends critically on duration of ischemia
Yang Xiao1, Qian Wang1, Coert J. Zuurbier1
Author Affiliations


- 1Department of Anesthesiology, Laboratory of Experimental Intensive Care & Anesthesiology (L.E.I.C.A.), Cardiovascular Science, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands.
Abstract
Since the discovery of ischemic preconditioning more than 35 years ago, a plethora of cardioprotective interventions have been developed and characterized in preclinical research. Nevertheless, successful translation of these cardioprotective interventions into the clinic has been almost non-existent, forcing the scientific community to think critically about what is missing in the preclinical models of ischemia/reperfusion (I/R) injury (IRI). Although several factors have been examined (comedications, comorbidities) little attention has been paid to the duration or severity of the ischemic insult. In this review we summarize the dependence of the efficacy of several well-known cardioprotective interventions on the duration of the ischemia. It has been found that reducing ischemic-induced oxidative stress and boosting endothelial nitric oxide synthase activity mostly provide protection against short duration ischemia (< 30 min), while it was found that ischemic preconditioning, postconditioning, mitochondrial permeability transition pore (mPTP) inhibition, opioids, sevoflurane, and metoprolol primarily provide protection against intermediate duration ischemia (30-60 min). Postconditioning and mPTP inhibition can even increase IRI after short duration ischemia. None of these cardioprotective interventions provide protection against long duration ischemia (> 60 min), although reducing IRI through other means, such as impairing inflammation, is still possible for long duration ischemic insults. In conclusion, cardioprotective interventions often target a single cellular mechanism that only contribute to IRI for a certain specific duration of ischemia. Given that the duration of ischemia during acute myocardial infarction in patients is highly variable, and the protection against long severe ischemia will be most advantageous for acute myocardial infarction (AMI) patients, we recommend simultaneously targeting multiple IRI mechanisms with various sensitivities towards ischemic duration to overcome poor translatability of preclinical cardioprotective interventions.
Keywords: Cardioprotection, Conditioning, Ischemia duration
Abstract
Since the discovery of ischemic preconditioning more than 35 years ago, a plethora of cardioprotective interventions have been developed and characterized in preclinical research. Nevertheless, successful translation of these cardioprotective interventions into the clinic has been almost non-existent, forcing the scientific community to think critically about what is missing in the preclinical models of ischemia/reperfusion (I/R) injury (IRI). Although several factors have been examined (comedications, comorbidities) little attention has been paid to the duration or severity of the ischemic insult. In this review we summarize the dependence of the efficacy of several well-known cardioprotective interventions on the duration of the ischemia. It has been found that reducing ischemic-induced oxidative stress and boosting endothelial nitric oxide synthase activity mostly provide protection against short duration ischemia (< 30 min), while it was found that ischemic preconditioning, postconditioning, mitochondrial permeability transition pore (mPTP) inhibition, opioids, sevoflurane, and metoprolol primarily provide protection against intermediate duration ischemia (30-60 min). Postconditioning and mPTP inhibition can even increase IRI after short duration ischemia. None of these cardioprotective interventions provide protection against long duration ischemia (> 60 min), although reducing IRI through other means, such as impairing inflammation, is still possible for long duration ischemic insults. In conclusion, cardioprotective interventions often target a single cellular mechanism that only contribute to IRI for a certain specific duration of ischemia. Given that the duration of ischemia during acute myocardial infarction in patients is highly variable, and the protection against long severe ischemia will be most advantageous for acute myocardial infarction (AMI) patients, we recommend simultaneously targeting multiple IRI mechanisms with various sensitivities towards ischemic duration to overcome poor translatability of preclinical cardioprotective interventions.
Keywords: Cardioprotection, Conditioning, Ischemia duration
Introduction
In 1986, it was first reported that the degree of cell death or infarct size due to an ischemic event of a fixed duration was amenable to ischemic therapy: pre-treating the heart with 3 x 5 min ischemia reduced infarct size due to 40 min of ischemia by 75% in dog heart (Murry et al., 1986). This phenomenon was named ischemic preconditioning (IPC) and constitute one of the highest cited research publications in the field of cardiovascular research (> 6,450 June 2022 CSI). The discovery ignited the field of cardioprotection, which now has over 10,000 publications and represented a tremendous gain in our understanding of underlying mechanisms and protection of ischemia/reperfusion (I/R) cardiac injury (IRI) (Hausenloy et al., 2016; Hausenloy and Yellon, 2016). Despite this intense research effort and the gain in knowledge, clinical translation has been rather disappointing.
Several reasons for this poor translation have been put forward, among which cardiovascular co-morbidities and risk factors (e.g., ageing, metabolic diseases, hypertension), co-medications, and peri-operative therapy have received the most attention (Ferdinandy et al., 2014). However, little attention has been paid to the duration of ischemia or the severity of the ischemic insult. This is somewhat surprising considering that in that first seminal publication by Murry et al., (1986), it was demonstrated that IPC protective effects were reduced from 75% to 0% when ischemia was extended to 3 h. Other infrequent reports have now also clearly demonstrated that cardioprotective efficacy of a specific protective treatment may strongly depend on the duration of ischemia, with some treatments being effective at short, or at mid-range, or at long duration ischemia (Ruiz-Meana et al., 2011; Zuurbier et al., 2014). This duration-dependency of protection is probably related to the specific targeted underlying mechanism of I/R damage that is mostly operative during early or late ischemia, but not both. For example, it was shown that the mPTP opening mainly dictates cell death developing during long ischemia, but not during short ischemia (Ruiz-Meana et al., 2011). Knowing that in general the applied ischemia duration in preclinical animal studies is often shorter than that observed in the clinical human condition (Vander Heide and Steenbergen, 2013), at least suggests that this mismatch in ischemia duration may also contribute to poor translation. It is possible that the protective interventions effective in animal studies with the shorter ischemia do not protect in the human condition with its longer duration ischemia. It may therefore be valuable for improvements in translation to summarize the literature on this ischemia duration-dependency of frequently applied cardioprotective interventions. This constitutes the subject of the current review.
Conditioning manoeuvres (IPC, RIPC, PostCond)
Ischemic preconditioning is a rather robust protective intervention, although Murry et al (1986) demonstrated in dogs that for very long ischemic periods (3 h) IPC lost protection. Dogs are known to have the highest resistance against I/R-induced infarction because of the presence of collaterals that can maintain residual flow to the ischemic region. Therefore, 3 h of ischemia in the dog still does not generate maximal infarction of the area at risk. For most other animals used in research, mice, rats, rabbits and pigs, maximal infarction is usually reached after an ischemic duration of 45-90 min (Schaper and Schaper, 1988; Schaper et al., 1988). Within this range of ischemia duration, IPC was largely effective in isolated mouse hearts after ischemic durations of 25, 35, and 45 min (Rossello et al., 2016) or in vivo rat hearts after ischemic durations of 30, 45 and 60 min, but not for 120 min (van den Doel et al., 1998). However, in another study using isolated rabbit hearts, IPC was effective after a 60 min duration of ischemia, but not for a 20-30 min duration (Kim et al., 1995).
In addition to IPC, remote IPC (RIPC), known as repeated brief cycles of I/R applied in distant tissues and organs, or postconditioning (PostCond), defined as brief episodes of reperfusion interrupted by ischemia applied at the onset of reperfusion, are also applied to render the myocardium resistant to a subsequent sustained episode of ischemia. We are unaware of studies that examined whether RIPC protective effects are dependent on ischemia duration. For postconditioning, however, one study demonstrated that it can be critically dependent on ischemia duration. Employing an isolated rat heart model, Manintveld et al. (2007), demonstrated effective PostCond for 45 min and 60 min ischemia duration. However, protection was lost when duration was extended to 90 min and 120 min, independent of the PostCond algorithm. Surprisingly, PostCond following short 15-30 min ischemia duration even enlarged infarct size relative to non-PostCond maneuvres (Manintveld et al., 2007). The loss of PostCond efficacy with shorter ischemia duration may be gender specific, as it was shown that PostCond reduced I/R injury following short duration ischemia in female, but not male, rats (Penna et al., 2009).
Overall, it seems that ischemic conditioning maneuvres can reduce infarct size for mid-range durations of ischemia, with loss of protection for very short (PostCond) and prolonged ischemia (PostCond and IPC).
Targeting oxidative stress
The generation of oxidative stress during I/R is, besides sodium and calcium overload, considered one of the main initial drivers of I/R-induced cell death. Mitochondrial reactive oxygen species (mitoROS) is increased during reperfusion as compared with during ischemia (Chouchani et al., 2013), and reduction of mitoROS protects against IR injury. However, we were unable to find literature that had examined a possible relation between ischemia duration and mitoROS. Although controversy sometimes exists whether ROS are produced during ischemia or during the reperfusion period, techniques allowing direct measurements of ROS demonstrated that free radical production does also occur during ischemia (Maupoil et al., 1990; Timoshin et al., 1994; Vanden Hoek et al., 1997). This is commensurate with the build-up of the irreversible oxidative stress marker 4-hydroxy-2-nonenal (4-HNE) during cardiac ischemia (Eaton et al., 1999). Employing this oxidative stress marker in cardiac I/R studies with different durations of ischemia, it was shown that there is a rapid accumulation of the marker between 10-30 min of ischemia without further increases with prolongation of ischemia (Eaton et al., 1999; Eaton et al., 2001). Additionally, no further increases in 4-HNE were observed with reperfusion, even when hearts were reperfused after only 10-15 min ischemia (preventing maximal ischemic accumulation of 4-HNE). The antioxidant N-(2-mercaptopropionyl)-glycine (MPG) could reduce 4-HNE only when administered before the start of ischemia but not at the onset of reperfusion. These data suggest that, for at least the I/R associated oxidative stress resulting in 4-HNE abduct formation, the oxidative stress is mainly occurring during the early period of ischemia, and not during the later period of prolonged ischemia or the reperfusion period. This is commensurate with the observation that ROS production increases during the initial period of ischemia but declines in the later ischemic period (Camara et al., 2007). Similar results were observed with the use of ferricytochrome C in the perfusate as a marker of vascular ROS production: total ROS production measured during the first 20 min reperfusion increased with ischemia durations from 15 to 30 min but decreased with further extension of ischemia duration to 45-60 min (Southworth et al., 1998). Thus, antioxidant strategies administered only at the end of ischemia or onset of reperfusion will be ineffective for this type of I/R-induced oxidative stress. In addition, antioxidant agents administered before the ischemic period are mostly effective for short periods of ischemia but not for long ischemic periods, as was also shown by Xiao et al., (2021). The newly developed redox modulating antioxidant Sonlicromanol was able to reduce cardiac I/R injury and 4-HNE levels when administration was started before ischemia and stopped at a 30 min reperfusion duration after a 20 min global ischemia period, but not for a 30 min duration global ischemia period in isolated mouse hearts (Xiao et al., 2021). Also, the classical antioxidant MPG provided relatively more protection against short versus longer ischemia in that same study (Xiao et al., 2021). In conclusion, the data suggest that oxidative stress maximally develops during the early part of ischemia (<30 min) following which it becomes saturated and does not further increase with prolongation of ischemia. Reducing this ischemic oxidative stress can reduce cardiac I/R injury, but protection can only be achieved by providing the antioxidant before the onset of ischemia. However, little to no protection is anticipated with antioxidant treatment when ischemia is of a lengthy duration or when the antioxidant is administered too late, that is during ischemia or at the onset of reperfusion. In addition, it is important to note that the use of an antioxidant may preclude protection offered by cardioprotective interventions known to protect through small increases in ROS, such as IPC or postconditioning maneuvres (Tullio et al., 2013). Thus, incorporating antioxidants in combination therapy against I/R injury should be carefully considered.
Targeting NO
Nitric oxide (NO) plays an important role in healthy cardiac physiology, from the regulation of vascular tone and immune system to the contractility of cardiomyocytes. NO plays a double-edge role in cardiac I/R injury, whereby both too little NO and too much NO can contribute to I/R injury (Sharp et al., 2002). Nitric oxide is generated by dimers of NO synthase (NOS), converting L-arginine and NADPH into L-citruline and NO. In 2008, Moens et al (2008) reported on an almost curing of cardiac infarction through the application of folic acid (FA). FA pre-treatment almost abolished the infarct following 30 min ischemia and reperfusion in both in vivo and ex vivo preclinical rat models. High-dose FA is supposed to increase the critical cofactor tetrahydrabiopterin (BH4) that is needed to maintain dimerization of the NOS to properly generate NO. Possible damaging effects of NOS during I/R are related to the breakdown of NOS dimers into monomers, followed then by ROS production of the monomers. We were able to reproduce these protective effects of FA in an isolated mouse heart model, or an in vivo rat model, employing 25 min of ischemia (Zuurbier et al., 2014). However, when ischemia duration was extended to a 35 min ischemia duration in the isolated mouse heart, all protection by FA was lost. The inability of FA to offer protection with longer ischemia probably relates to irreversible oxidation of BH4 after a 30 min ischemia duration (Dumitrescu et al., 2007) and the irreversible breakdown of NADP(H) due to CD38 activation with longer ischemia (Reyes et al., 2015). Therefore, it seems that activation of endothelial NOS (eNOS) activity can only protect against shorter, but not extended, durations of ischemia, due to the critical loss of cofactors and cosubstrates of eNOS during the initial phase of ischemia.
Targeting the mPTP
It is currently believed that many cardioprotective interventions share as end-effector the mitochondria, and particularly the prevention of the opening of a non-specific pore in the inner mitochondrial membrane, the mitochondrial permeability transition pore (mPTP) (Halestrap and Richardson, 2015; Heusch, 2015). Although the precise molecular structure of this mPTP is still uncertain, the FoF1 ATP synthase, the adenine nucleotide translocase (ANT), and the phosphate carrier (PiC) are supposed to be part of the pore, with cyclophilin D (CypD) as an important regulator (Halestrap and Richardson, 2015). Hexokinase II and creatine kinase also function as important regulators of the pore within the heart (Nederlof et al., 2014; Zuurbier et al., 2020) . Following the first discovery of the importance of the mPTP in mediating cardiac I/R injury, using cyclosporine A treatment to inhibit CypD (Griffiths and Halestrap, 1993), most published experimental studies were able to confirm this finding. However, some studies soon appeared showing that mPTP inhibition through targeting of CypD does not always offer protection, or even result in increased cardiac I/R injury. For example, increased infarct size following 30 min of ischemia was observed in isolated mouse hearts with ablation of CypD. Only when ischemia duration was prolonged to 60 min did CypD ablation caused reduced infarct size (Ruiz-Meana et al., 2011). A possible explanation for the loss of protection by CypD ablation at shorter ischemia duration, may be related to the fact that at shorter ischemia durations the cell death occurring is mostly determined by a sarcolemmal disruptive mechanism that is independent on mPTP opening. Following reperfusion after a short ischemia duration, the mitochondria can still quickly regenerate ATP, thereby fueling the highly Ca2+-induced activated contractile machinery that then start rupturing the membrane (Garcia-Dorado et al., 2006). In another study CypD deletion protected against a 30-45 min ischemia duration, but not against 60-90 min ischemia in an in vivo model of cardiac I/R (Ikeda et al., 2021). The infarct size developed from a 60-90 min ischemia duration was still reduced with deletion of the C-C chemokine receptor 2 (CCR2), showing that protection at these longer ischemic periods could still be attained through reduction of the inflammatory component of I/R.
In summary, although mPTP inhibition is most often associated with protection against cardiac I/R injury, infarct size reduction is not always observed with increased resistance of mPTP to opening. One important factor that seems to disturb this relation between mPTP sensitivity and infarct size appears to be the duration or severity of the ischemic insult, such that at shorter ischemic durations (< 30 min) the inhibition of the mPTP does not provide cardioprotection.
Opioids and volatile anesthetics
Opioids protect against cardiac infarction in preclinical models (Schultz et al., 1996). Protection by an enkephalin analog is strongly dependent on time of administration, with protection only observed for administration before or in the early phase of ischemia, but not during the late phase of ischemia or at reperfusion (Spanos et al., 2015). Sevoflurane pretreatment protected against cardiac I/R injury for 25-40 min global ischemia in the isolated guinea pig heart, but not for shorter (20 min) or longer (45 min) periods of global ischemia (Kevin et al., 2003). However, sevoflurane protection against longer ischemia was restored upon baseline activation of autophagy, suggesting that with longer ischemia durations it is the activation of autophagy that can provide sevoflurane-protection (Shiomi et al., 2014). Interestingly, volatile anesthetics even seem to be more protective than cyclosporine A when given at the start of reperfusion, maybe indicating the importance of decreasing mitochondrial activity during early reperfusion above mPTP inhibition (De Paulis et al., 2013).
Metoprolol
The use of β-blockers in the setting of ST-elevation myocardial infarction (STEMI) has a long history, with initial reports of beneficial effects followed by neutral effects in later trials (Ibanez, 2020). Currently, the cardioselective β-blocker metoprolol, in contrast to other cardioselective β-blockers, holds promise as protective agent against acute I/R damage of the heart (Ibanez, 2020). Crucial for the efficacy of metoprolol is the early as possible administration of this drug following symptoms of pain or chest discomfort. In a preclinical cardiac I/R pig model, the bolus administration of metoprolol after 20 min of ischemia reduced infarct size for ischemia durations between 30-50 min, but not for shorter or longer ischemia durations (Lobo-Gonzalez et al., 2020) . The data indicates that metoprolol protects mainly against reversible ischemic injury, and not against reperfusion injury because otherwise protection should have also been observed for the 20 to 25 min ischemic intervention. For the 25 min ischemia duration, the presence of metoprolol for only the last 5 min of ischemia is likely insufficient for metoprolol to impact the on-going mechanisms causing the ischemic injury.
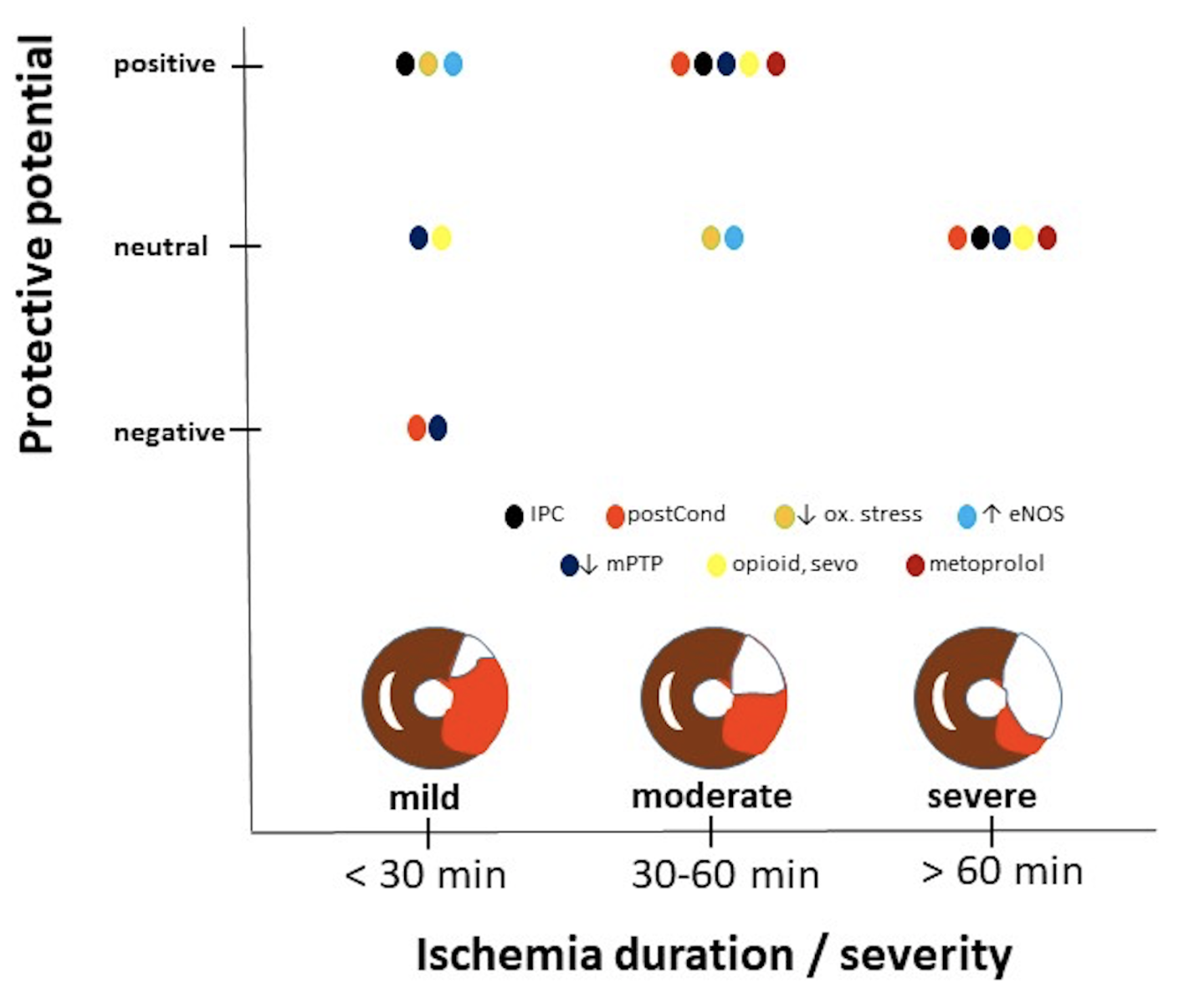
In a new window | Download PPT
Figure 1: Protective potential of various well-known interventions as function of duration or severity of ischemia. Data obtained from studies examining mostly male animals, indicating that information displayed has a gender bias. Interventions can reduce (positive protective potential), have no effect (neutral potential) or increase (negative potential) infarct size relative to non-treated hearts. Ischemic insults are divided into short duration (< 30 min) causing in general small infarct sizes (as reflected by the small white area in the area at risk (red) within the schematic drawing of a heart), moderate durations (30-60 min) causing ~50% infarct size, and long duration associated with severe ischemia and large infarct sizes (~75%). Cardioprotective interventions displayed, IPC: ischemic preconditioning; postcond: post-conditioning; ↓ ox. stress, anti-oxidant treatment; ↑ eNOS, activating endothelia nitric oxide synthase; ↓ mPTP, inhibition of the mitochondrial permeability transition pore; opioid, sevo and metoprolol: administration of opioids, sevoflurane or the β-blocker metoprolol to the heart.
Discussion
Examining several well-studied preclinical protective interventions against acute cardiac I/R injury for their efficacy dependency on duration or severity of ischemia, most interventions were only protective against a specific duration of ischemia. IPC, reductions in oxidative stress, and boosting eNOS activity were protective against short duration (< 30 min) ischemia, whereas IPC, postconditioning, inhibition of mPTP, opioids, sevoflurane, and metoprolol mostly offer protection against moderate duration (30-60 min) of ischemia. None of the discussed cardioprotective interventions protected against prolonged (> 60 min) or severe ischemia (see Figure 1 and Table 1).
Infarct evolution as a function of ischemia duration is species-specific.
The time to reach 50% infarction of the area at risk within the heart was reported to be 288 min for humans (Hedstrom et al., 2009). The estimated time for acute infarction patients between pain symptoms and balloon time is 150-250 min nowadays in the Western world (Hausenloy et al., 2019; Park et al., 2019; Zahler et al., 2022). Median infarct size relative to area at risk or relative to left ventricle in AMI patients was reported to be 50% and 15-17%, respectively (Miura and Miki, 2008; Pasupathy et al., 2017; Dia et al., 2021). It has been estimated that the human heart can benefit from cardioprotective interventions when these interventions are applied within 2-3 hours after symptom onset, when approximately 50% of the region at risk has become infarcted (Gersh et al., 2005).
For animals such as rodents (mice and rats) (25-40 min) and pigs (40-60 min), the time to reach 50% infarction was estimated to be much shorter (Hedstrom et al., 2009), although this also critically depends on the anesthetic regimen applied. This large difference between humans and animals, could indicate that within the clinical condition of AMI, the human heart is less sensitive to an ischemic insult than smaller animals. Several factors can contribute to this difference: 1) the presence of perioperative medications (heparin, aspirin, nitrates, P2Y12 antagonists) in the human condition, 2) the presence of comorbidities and its medications (diabetes, aging, hypertension, hypocholesteremia) in the human condition (Ferdinandy et al., 2014; Dia et al., 2021), 3) the lower metabolic rate of the human heart versus the mouse and rat heart (Zuurbier et al., 2020), and 4) the observation that the area at risk often still receives some lingering flow through the obstructed artery and/or from collaterals during natural occurring infarction in the aged human heart (Sabia et al., 1992) versus human-induced infarction in preclinical IRI models. Preclinical models mimicking the clinical condition of cardiac I/R almost always employ either complete occlusion of a coronary artery in in vivo models, or 100% stopped flow in ex vivo heart models, thus resulting in a complete halt of perfusion of the downstream vascular bed. However, this is frequently not the case in the human condition of cardiac ischemic events (Sabia et al., 1992). Thus, preclinical models of low-flow ischemia may represent the clinical condition better. Such preclinical models of low-flow ischemia will delay infarct developing with time to a large extent. We demonstrated that a 40 min low-flow (5% residual flow) ischemia model caused ~ 1% cell death, whereas 40 min no-flow caused 20 times more cell death in isolated mice hearts (Smeele et al., 2011). We hypothesize that this residual flow, although marginal, that often is still present during acute human AMI (Sabia et al., 1992) explains largely the difference in ischemic sensitivity between preclinical animal models and human AMI.
Developing preclinical models with potential for clinical benefits
Although the median infarct size in human AMI may be 50%, there is a huge variation in infarct size between AMI patients. Miura and Miki (2008) have argued that only in those patients with a 75% infarct size of a 30% area at risk (1/4 of all AMI patients) is it possible to obtain meaningful reductions in mortality and morbidity with cardioprotection. This suggests that cardioprotective research should shift its attention towards preclinical IRI small animal models with such large infarct sizes, i.e. employing relative long durations (> 40 min) of ischemia. Interestingly, most small animal models so far have employed shorter durations of ischemia. In addition, our review suggests that especially for these longer ischemia durations most of the cardioprotective interventions that are discussed in our review start losing its potential to protect. However, interventions targeting inflammation or immunity may have protective potential for these longer and more severe periods of ischemia (Ikeda et al., 2021).
In conclusion, many cardioprotective interventions have only limited efficacy concerning the full possible time scale of ischemia durations. Most previous preclinical cardioprotective research have used IRI models of short or moderate ischemia duration, whereas it is anticipated that especially interventions reducing IRI from long and severe ischemia have the potential to provide clinical benefits. It may therefore be recommended that preclinical models of IRI focus on long and severe ischemia, and clinical cardioprotective therapy should incorporate simultaneous targeting of various underlying IRI mechanisms with different sensitivities to durations or severity of ischemia.
Conflict of Interest
The authors declare that they have no conflicts of interests
Acknowledgements
None
References
Yang Xiao1
1Department of Anesthesiology, Laboratory of Experimental Intensive Care & Anesthesiology (L.E.I.C.A.), Cardiovascular Science, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands.
Qian Wang1
1Department of Anesthesiology, Laboratory of Experimental Intensive Care & Anesthesiology (L.E.I.C.A.), Cardiovascular Science, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands.
Coert J. Zuurbier1
1Department of Anesthesiology, Laboratory of Experimental Intensive Care & Anesthesiology (L.E.I.C.A.), Cardiovascular Science, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands.
Corresponding author:
Coert J. Zuurbier
Email: c.j.zuurbier@amc.uva.nl

In a new window | Download PPT
Figure 1: Protective potential of various well-known interventions as function of duration or severity of ischemia. Data obtained from studies examining mostly male animals, indicating that information displayed has a gender bias. Interventions can reduce (positive protective potential), have no effect (neutral potential) or increase (negative potential) infarct size relative to non-treated hearts. Ischemic insults are divided into short duration (< 30 min) causing in general small infarct sizes (as reflected by the small white area in the area at risk (red) within the schematic drawing of a heart), moderate durations (30-60 min) causing ~50% infarct size, and long duration associated with severe ischemia and large infarct sizes (~75%). Cardioprotective interventions displayed, IPC: ischemic preconditioning; postcond: post-conditioning; ↓ ox. stress, anti-oxidant treatment; ↑ eNOS, activating endothelia nitric oxide synthase; ↓ mPTP, inhibition of the mitochondrial permeability transition pore; opioid, sevo and metoprolol: administration of opioids, sevoflurane or the β-blocker metoprolol to the heart.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6497 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA