Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Conditioning against cognitive impairment: present evidence and future directions
Time:2022-10-15
Number:8549
Hahn Young Kim1, Dong Bin Back1, Bo-Ryoung Choi1, Dong-Hee Choi2, Kyoung Ja Kwon2
Author Affiliations


- 1Department of Neurology, Konkuk University School of Medicine, Seoul, Republic of Korea.
- 2Department of Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea.
Conditioning Medicine 2022. 5(3): 85-92.
Abstract
Conditioning medicine is an emerging field of therapeutics. Remote ischemic conditioning, initially developed to protect the heart, is now being applied to the brain and other solid organ transplantations, such as those of the heart, kidney, liver, and lungs. As sublethal ischemic injury could act as a beneficial stimulus to activate ischemia-associated diverse protective mechanisms, ischemic preconditioning may be ideal for diseases with vascular compromised conditions, such as stroke, coronary heart disease, or peripheral vascular disease. Vascular dementia, Alzheimer’s disease, and mixed dementia are the most common types of cognitive impairment. Considering the insidious onset and chronic progressive nature of cognitive impairment, conditioning methods for cognitive impairment should be safe, feasible, cost-effective, and long-term applicable. Here, we reviewed current experimental evidence on the effects of remote ischemic conditioning, intermittent brain ischemic conditioning, exercise-induced conditioning, pharmacological (chemical) conditioning, and hypoxic conditioning against cognitive impairment. The results of clinical studies and trials focusing on conditioning as a cognitive therapeutic option have also been included in this review. The effects of conditioning against cognitive impairment can be better understood with more experimental evidence and clinical applications. In addition to the effects of conditioning on the vascular aspect of cognitive impairment, further studies focusing on various conditioning effects on the chronic neurodegenerative aspect of cognitive impairment are needed.
Keywords: Conditioning, Cognitive impairment, Vascular dementia, Stroke, Neurodegeneration
Abstract
Conditioning medicine is an emerging field of therapeutics. Remote ischemic conditioning, initially developed to protect the heart, is now being applied to the brain and other solid organ transplantations, such as those of the heart, kidney, liver, and lungs. As sublethal ischemic injury could act as a beneficial stimulus to activate ischemia-associated diverse protective mechanisms, ischemic preconditioning may be ideal for diseases with vascular compromised conditions, such as stroke, coronary heart disease, or peripheral vascular disease. Vascular dementia, Alzheimer’s disease, and mixed dementia are the most common types of cognitive impairment. Considering the insidious onset and chronic progressive nature of cognitive impairment, conditioning methods for cognitive impairment should be safe, feasible, cost-effective, and long-term applicable. Here, we reviewed current experimental evidence on the effects of remote ischemic conditioning, intermittent brain ischemic conditioning, exercise-induced conditioning, pharmacological (chemical) conditioning, and hypoxic conditioning against cognitive impairment. The results of clinical studies and trials focusing on conditioning as a cognitive therapeutic option have also been included in this review. The effects of conditioning against cognitive impairment can be better understood with more experimental evidence and clinical applications. In addition to the effects of conditioning on the vascular aspect of cognitive impairment, further studies focusing on various conditioning effects on the chronic neurodegenerative aspect of cognitive impairment are needed.
Keywords: Conditioning, Cognitive impairment, Vascular dementia, Stroke, Neurodegeneration
Introduction
While cognitive impairment can develop abruptly in cases of post-traumatic brain injury dementia or post-stroke dementia (Leys et al., 2005), it usually develops insidiously and progressively in cases of Alzheimer’s disease (AD), vascular dementia, and other neurodegenerative diseases (O'Brien and Thomas, 2015). As the conditioning effect on ischemic or hemorrhagic stroke has already been established (Hess et al., 2021), the conditioning effect on stroke could also act against cognitive impairment associated with stroke or vascular compromised conditions. However, the benefits of conditioning effects on cognitive impairment is poorly understood owing to the insidious onset and chronic progressive nature of the cognitive impairment in patients with AD, vascular dementia, and other neurodegenerative diseases.
Based on the strong evidence supporting a positive effect of conditioning on brain and heart ischemia, a similar conditioning effect could act against vascular cognitive impairment or vascular dementia, the main pathophysiology of which is ischemia. Several risk factors, such as hypertension, obesity, dyslipidemia, diabetes, physical inactivity, and smoking, are common between cardiovascular diseases and cognitive impairment in midlife or old age (Rovio et al., 2019). These risk factors negatively affect the brain and heart pathophysiology in a similar way by provoking atherosclerosis induced by oxidative stress, endothelial dysfunction, and adverse immune response (Rovio et al., 2019). In the brain, the altered pathophysiology leads to blood-brain barrier damage, cerebral hypoperfusion, and neurodegenerative processes, including cerebral accumulation and impaired clearance of amyloid beta deposit (Rovio et al., 2019). Cardiogenic dementia refers to the cognitive impairment associated with heart diseases induced by cerebral hypoperfusion, atrial fibrillation, microemboli, thromboembolism, and genetic predisposition such as that caused by apolipoprotein E4 (Dikic et al., 2021; Selnes et al., 2006). Cognitive impairment has been suggested in cases of myocardial ischemia due to myocardial infarction (Dikic et al., 2021), incident coronary artery disease (Rovio et al., 2019), or cardiac surgery including coronary artery bypass grafting (Selnes et al., 2006; Whitlock et al., 2021). Considering the causal relation between the heart and brain and the pathophysiological similarity between the cerebrovascular- and cardiovascular system, conditioning that works on heart ischemia may also work on cognitive impairment in the brain.
In addition, considering that vascular compromise is a major risk factor for various neurodegenerative diseases, including AD (Skoog, 2000), the conditioning effect that works on ischemia may help in the prevention of neurodegenerative cognitive impairment. Based on the strong interaction between vascular dementia and AD, more specific targeting of the conditioning stimuli to major AD pathologies, such as amyloid and tau, could be expected. Furthermore, considering the insidious onset and chronic progressive nature of the cognitive impairment, clinical studies or animal experiments mimicking these conditions should be designed to investigate the benefit of long-term application of the conditioning methods. Based on the timing of conditioning against cognitive impairment, pre-, per-, or post-conditioning could be distinguishable in cases of dementia with abrupt onsets, such as traumatic brain injury or stroke; however, this distinction may be uncertain in cases of chronic progressive cognitive impairment or dementia.
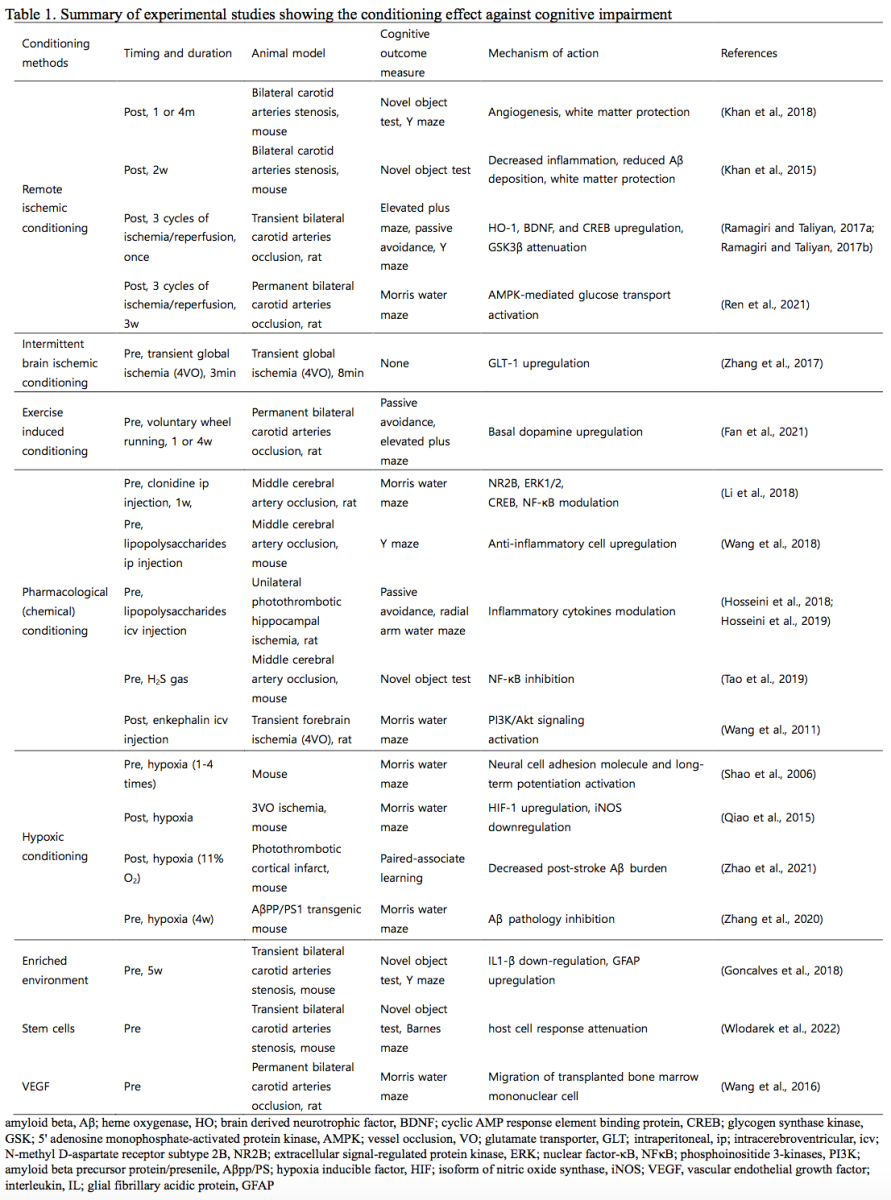
We exclusively reviewed experimental studies that were predesigned as per the concept of conditioning effect and included cognitive measurements. Most evidence of the conditioning effect on cognitive impairment is obtained from studies with acute stroke animal models that concomitantly implemented cognitive behavioral tests (Ding et al., 2017; Hosseini et al., 2018; Hosseini et al., 2019; Huang et al., 2019; Miao et al., 2016; Sumien et al., 2020; Tao et al., 2019; Wang et al., 2018). Predesigned animal experiments as per the conditioning effects on cognitive impairment are scarce (Khan et al., 2018; Khan et al., 2015; Ramagiri and Taliyan, 2017a; Ramagiri and Taliyan, 2017b; Ren et al., 2021).
In the broad concept of conditioning, lifestyle modification by regular exercise or environmental enrichment for the primary prevention of cognitive impairment could be considered as preconditioning. Depending on the timing of intake of specific ingredients or drugs, conditioning can be classified as pre-, per-, or post-chemical conditioning. Furthermore, additional special procedures, such as remote ischemic conditioning (RIC) and medical gas therapy, have been reported as conditioning. Here we have reviewed experimental evidence of various conditioning modalities as therapeutic for cognitive impairment and clinical studies focusing on the therapeutic effect of conditioning on patients with cognitive impairment in various conditions. Diverse experimental conditioning modalities and clinical studies targeting cognitive impairment in various conditions are summarized in the Figure 1. Finally, we have also discussed the future direction of experimental and clinical studies.
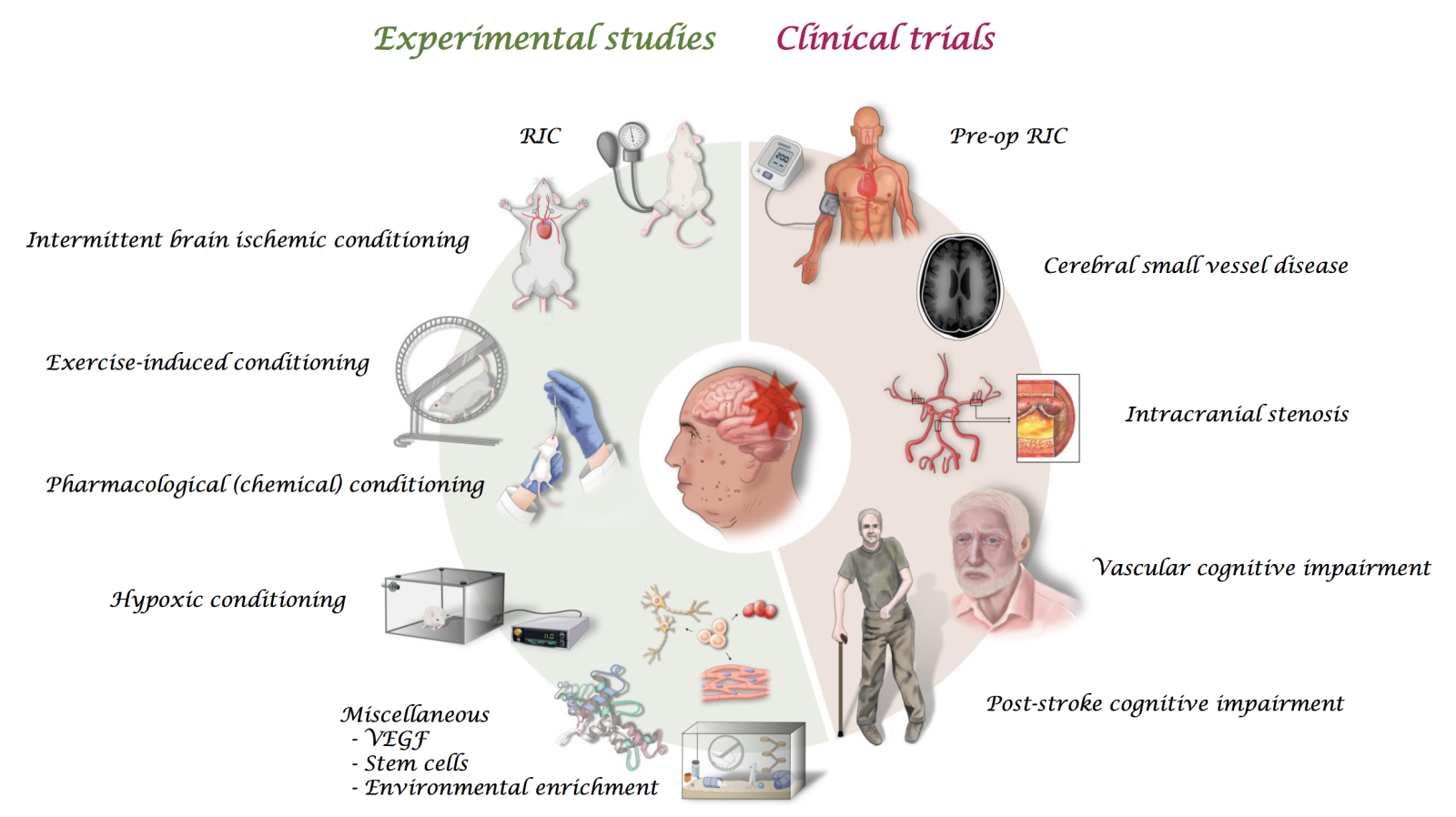
In a new window | Download PPT
Figure 1: Summary of diverse experimental studies and clinical trials targeting cognitive impairment in various conditions (remote ischemic conditioning, RIC; vascular endothelial growth factor, VEGF).
RIC against cognitive impairment
RIC was originally developed to protect the heart against acute ischemic and reperfusion injury (Przyklenk et al., 1993). The endogenous cardioprotective effect is induced by artificial ischemia in a tissue or organ remote from the heart by the process termed RIC (Chong et al., 2018). The alternating inflation and deflation of the cuff applied to the arm or thigh could be used to construct as a simple clinical RIC model (Chong et al., 2018). Although most experimental RIC studies on the brain have focused on stroke (Zhao et al., 2019), some studies have reported the beneficial effect of RIC on cognitive impairment (Khan et al., 2018; Khan et al., 2015; Ramagiri and Taliyan, 2017a; Ramagiri and Taliyan, 2017b; Ren et al., 2021). Using a mouse model of vascular dementia induced by stenosis of both carotid arteries, daily post-conditioning RIC was shown to benefit cognitive function by improving cerebral blood flow, new collateral formation, and white matter protection (Khan et al., 2018; Khan et al., 2015). In a rat model of bilateral common carotid arterial occlusion, post-conditioning RIC showed neuroprotective effects against cognitive impairment via upregulation of heme oxygenase-1 (HO-1), brain derived neurotrophic factor (BDNF), cAMP response element-binding protein (CREB), and 5' adenosine monophosphate-activated protein kinase (AMPK)-mediated glucose transport and downregulation of glycogen synthase kinase-3 beta (GSK-3b) expression (Ramagiri and Taliyan, 2017a; Ramagiri and Taliyan, 2017b; Ren et al., 2021). Because of the insidious onset and chronic progressive course of cognitive impairment, RIC as a preconditioning method to prevent cognitive impairment should be a safe, tolerable, and feasible method for periods longer than a year.
A recent review on the effect of RIC on vascular cognitive impairment has highlighted the therapeutic potential of RIC in terms of risk reduction of recurrent stroke, control of blood pressure, improvement of cerebral blood flow, restoration of white matter integration, protection of the neurovascular unit, attenuation of oxidative stress, and inhibition of inflammatory process (Hess et al., 2015b). RIC may be a safe, feasible, cost-effective, and long-term therapeutic option for vascular cognitive impairment (Hess et al., 2015a). In addition, cardiovascular risk factors and ischemic injury are the major factors attributable to dementia, including AD (Hess et al., 2015a). The potential improvement of cerebral blood flow and other neuroprotective mechanisms induced by RIC in preclinical and human studies could suggest RIC as an ideal therapeutic option to treat vascular cognitive impairment, mixed dementia, or even sporadic AD, and other neurodegenerative diseases (Hess et al., 2015b).
Intermittent brain ischemic conditioning against cognitive impairment
Most experimental studies on brain ischemic tolerance, using the concept of ischemic preconditioning, have focused on acute ischemic injury and related neurological functional recovery, which was mostly motor function recovery (Hao et al., 2020; Zhang et al., 2017). In the clinical setting, intermittent direct brain ischemia due to transient ischemic attack prior to stroke has been studied with respect to brain ischemic tolerance, which could also be interpreted as ischemic preconditioning (Ghozy et al., 2021). However, the effect of ischemic tolerance or transient ischemic attack as ischemic preconditioning on cognitive impairment has not been investigated.
Exercise-induced conditioning against cognitive impairment
Exercise is a strong cardioprotectant and neuroprotectant (Hess et al., 2015a). The benefits of exercise on the prevention of cognitive impairment and dementia have been widely accepted and supported by a large body of experimental and clinical evidence (Livingston et al., 2020; Sujkowski et al., 2022). Maintaining regular exercise in midlife could prevent dementia in late life by decreasing obesity, diabetes, and other cardiovascular risk factors (Livingston et al., 2020). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study found a reduction of cognitive decline in the intervention group, including exercise training, compared to that in the control group (Rosenberg et al., 2018). Preclinical studies have shown that the preconditioning effect of exercise benefits patient with stroke via mechanisms such as protecting the brain vasculature and blood-brain barrier integrity, neurogenesis, and ameliorating neuroinflammation and excitotoxicity (Islam et al., 2017; Sakakima, 2019). Therefore, similar neuroprotective mechanisms could also be effective against cognitive impairment associated with stroke or other vascular compromised conditions. Interestingly, exercise may be considered equivalent to an RIC, as they share common mechanisms, humoral mediators, and neuroprotective pathways (Hess et al., 2015b). Exercise preconditioning using voluntary wheel running by a rat with bilateral common carotid occlusion improved its neurocognitive function related to striatal dopamine levels (Fan et al., 2021). Thus, intense exercise preconditioning could act as a form of remote conditioning (Hess et al., 2015b), and RIC could also be considered as an exercise equivalent and vice versa (Hess et al., 2015a).
Pharmacological (chemical) conditioning against cognitive impairment
In the broad concept of conditioning, many drugs with neuroprotective effects are considered to induce pharmacological (chemical) conditioning against cognitive impairment. By definition, preconditioning agents should work as tolerable stressors that trigger endogenous neuroprotective pathways against a sub-lethal injury, although they could be lethal when administered at a higher dosage. Pharmacological conditioning involves artificial ischemic preconditioning using chemical agents that can activate neuroprotective pathways without directly inducing ischemia (Rehni and Dave, 2018). Based on this concept, volatile anesthetic agents such as isoflurane and sevoflurane, opioids, and bradykinin can function as pharmacological pre- or post-conditioning agents against ischemic injury (Hess et al., 2015a; Wang et al., 2011). In addition to these anesthetic drugs, other pharmacological agents focusing on cognitive impairment have been studied in preclinical experiments. In the preconditioning setting, alpha-2-adrenoceptor agents, such as clonidine, prevented memory deficits in rats with bilateral common carotid arterial occlusion via the extracellular signal-regulated protein kinase (ERK1/2)-CREB/nuclear factor-κB (NF-κB)-N-methyl D-aspartate receptor subtype 2B (NR 2B) pathway (Li et al., 2018). The inflammatory response was shown to have a preconditioning effect on cognitive impairment. Preconditioning using lipopolysaccharides exerted neuroprotective effects on cognitive dysfunction via spleen-mediated immunomodulation in rats with transient middle cerebral artery occlusion (Wang et al., 2018) or via down-regulation of the expression of pro-inflammatory cytokines, such as NF-κB and tumor necrosis factor (TNF)-α, and up-regulation of the expression of anti-inflammatory cytokines, such as interferon regulatory factor (IRF) 3, interferon (IFN)-β, and transforming growth factor (TGF)-β, in rats with unilateral photothrombotic hippocampal ischemia (Hosseini et al., 2018; Hosseini et al., 2019).
Preconditioning with endogenous and exogenous hydrogen sulfide attenuates cerebral ischemia-reperfusion injury-related cognitive impairment via inhibition of NF-κB (Tao et al., 2019). In the post-conditioning setting, opioids, such as delta opioid peptide enkephalin, showed cognitive benefits via activation of the delta opioid receptor-dependent and phosphoinositide 3-kinases (PI3K)/Akt signaling pathway in rats with transient forebrain ischemia (Wang et al., 2011). As pharmacological conditioning has been studied mainly with respect to ischemic damage (Rehni and Dave, 2018), its effect on cognitive impairment is poorly understood. In experimental studies comprising mostly acute ischemic injury models, the protective mechanisms of cognitive function are based on the alleviation of ischemia-reperfusion injury instead of chronic neurodegenerative changes, such as amyloidopathies, tauopathies, synucleinopathies, or transactive response DNA binding protein of 43 kDa (TDP-43)-related proteinopathies. Therefore, further experimental studies using clinically relevant animal models with various neurodegenerative proteinopathies are needed to investigate the conditioning effect on chronic and progressive neurodegenerative cognitive impairment.
Hypoxic conditioning against cognitive impairment
Preconditioning resulting from intermittent hypoxia enhances the recovery of cognitive function after ischemic injury via up-regulation of hypoxia-inducible factor (HIF)-1 expression (Qiao et al., 2015). In another study, compared to the once hypoxic exposure, repetitive hypoxic preconditioning (four times) enhanced the spatial memory function in the Morris water maze task by restoring the expression of the neural cell adhesion molecule and long-term potentiation (Shao et al., 2006). Using a mouse model with photothrombotic stroke, low-oxygen post-conditioning treatment enhanced learning and memory compared to those of the control group maintained in atmospheric air and reduced the post-stroke amyloid beta burden (Zhao et al., 2021). Using transgenic AD mice, hypoxic preconditioning for four weeks ameliorated spatial memory decline in the Morris water maze task and amyloid β pathology with a reduction of amyloid precursor protein (APP) and β-site APP cleaving enzyme 1 (BACE1) levels (Zhang et al., 2020). An interesting clinical report showed that intermittent asphyxiation in athletes caused elevated cerebral perfusion and preserved their cognition, suggesting the neuroprotective role of intermittent hypoxia (Stacey et al., 2021).
Miscellaneous
An enriched environment for five weeks has been proposed as a preconditioning method for improving short-term memory with decreased levels of inflammatory cytokines, such as interleukin1-β (IL1-β), in rats with bilateral carotid arterial occlusion (Goncalves et al., 2018). The attenuation of stroke-induced neurological cognitive dysfunction in aged mice by pretreatment with bone marrow stem cells is known as preconditioning using stem cells (Wlodarek et al., 2022). Preconditioning using vascular endothelial growth factor (VEGF) for bone marrow mononuclear cell transplantation in rats with chronic cerebral hypoperfusion has shown better performance in spatial memory tests, enhanced cerebral vascular proliferation, and amelioration of neuronal degeneration compared to control rats (Wang et al., 2016). Estrogen preconditioning by the supplementation of estrogen, 17β-estradiol, in postmenopausal women was shown to be protective against ischemic brain injury and related cognitive decline (de Rivero Vaccari et al., 2019).
Clinical trials: Conditioning against cognitive impairment
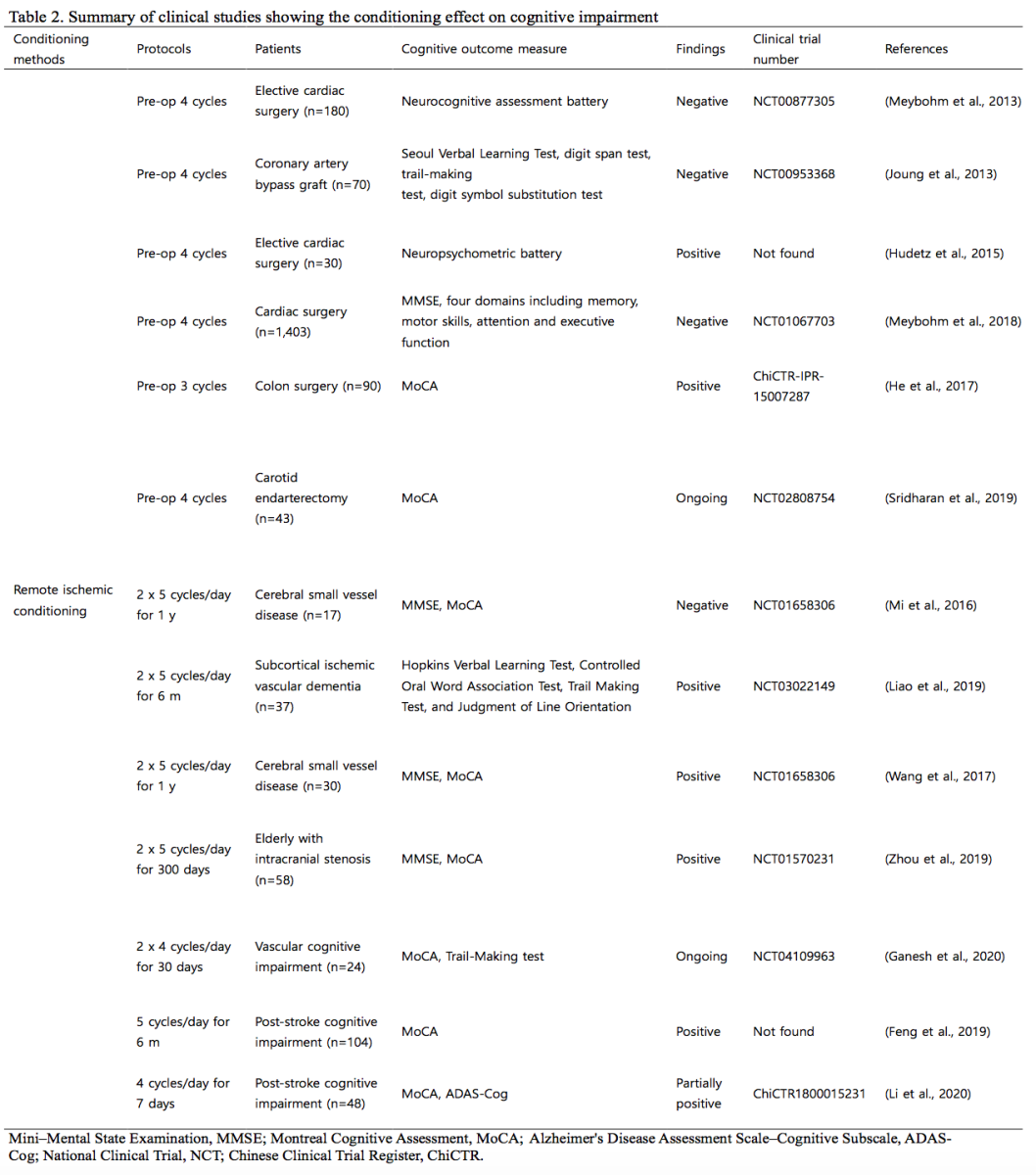
Among the conditioning interventions, preconditioning RIC has been widely studied as a safe, feasible, and cost-effective therapeutic modality for various clinical conditions (Chong et al., 2018; Hess et al., 2015a; Zhao et al., 2019; Zhao et al., 2018). Several clinical trials in which the protective role of RIC on postoperative neurocognitive dysfunction was investigated in patients undergoing cardiac surgery have shown controversial results (Hudetz et al., 2015; Jing et al., 2021; Joung et al., 2013, NCT00953368; Meybohm et al., 2013, NCT00877305). Most of these studies, including meta-analyses and trials with a large sample size, have shown negative results (Jing et al., 2021; Joung et al., 2013; Meybohm et al., 2013) except for one small pilot study (Hudetz et al., 2015). A long-term follow-up of one year in patients who underwent remote ischemic preconditioning for heart surgery showed no additional benefits on their cognitive function (Meybohm et al., 2018, NCT01067703). In another small-scale study involving patients who underwent colon surgery, RIC improved their postoperative cognitive function (He et al., 2017, ChiCTR-IPR-15007287). A clinical trial on the role of RIC in the prevention of postoperative cognitive decline in patients undergoing carotid endarterectomy is ongoing (Sridharan et al., 2019, NCT02808754). In these studies, the RIC has been applied for short-term preconditioning design to prevent postoperative cognitive decline; however, a more long-term application may be required to study the neuroprotective effects on postoperative cognitive decline.
Several small-scale studies have focused on the cognitive function of specific patients vulnerable to cognitive decline (Ganesh et al., 2020, NCT04109963; Liao et al., 2019, NCT03022149; Mi et al., 2016, NCT01658306; Wang et al., 2017, NCT01658306; Zhou et al., 2019, NCT01570231). Application of RIC for one year in a small number of patients with underlying cerebral small vessel disease suggested favorable outcomes due to possible mechanisms of improved flow velocity in the middle cerebral artery and decreased cerebral white matter lesions, although there were no significant differences in the cognitive function of the patients (Mi et al., 2016). In patients with subcortical ischemic vascular dementia, an RIC for sic months showed significant improvement in some neuropsychological tests (Liao et al., 2019). In a small-scale study in patients with small vessel disease, an RIC for five minutes two times a day on both upper arms for one year showed a reduction in white matter hyperintensities and improved their visuospatial and executive function compared to those of the control group (Wang et al., 2017). An RIC consisting of five cycles of alternating ischemia and reperfusion for five minutes each on both upper arms for 300 days effectively prevented the progression of white matter hyperintensities and ameliorated cognitive impairment in very elderly patients with intracranial atherosclerosis (Zhou et al., 2019). A clinical trial using RIC for 30 days in a small number of patients with cerebral small vessel disease to observe the changes in cerebral blood flow, imaging markers such as white matter hyperintensity volume or diffusion tensor imaging, and cognitive scale is ongoing (Ganesh et al., 2020).
The effect of remote ischemic post-conditioning has also been reported in cases of post-stroke cognitive impairment, (Feng et al., 2019; Li et al., 2020, ChiCTR1800015231). Long-term application of remote ischemic post-conditioning for six months improved visuospatial function, executive function, and attention scores (Feng et al., 2019). In another study, remote ischemic post-conditioning for seven days showed significant improvement in cognitive scale scores such as the Montreal Cognitive Assessment scale (MoCA) and Alzheimer's Disease Assessment Scale Cognitive subscale (ADAS-Cog) at 180 days (Li et al., 2020). However, these clinical studies were performed on a relatively small number of patients and had a short intervention period. A large-scale trial with a longer intervention period should be performed to determine the effect of conditioning on the prevention of poststroke cognitive impairment. Furthermore, clinical trials should focus on the neuroprotective conditioning effect on patients with chronic and progressive neurodegenerative cognitive impairment.
Future directions
Current experimental evidence (Table 1) and clinical study reports (Table 2) are summarized in this review. The field of conditioning for cognitive impairment will expand with more data from experimental evidence and clinical applications. RIC and exercise-induced conditioning may be safe, beneficial, and cost-effective therapeutic options for cognitive impairment. Most experimental studies on the effects of conditioning on cognitive decline have showed concomitant results, in addition to those on ischemic injury. As a large body of evidence suggests that ischemic conditioning is remarkable in cases of ischemic heart disease and ischemic stroke, long-term consequences of ischemic brain injury, such as vascular cognitive impairment or vascular dementia, could be also promising candidates for the benefits of ischemic conditioning. In addition, other chronic neurodegenerative diseases such as AD, Parkinson’s disease, and other neurodegenerative diseases associated with proteinopathies, which are probably affected by a vascular compromise, could also be potential candidates for the benefits of ischemic conditioning. Further studies mainly focusing on various conditioning effects on chronic neurodegenerative diseases, such as amyloidopathies, tauopathies, synucleinopathies, and TDP-43-related proteinopathies, are anticipated.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1F1A1076085).
Acknowledgements
We thank Ms. Seo-U Kim at Medizin Atelier for her contribution with the Figure.
Conflict of interest
The authors declare no conflict of interest.
References
Hahn Young Kim1,*
1Department of Neurology, Konkuk University School of Medicine, Seoul, Republic of Korea.
Dong Bin Back1
1Department of Neurology, Konkuk University School of Medicine, Seoul, Republic of Korea.
Bo-Ryoung Choi1
1Department of Neurology, Konkuk University School of Medicine, Seoul, Republic of Korea.
Dong-Hee Choi2
2Department of Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea.
Kyoung Ja Kwon2
2Department of Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea.
Corresponding author:
Hahn Young Kim, MD, PhD
Email: hkimmd@kuh.ac.kr

In a new window | Download PPT
Figure 1: Summary of diverse experimental studies and clinical trials targeting cognitive impairment in various conditions (remote ischemic conditioning, RIC; vascular endothelial growth factor, VEGF).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8549 | 30 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA