Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Blood Flow Restriction Training and Stroke: Review and Evaluation of Safety from a Hemodynamic and Hematological Perspective
Time:2022-12-04
Number:7029
Luke J. Schmidt1, Ann M. Stowe2,3, Gregory J. Bix4,5, Jonathan M. Peake1, Tony J. Parker1
Author Affiliations


- 1Queensland University of Technology, Tissue Repair and Translational Physiology Group, School of Biomedical Sciences, Faculty of Health, Brisbane, Queensland, Australia.
- 2Department of Neurology, University of Kentucky, Lexington, Kentucky, USA.
- 3Department of Neurology and Neurotherapeutics, Peter O’Donnell Jr. Brain Institute, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
- 4Clinical Neuroscience Research Center, Departments of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, Louisiana, USA.
- 5Tulane Brain Institute, Tulane University, New Orleans, Louisiana, USA.
Conditioning Medicine 2022. 5(4): 131-143.
Abstract
Blood flow restriction training is a physical intervention that promotes many beneficial muscular and cardiovascular adaptations when low load/intensity is used in healthy and clinical populations. To date, no studies have investigated the safety of blood flow restriction training in a stroke population, yet medical history of stroke is commonly regarded as a contraindication for the application of blood flow restriction training. This review examines the current available literature regarding hemodynamic and hematological changes in response to blood flow restriction training in young and old, healthy, and hypertensive participants to draw conclusions as to the safety of such an intervention in a stroke population. This review also fills a current knowledge gap needed to progress clinical application of blood flow restriction training, specifically, in a stroke population.
Keywords: Stroke, Blood flow restriction training, Safety, Blood pressure, Coagulation
Abstract
Blood flow restriction training is a physical intervention that promotes many beneficial muscular and cardiovascular adaptations when low load/intensity is used in healthy and clinical populations. To date, no studies have investigated the safety of blood flow restriction training in a stroke population, yet medical history of stroke is commonly regarded as a contraindication for the application of blood flow restriction training. This review examines the current available literature regarding hemodynamic and hematological changes in response to blood flow restriction training in young and old, healthy, and hypertensive participants to draw conclusions as to the safety of such an intervention in a stroke population. This review also fills a current knowledge gap needed to progress clinical application of blood flow restriction training, specifically, in a stroke population.
Keywords: Stroke, Blood flow restriction training, Safety, Blood pressure, Coagulation
Introduction
Approximately 3% of males and 2% of females in the United States of America have a disability secondary to either ischemic or hemorrhagic stroke, according to the 2019 update from the American Heart Association (Centers for Disease Control and Prevention, 2009; Benjamin et al., 2019). Ischemic etiology accounts for approximately 87% of strokes, whereas hemorrhagic etiology constitutes approximately 13% of all strokes. The prevalence of hemorrhagic strokes can be further subdivided into intracerebral hemorrhage (10%) and subarachnoid hemorrhage (3%) (Benjamin et al., 2019; Benjamin et al., 2018). Short-term sequelae caused by stroke include deep vein thrombosis and seizures. By contrast, long-term sequelae include gait instability, increased fall risk, reduced muscle mass, reduced cardiorespiratory fitness, increased fatigue, and spasticity (Benjamin et al., 2019; Ivey et al., 2005; Scherbakov et al., 2013; Xu et al., 2018; Zorowitz et al., 2013). There are numerous risk factors that are associated with stroke etiology, including: physical inactivity, high blood pressure, diabetes mellitus, disordered heart rhythm, high blood lipids, smoking, diet, and genetics (Benjamin et al., 2019). With these stroke outcomes, risk factors and statistics in mind, exercise has beneficial outcomes for stroke patients.
A recent Cochrane review summarized the current evidence on exercise in a stroke population (Saunders et al., 2020). Exercise training in stroke populations was considered safe. The most effective modes for improving disability were aerobic and mixed training. These modes of training improved walking, balance, and fitness outcomes. Importantly, the fitness improvement as a result of aerobic exercise was estimated to reduce future stroke-related hospitalizations by 7%. There were, however, insufficient data to draw solid conclusions in other areas such as cognition, mood, quality of life (QOL), or the impact of resistance exercise on disability (Saunders et al., 2020). Nevertheless, other literature exists regarding further benefits of exercise for stroke populations, and it is clear that exercise plays an important role in the prevention and reduction of stoke recurrence. What is less clear is how more novel exercise-based rehabilitation methodologies may benefit a stroke patient.
Blood Flow Restriction (BFR) training is a relatively new form of exercise that facilitates adaptations that may be beneficial in a stroke population. Not only could BFR training act as a rehabilitation tool following stroke; it could also reduce the severity of a stroke if one reoccurs. In this mode of exercise, BFR is applied to a limb through the use of modified pneumatic cuffs, belts, or wraps, either uni- or bilaterally. Exercise is then performed with a low load, typically ≤30% of one repetition maximum (RM), in four sets totaling 75 repetitions (30, 15, 15, 15 repetitions) (Mattocks et al., 2018). Practically, it has been reported that a subjective seven out of ten sensations of pressure without pain or neural symptoms could be used to gauge appropriate tightness of the cuff or band (Mattocks et al., 2018). This approach is often used in rehabilitation settings for musculoskeletal injuries. BFR can facilitate hypertrophy and strength gains with loads ≤30% of one RM (Hughes et al., 2017). It can also potentially improve bone mineral density, factors indicating osteoblast activity and neural adaptations (Hughes et al., 2017; Loenneke et al., 2012). Significantly, BFR has been identified as a promising intervention for stroke patients (Vanwye et al., 2017), although direct evidence is currently lacking. Despite a growing body of research indicating that BFR training can be safe in healthy young and elderly populations (Loenneke et al., 2011; Vanwye et al., 2017), to date there are also no published studies evaluating the safety of this approach in stroke patients, let alone the potential positive effects that it may have.
In view of valid concerns in the literature as to the safety of BFR training for clinical populations (Heitkamp, 2015; Loenneke et al., 2011; Spranger et al., 2015), this review aims herein to review the safety concerns of BFR training as they would apply specifically to the stroke population. Thus, this review fills a critical gap in the literature by moving away from speculation, which involves questioning the validity of this training intervention, specifically in a stroke population, without widely consulting the available literature toward clinical justification for investigating BFR training in stroke research and clinical practice, which has been lacking in the literature thus far. This review will initially discuss the potential benefits of BFR training in a stroke population. It will then focus on the blood pressure (BP) alterations associated with BFR training (immediately before, during, and immediately after the exercise session) in young and elderly populations, as well as normotensive and hypertensive populations. Finally, the literature regarding coagulation changes with BFR training will be investigated. Throughout this review, gaps in the literature will also be highlighted so that future research may further advance knowledge around BFR training in stroke populations.
BFR training and stroke populations
About 60% of stroke survivors experience disability following stroke (Scherbakov et al., 2013). Because of this, the primary goal of post-stroke exercise interventions is to improve the patient’s competence with activities of daily living (ADL) (American College of Sports Medicine, 2018). In addition to exercises that improve the patient’s capacity to perform ADL, a secondary concomitant focus is to improve muscular strength, aerobic fitness, and balance. This approach is intended not only to improve function, but also to prevent a stroke recurrence (American College of Sports Medicine, 2018). Because no research has been conducted on the safety or effect of BFR training in a stroke population, one can only hypothesize regarding the safety and possible benefits based on trends observed in younger populations, and the few studies conducted in elderly populations. Considering the goals of stroke rehabilitation, particularly in elderly populations, BFR training has the potential to improve muscular strength (Cook et al., 2017), muscle size (Cook et al., 2017), walking speed (Cook et al., 2017), cardiovascular fitness (Conceição et al., 2019), and scores on functional test such as the 30 sit-to-stand and 6-minute walk tests (Conceição et al., 2019), while reducing muscle wastage associated with bed rest (Conceição et al., 2019). In additon to the potential physical improvements, there are possibly neuroprotective and neurotrophic benefits of BFR training. The evidence for this comes from studies investigating remote ischemic conditioning (RIC). When applied chronically to the arms twice daily prior to a stroke in much the same way that BFR training would be applied, applying an occlusion device proximally on the limb reduces the recurrence of stroke and improves recovery outcomes for stroke patients (Chen et al., 2022; Meng et al., 2012; Meng et al., 2015). Biochemical and immunological mechanisims behind the neuroprotective effect of RIC, and parallels beween RIC, BFR training, and exercise are outside the scope of this review, but have been previously discussed (Schmidt et al., 2021; Zhao et al., 2018).
Current recommendations by the American Heart Association and American Stroke Association indicate that for stroke inpatient or outpatient exercise rehabilitation, aerobic exercise (e.g., treadmill or ground walking, arm and/or leg ergometry, etc.) should be conducted three to five days per week (Billinger et al., 2014). Aerobic exercise should total 20 to 60 minutes per day and can be comprised of multiple ten-minute bouts (Billinger et al., 2014). Additionally, aerobic exercise should be conducted at an intensity of 40 to 70% heart rate reserve (HRR), 55 to 80% HR maximum, or at a rating of perceived exertion (RPE) of 11 to 14 (on a 6 to 20 Borg scale) (Billinger et al., 2014). Furthermore, a prolonged warmup, lasting up to ten-minutes, should be performed (Billinger et al., 2014). Resistance exercise (e.g., free weights, body/partial body weight, machine, etc.) primarily focusing on functional upper/lower extremity and trunk exercises should be performed two to three days per week, consisting of one to three sets of 10 to 15 repetitions at 50% to 80% 1RM (Billinger et al., 2014). These guidelines also recommend performing eight to ten different resistance exercises during sessions. As well as aerobic and resistance exercise, flexibility and neuromuscular training should be performed two to three times per week (Billinger et al., 2014).
To date, no specific limits for maximal exercising BP have been determined for stroke, diabetic, hypertensive, or cardiac patients (American College of Sports Medicine, 2018; Billinger et al., 2014; Colberg et al., 2010; Sharman et al., 2019; Sharman et al., 2009). With regard to stroke, it is recommended in a joint position statement by the American Heart association and the American Stroke Association to follow guidelines for exercising with cardiovascular disease. Such guidelines merely state that a safe exercise intensity is a HR at least ten beats per minute less than the ischemic threshold, the HR at which angina occurs (American College of Sports Medicine, 2018; Billinger et al., 2014). The guidelines lack specificity regarding the pathophysiology of stroke etiology, and therefore the safe prescription of exercise for a stroke population. With this in mind, the general recommendation for exercise termination with regard to BP is 250/115 mmHg in normotensive and hypertensive participants (Sharman et al., 2019), or 220/105 mmHg for hypertensive participants, depending on which guidelines are followed (American College of Sports Medicine, 2018).
Traditional exercise and blood pressure alterations
During exercise, blood flow to the working muscles and heart increases disproportionately compared with other organs of the body (Joyner et al., 2015). Muscles and the heart receive most of the increase in blood flow due to an increased cardiac output, which is primarily driven by an increase in HR. By contrast, blood flow to the brain is maintained or slightly increased, while blood flow to the kidneys, liver, and spleen can decrease by 25% (Joyner et al., 2015). This redirection and subsequent increase of blood is termed hyperemia and is due to a complex (and incompletely understood) interplay between vasoconstrictors and vasodilators. This interplay attempts to meet the metabolic demands placed on the exercising muscles and heart (Gliemann et al., 2019).
Similar to blood flow, BP is also modulated during exercise. The type of exercise has a predictable influence on the BP response over the duration of an exercise session (Falz et al., 2019). When exercising in a steady state, such as walking, jogging, or cycling at a constant speed and incline, systolic blood pressure (SBP) will sharply increase and then plateau, while diastolic blood pressure (DBP) will remain steady or slightly decrease (Falz et al., 2019). If the exercise intensity is continuously increased, such as during a ramped VO2 max test, or while increasing the intensity of the exercise in a linear fashion, SBP will steadily increase and eventually plateau with peak exercise capacity, while DBP may be maintained or decrease with increasing intensity (Sharman et al., 2015). During resistance training or high-intensity interval training, BP sharply rises and falls, corresponding to periods of exercise and rest (Falz et al., 2019; Paulo et al., 2019). With any type of exercise, changes in BP are directly related to the intensity and duration of the exercise. Intensity, including the speed, gradient and/or resistance during cardiovascular exercise or load and rest in resistance training will affect the magnitude by which BP will change (Falz et al., 2019; Paulo et al., 2019). Time, including the duration of cardiovascular exercise and sets, repetitions, and rest periods in resistance exercise, can alter the magnitude and frequency of BP changes during an exercise session (Paulo et al., 2019). With these exercise related factors in mind, BP alterations also have a physiological phenomenon driving the response, known as the exercise pressor reflex.
The exercise pressor reflex modulates alterations in BP in response to changes in the intramuscular environment that are detected by mechanoreceptors and metaboreceptors (Secher et al., 2012; Smith et al., 2006; Spranger et al., 2015). These receptors can generate action potentials due to external forces that deform the tissue, internal forces generated by muscle contractions, or when metabolite concentrations are sufficiently elevated (Boushel, 2010; Drew, 2017; Drew et al., 2017). These peripheral action potentials (together with action potentials from baroreceptors in the carotid sinus and aortic arch, and action potentials propagating from the cerebral cortex) act on the cardiovascular control center in the brainstem (Drew, 2017; Spranger et al., 2015). This results in an increased HR, cardiac output, contractility of the heart, arterial resistance, and decreased venous compliance. These alterations ultimately increase arterial BP through a coordinated increase in sympathetic outflow, and a decrease in parasympathetic outflow (Spranger et al., 2015). Any type of exercise training that could significantly increase the activity of mechanoreceptors and metaboreceptors (above what would be considered normal for an exercise intervention) could have potentially serious implications for BP, particularly in clinical populations. One such training type that may have serious implications for BP alterations is BFR training.
BFR training and blood pressure alterations
BFR training and blood pressure alterations – a first principles perspective
BFR training requires the partial occlusion of a limb or limbs, then exercising while the pneumatic cuff, belt, or wrap continues to partially occlude the limb. In the context of the exercise pressor reflex, it would be logical that this form of training would lead to additional increases in BP when compared with exercise without BFR (Figure 1). This assumption is based on the previously outlined literature, and with the understanding that further research is needed to confirm how BFR affects the exercise pressor reflex. More specifically, based on the presented literature, it is plausible that mechanoreceptors may increase their firing rates above and beyond what would be expected for a particular load, because the pressure cuff itself could deform the receptors. A decrease in venous return with BFR may also increase peripheral intravascular and intramuscular pressure of the occluded limb, which could again increase the firing rate of mechanoreceptors. Similarly, metaboreceptors may also increase their firing rates, because BFR training relies on an increased metabolic stimulus in place of a load stimulus to effect positive training adaptations. Based on the theoretical understanding of how BP is modulated during exercise, and the hypothetical mechanisms described above, BFR training may cause unsafe alterations in BP, particularly in populations who already have elevated BP, or those who are more sensitive to the effects of increased BP. This is why it is important to consider not only theoretical perspectives (as many papers criticising BFR training have done), but also to consider absolute values regarding BP alterations. Ultimately this will provide a more meaningful evaluation of the safety of BFR training.
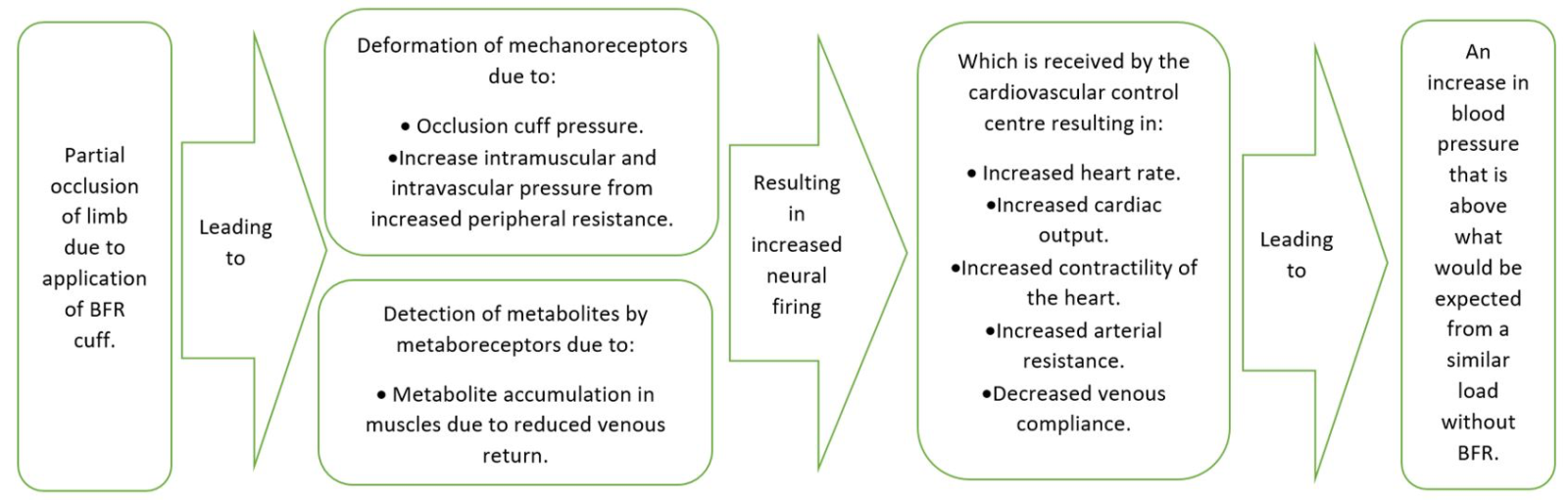
In a new window | Download PPT
Figure 1: Hypothetical mechanisms behind the exercise pressor reflex associated with BFR that may explain an increase in BP beyond what would be expected by a similar load without BFR.
BFR training and blood pressure alterations – exercise prescription confusion
There is currently conflicting advice around the safe upper limit of BP for exercise in hypertensive populations. The American College of Sports Medicine (ACSM) recommends the termination of exercise sessions when a healthy participant reaches a SBP of >250 mmHg, or a DBP of >115 mmHg (American College of Sports Medicine, 2018). In addition to this, the ACSM recommends that SBP should not exceed 220 mmHg, or the DBP should not exceed 105 mmHg in an exercise session for hypertensive participants (American College of Sports Medicine, 2018). However, the governing body for Exercise Physiologists in Australia, Exercise and Sports Science Australia (ESSA), make the recommendation that exercise for a hypertensive population should be terminated if a SBP of >250 mmHg, or a DBP of >115 mmHg is reached (Sharman et al., 2019). These upper limits of safe exercising BP should be considered when prescribing and implementing an exercise program for clinical populations. In light of this, it is important to consider how BFR training affects BP in normotensive and hypertensive participants, particularly compared with resistance training loads of >60% 1RM. These loads are commonly used to improve strength and muscle hypertrophy in healthy populations, and they comply with the American Heart Association and American Stroke Association recommendations for exercise in a stroke population (Billinger et al., 2014; Garber et al., 2011).
BFR training and blood pressure alterations – an evidence-based practice approach
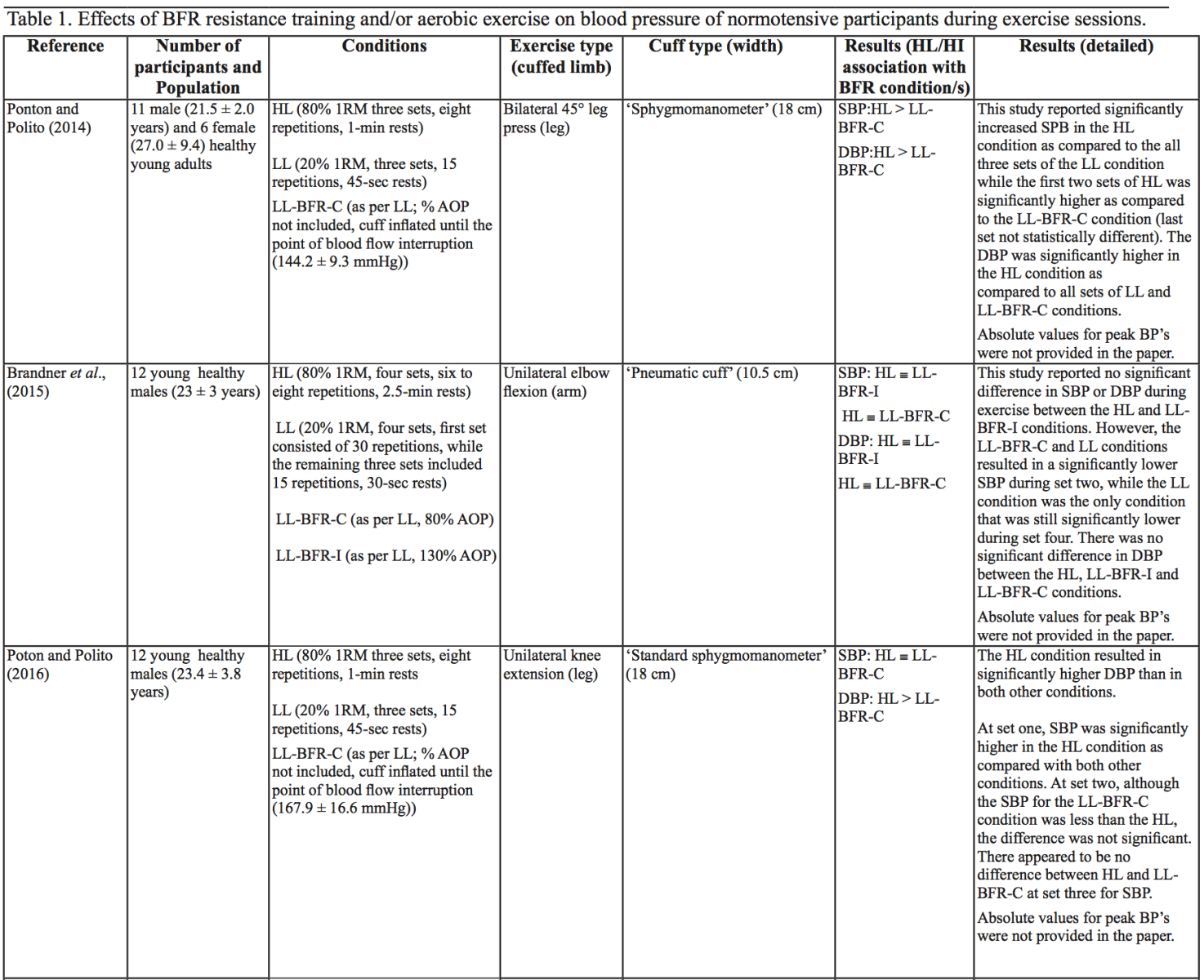
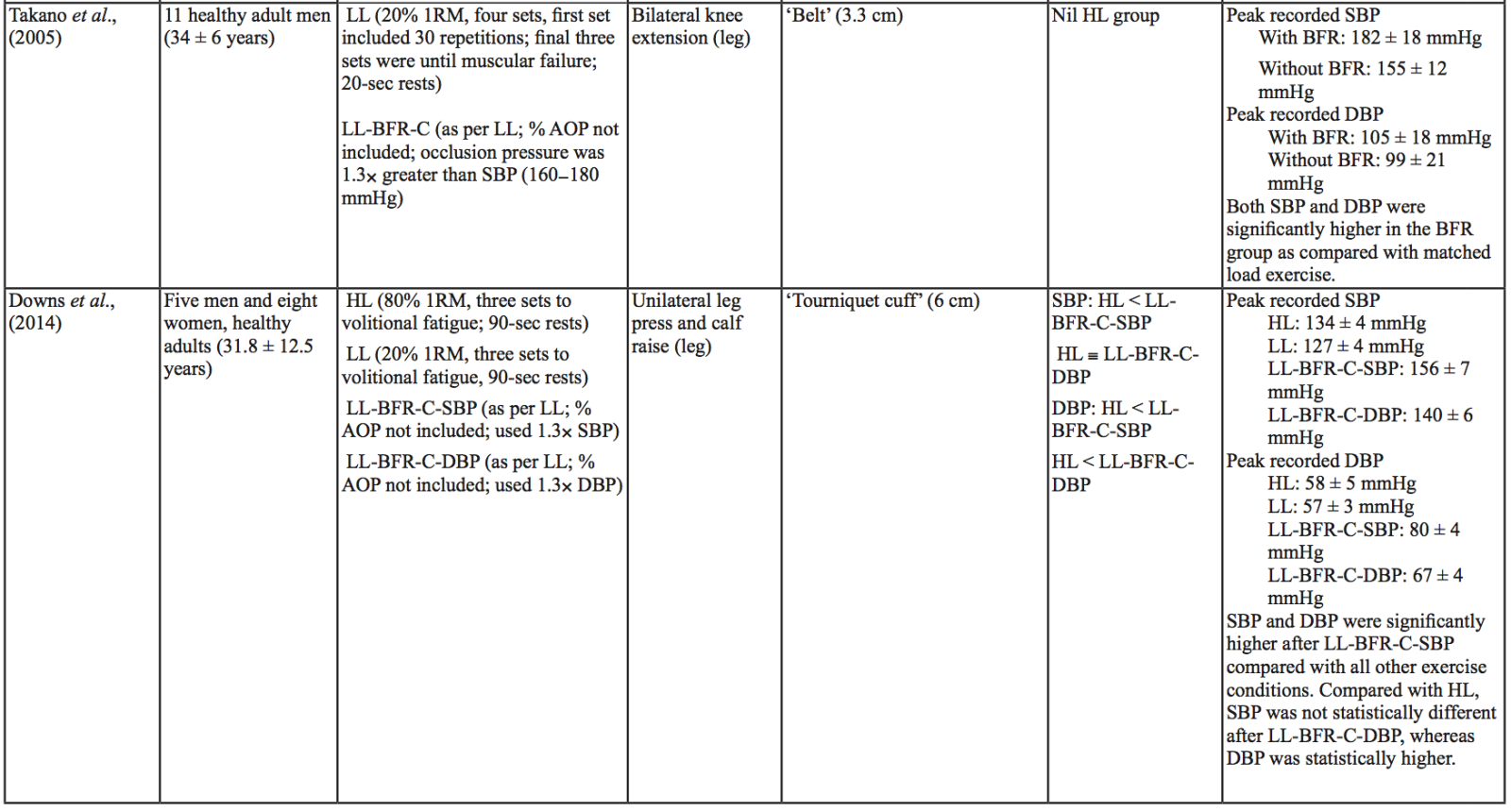
The evidence to date is currently not definitive as to whether BFR training leads to unsafe BP alterations. A systematic review and meta-analysis by Domingos et al.,(2018) investigated how resistance exercise with and without BFR affects BP during and after exercise sessions (Domingos et al., 2018). The overall findings indicate that during BFR exercise sessions, SBP does not significantly differ from SBP resulting from >60% 1RM loads; however, DBP is significantly higher in response to resistance exercise with BFR (Domingos et al., 2018). Conversely, BFR training of the upper arms results in a statistically greater post-exercise reduction of BP compared with traditional >60% 1RM load exercise (Domingos et al., 2018). These alterations are on average in the magnitude of 1.37 mmHg (SBP) and 1.66 mmHg (DBP) more for the BFR training condition (Domingos et al., 2018). Among the papers included in the review article by Domingos et al.,(2018), ten studies assessed the effects of BFR training on BP during exercise sessions, not just pre- and post-exercise. Among these ten papers, eight studies investigated the effects of BFR resistance training and/or aerobic exercise on normotensive participants. The findings from these studies, and additional papers uncovered from literature searches are summarized in Table 1. Figure 2 gives a graphic representation of the absolute values described in Table 1. The findings from hypertensive studies are reported in Table 2.
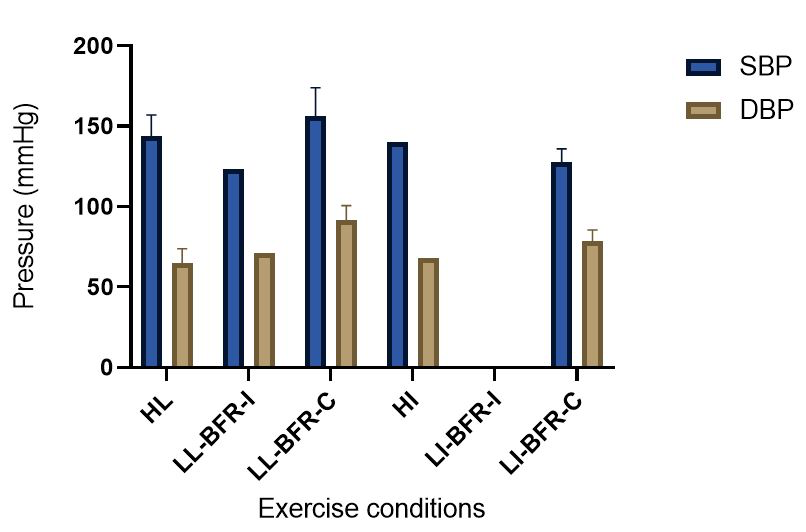
In a new window | Download PPT
Figure 2: Absolute BP values compiled from resistance and aerobic exercise trials (mean ± SD) (Bazgir et al., 2016; Downs et al., 2014; May et al., 2017; Picón et al., 2018; Staunton et al., 2015; Sugawara et al., 2015; Takano et al., 2005). This figure presents the means of all the peak absolute values for the HL/HI and BFR conditions presented in Table 1. When there was only one study that provided absolute values for an exercise condition, there are no SD bars included. Additionally, when no absolute data were presented in any of the studies contained in Table 1 for a particular exercise condition, there were no data included in this figure. HI = High-intensity aerobic training; HL = High load resistance training; LL-BFR-C = continuous blood flow restriction with low-load resistance training; LL-BFR-I = intermittent blood flow restriction with low-load resistance training; LI-BFR-C = continuous blood flow restriction with low-intensity aerobic training; LI-BFR-I = intermittent blood flow restriction with low-intensity aerobic training.
Taken together, it is clear from the studies cited in Table 1 that there is an overall trend that low load BFR (LL-BFR) exerts a similar effect on SBP as high load (HL) training conditions (Brandner et al., 2015; May et al., 2017; Poton et al., 2016; Sardeli et al., 2017; Staunton et al., 2015). There are, however, some notable exceptions where SBP has been reported as higher (Downs et al., 2014; Sardeli et al., 2017; Scott et al., 2018), or lower in the LL-BFR condition (Libardi et al., 2017; Poton et al., 2014). Due to the variability in the methodologies for these studies, it is difficult to speculate as to why these studies resulted in divergent SBP for the LL-BFR condition. Although, based on findings presented in Figure 2, it appears that low load exercise with intermittent blood flow restriction (LL-BFR-I) results in lower SBP compared with HL or low load exercise with continuous blood flow restriction (LL-BFR-C). Additionally, based on data presented in Table 1, it appears that there is a smaller rise in SBP for LL-BFR associated with the first one to two sets (Brandner et al., 2015; Poton et al., 2014; Poton et al., 2016); however, this is not a consistent finding (Scott et al., 2018). Whereas DBP in some studies tended to increase more in the LL-BFR conditions than in HL training conditions (Downs et al., 2014; May et al., 2017; Sardeli et al., 2017; Scott et al., 2018; Staunton et al., 2015), other studies observed non-significant differences between the conditions (Bazgir et al., 2016; May et al., 2017), or significantly lower DBP in the LL-BFR condition (Libardi et al., 2017; Poton et al., 2014; Poton et al., 2016). This finding (i.e., lower DBP for the LL-BFR condition) is not reflected in Figure 2, because the studies that reported this did not present absolute values (as reported in Table 1). Additionally, some studies reported that there were non-significant increases in SBP and DBP in the LL-BFR condition compared with baseline (Picón et al., 2018). Furthermore, there were trials in which low load (LL) and LL-BFR were not statistically different with regard to SBP and DBP (Bazgir et al., 2016; Vieira et al., 2013).
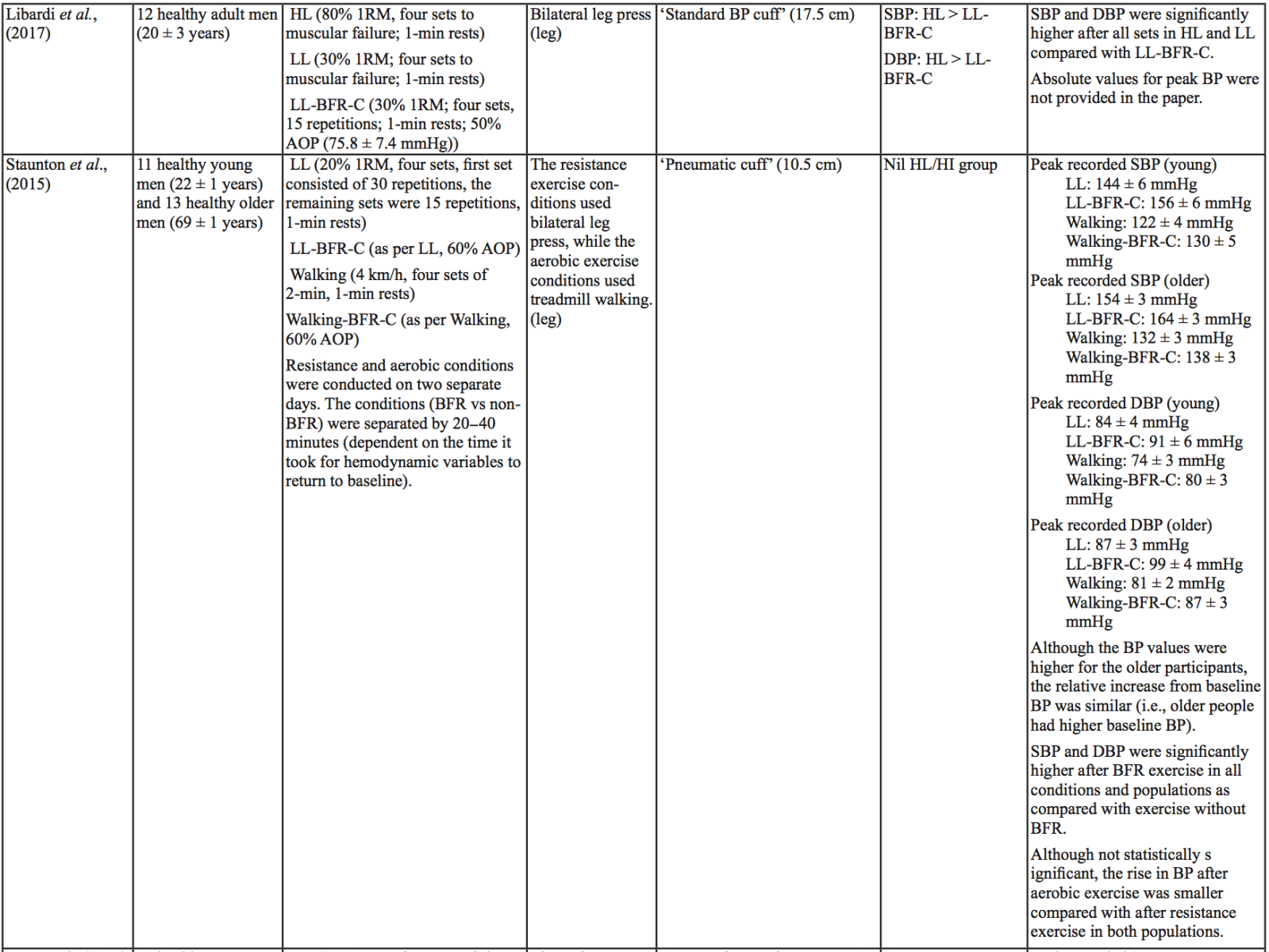
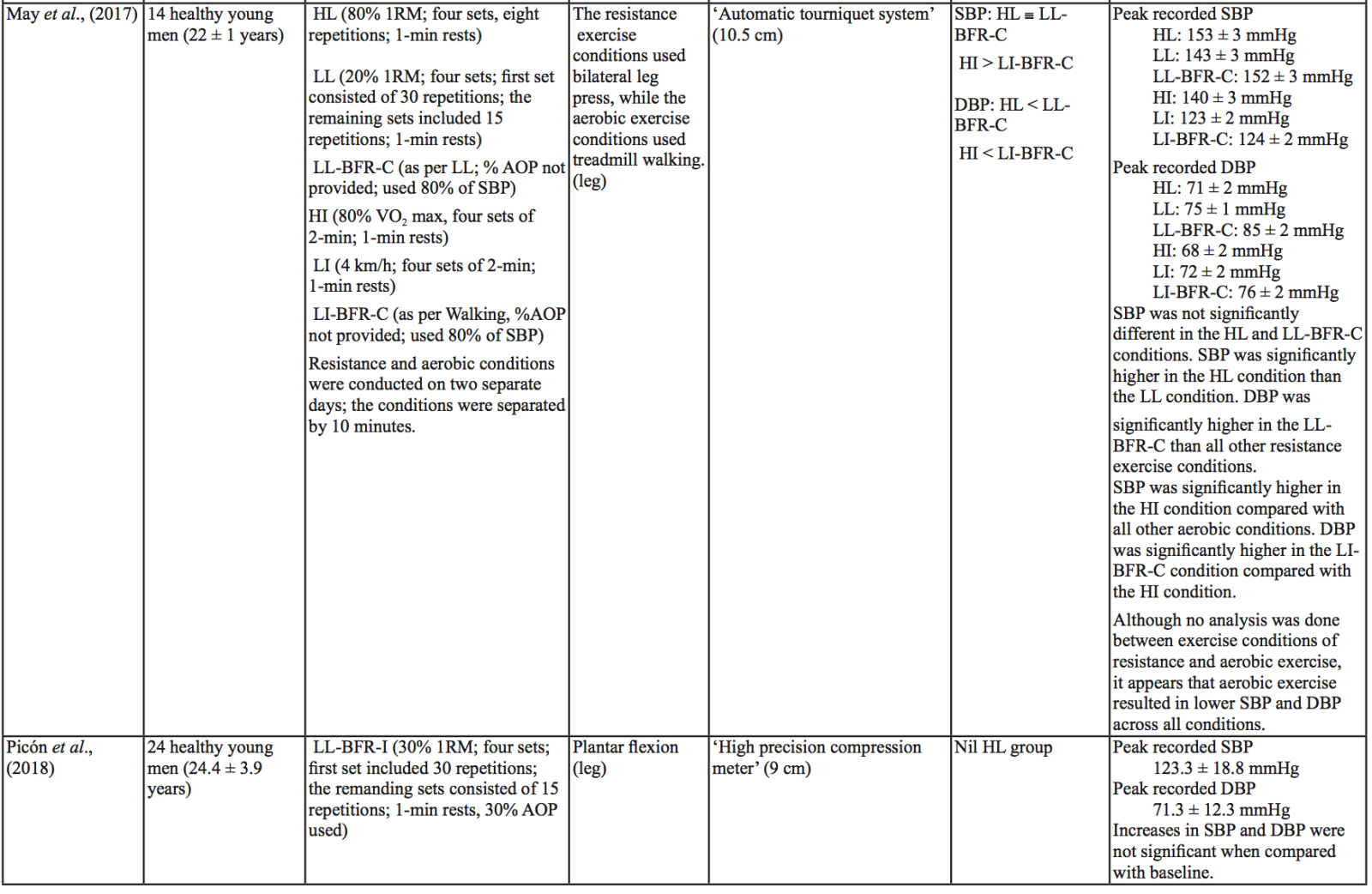
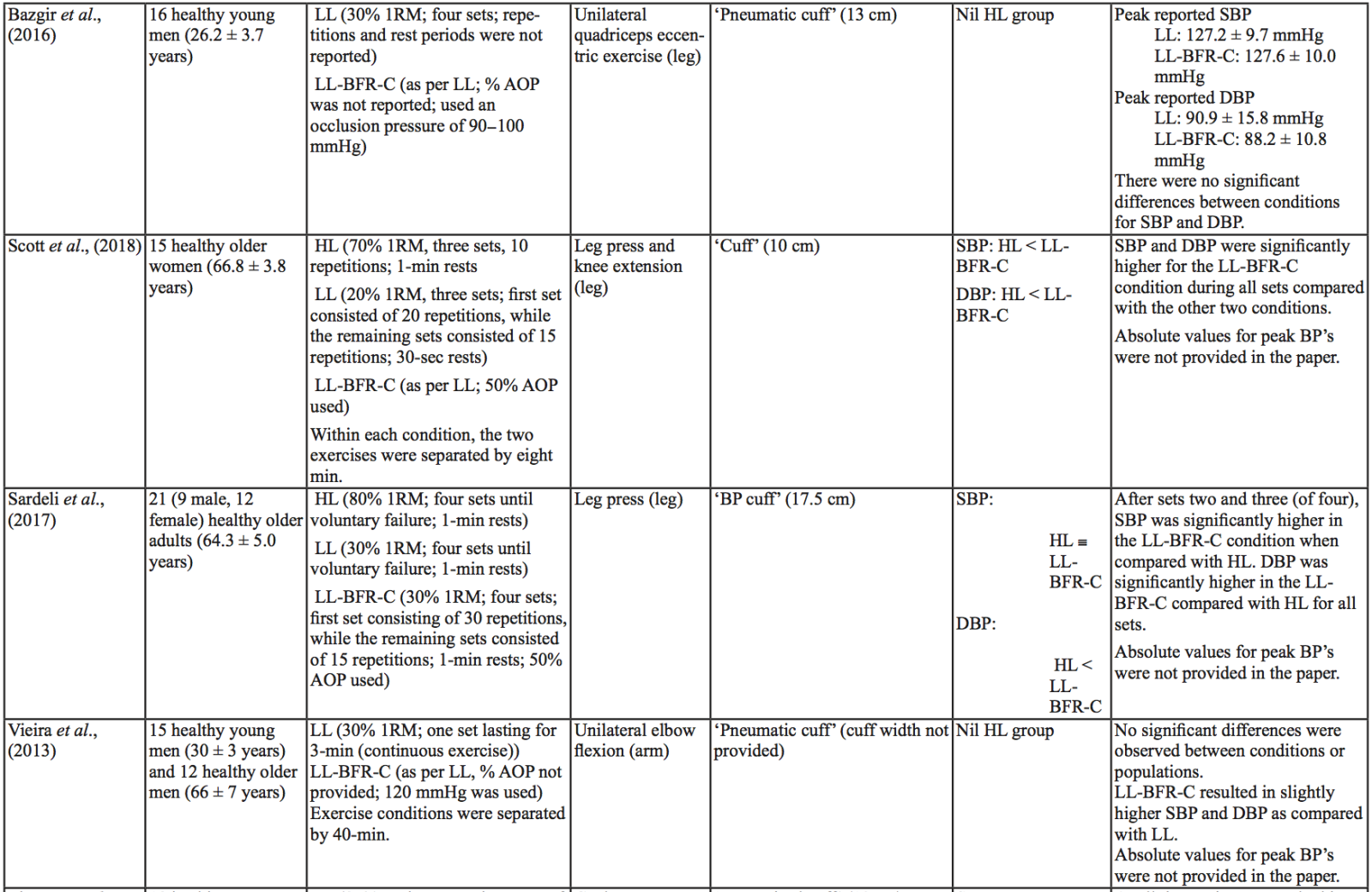
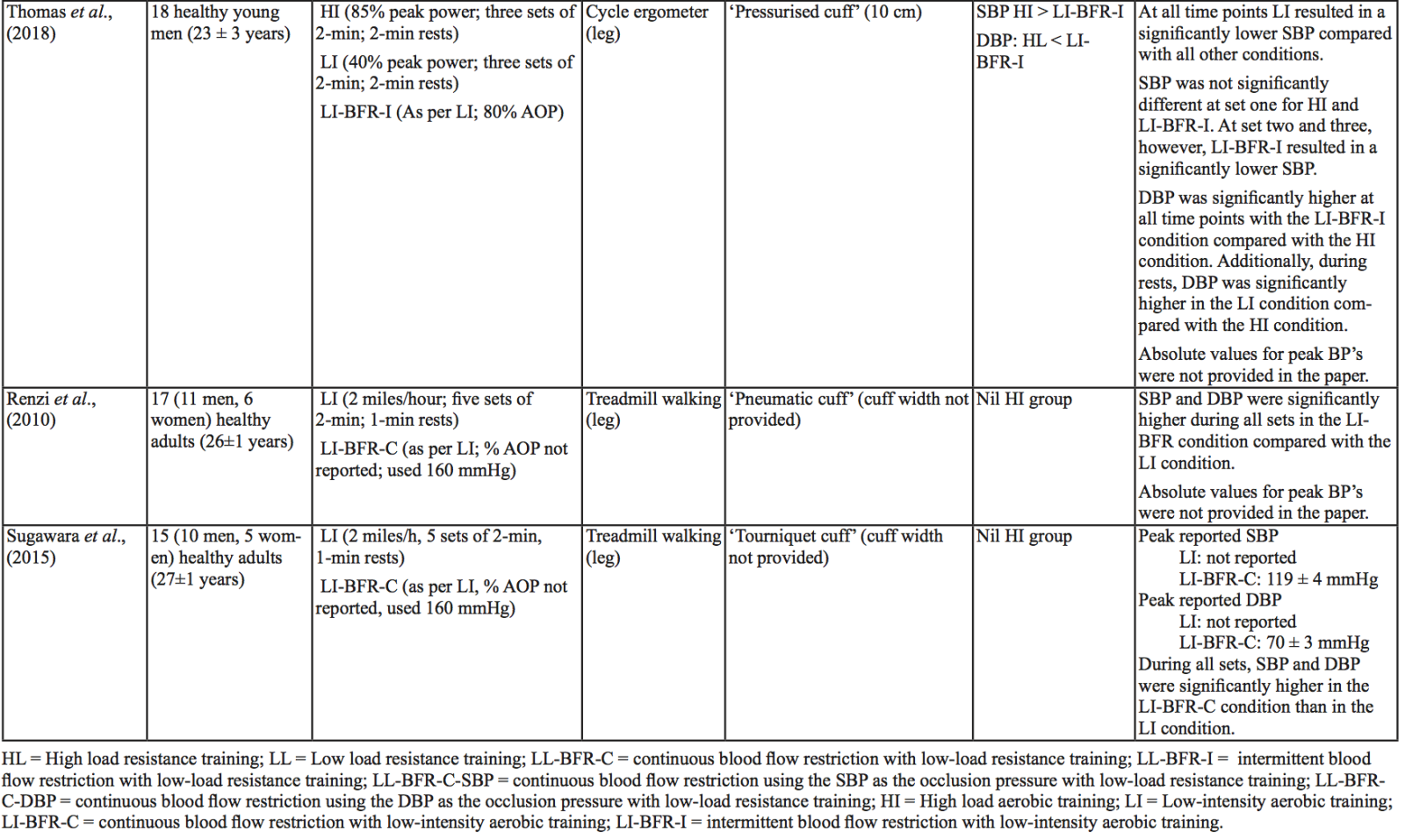
For aerobic exercise with BFR, the current literature indicates that the increase in both SBP and DBP is smaller than that for resistance training with BFR. Furthermore, the increase in SBP is smaller in magnitude than LL resistance training (without BFR), whereas changes in DBP are similar between conditions (May et al., 2017; Staunton et al., 2015). When comparing aerobic BFR exercise to other aerobic exercise conditions, low-intensity blood flow restriction (LI-BFR) tends to increase SBP to a similar (or slightly smaller) extent than does high-intensity (HI) exercise (although the differences are not statistically significant at all time points) (Thomas et al., 2018). Conversely, when comparing the relative change in BP for LI-BFR against other LI conditions, SBP is significantly greater in the LI-BFR condition (Renzi et al., 2010; Sugawara et al., 2015). DBP increases significantly more in LI-BFR in comparison to HI and LI conditions without BFR (Renzi et al., 2010; Sugawara et al., 2015; Thomas et al., 2018).
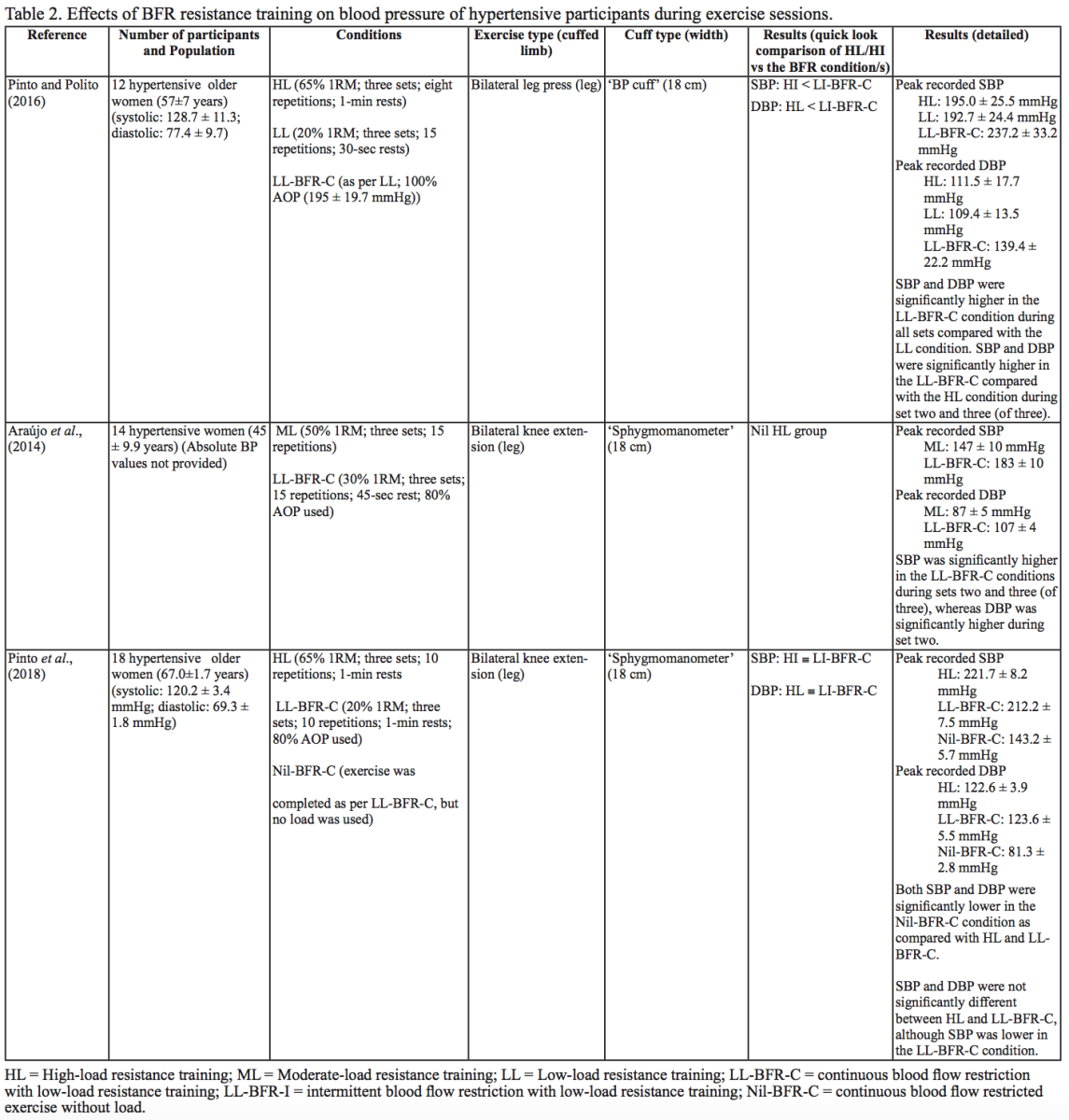
Interestingly, in both resistance and aerobic BFR exercise, age does not appear to influence the overall increase in SBP and DBP (Staunton et al., 2015; Vieira et al., 2013). However, initial BP appears to affect the peak BP that occurs (Staunton et al., 2015). The effects of initial, or resting BP on the magnitude of the BP alteration in aged populations are displayed in Tables 1 and 2. When comparing absolute data for healthy aged participants to that of absolute data for hypertensive aged participant (Araújo et al., 2014; Pinto et al., 2016; Pinto et al., 2018; Staunton et al., 2015), more studies would be required to draw a definitive conclusion. However, based on the presented data, it seems unlikely that age should be considered when determining the safety of the intervention from a hemodynamic perspective.
Based on the data presented in Table 1, no clear differences exist between LL-BFR-C and LL-BFR-I. Interestingly though, Figure 2 does show that LL-BFR-I results in a lower SBP as compared with HL and LL-BFR-C conditions, whereas DBP is only lower in comparison with the LL-BFR-C condition. The lack of any differences observed in the studies detailed in Table 1 and the subsequently conflicting results presented in Figure 2 may reflect variation in the protocols that were used between studies in Table 1 and those studies that did (or did not) report absolute BP values.
BP appears to be modulated differently for SBP and DBP in hypertensive participants as compared with normotensive participants (Table 2). As previously discussed, normotensive participants tend to exhibit a similar increase in SBP with HL and LL-BFR conditions. However, in hypertensive participants, there is a trend toward a higher SBP in the LL-BFR condition compared with HL and moderate load (ML; not reported in normotensive studies) conditions (Araújo et al., 2014; Pinto et al., 2016). Similar to normotensive participants, hypertensive participants also tend to have a larger increase in DBP during the session with LL-BFR compared to HL and ML conditions (Araújo et al., 2014; Pinto et al., 2016). One study reported that SBP and DBP were significantly different between HL and LL-BFR. Although not statistically different, this study reported that SBP was lower in the LL-BFR condition by about 9 mmHg (Pinto et al., 2018).
Variables that influence SBP and DBP increase during BFR exercise sessions
There are several variables that could alter the BP response during a BFR exercise session, including cuff width and percentage of arterial occlusion pressure (% AOP), with AOP defined as the pressure at which the arterial blood flow is completely occluded. Cuff width and % AOP are highly interrelated (Mattocks et al., 2018). When using the same cuff pressure with two different width cuffs, the wider cuff occludes more blood flow to the limb compared with the narrower cuff (Jessee et al., 2016; Weatherholt et al., 2019). This means that the wider cuff induces a higher % AOP than a narrower cuff inflated to the same pressure (Mattocks et al., 2018). A wider cuff (e.g., 13.5 cm) inflated to the same pressure as a narrow cuff (e.g., 5 cm) leads to significantly higher SBP and DBP during exercise in a healthy population (Rossow et al., 2012). By contrast, a study investigating SBP changes in an obese population with different % AOP (same cuff width) reported that increasing % AOP leads to a significant increase in SBP at the same aerobic workload (Karabulut et al., 2017). Similarly, Downs et al., (2014) observed a significantly lower SBP and DBP with a comparatively lower % AOP (Downs et al., 2014).
Other variables may also influence BP during a BFR exercise session. Minimal literature exists on the relative difference in BP between upper body versus lower body BFR exercise, or between intermittent versus continuous pressure. Nevertheless, there are some indications that there may be differences (although non-significant) in how these variables affect BP. Vilaça-Alves et al., (2016) reported that immediately after exercise, upper limb BFR resistance exercise resulted in a higher SBP and DBP compared with a lower limb BFR resistance exercise condition (Vilaça-Alves et al., 2016). Rodrigues Neto et al., (2016) investigated the effects of intermittent and continuous BFR training, as well as a HL condition, on SBP and DBP immediately after exercise (Rodrigues Neto et al., 2016). They found that SBP and DBP were lower in the intermittent condition compared with the continuous BFR and HL conditions. Further studies are needed to clarify how these (and other) variables influence SBP and DBP alterations during exercise sessions. Based on the current literature, it appears that intermittent BFR of the lower limb, resulting in a lower % AOP, may result in smaller BP changes. Understanding which variables influence the safety and efficacy of exercise interventions such as BFR is particularly important before considering using such interventions in at-risk populations.
BFR training and clotting implications
In addition to BP, the risk of thrombosis is also important to consider when using BFR as an adjunct to exercise training, because most strokes are caused by a thrombus becoming lodged in the brain’s vasculature. There are three main factors affecting thrombus formation, including stasis of blood, changes/damage to the vessel wall, and increases in clotting factors. Together, these factors are known as Virchow’s triad (Esmon, 2009). These three factors are relevant considerations for the safety of BFR, especially with regard to thrombus formation. BFR is used to alter blood flow. Blood vessels may be damaged due to the pressure of the cuff and altered hemodynamics. Beyond knowledge of Virchow’s triad, it is important to understand the molecular process of clotting to fully assess the implications of BFR on thrombus formation.
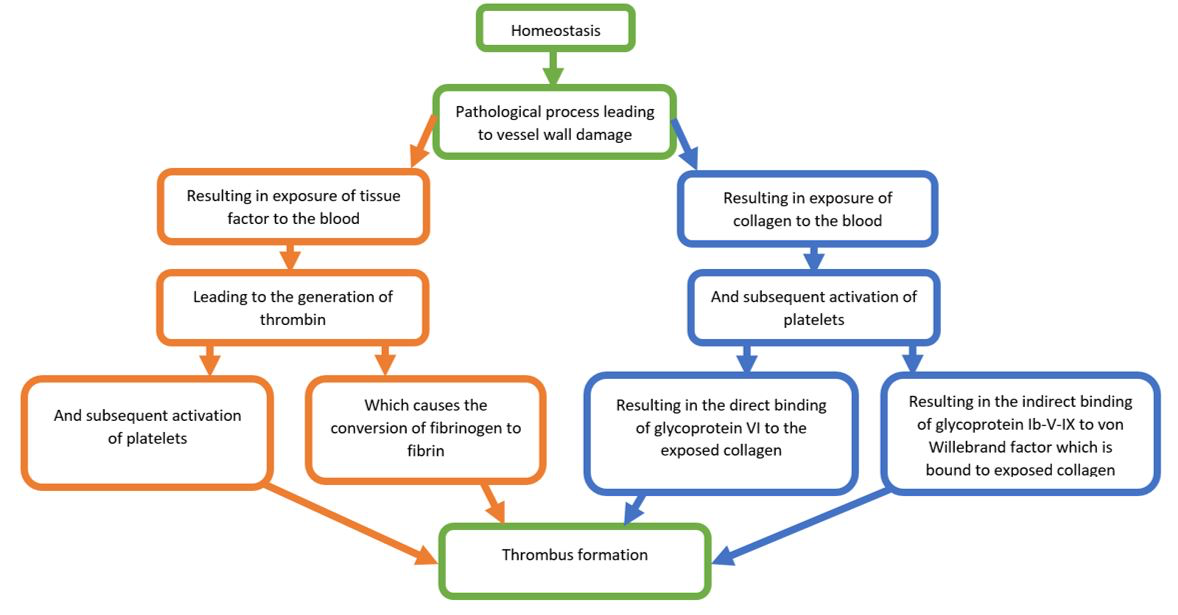
A review paper by Furie et al., (2008) clearly and succinctly details the relevant literature with regard to thrombus formation (Furie et al., 2008). Figure 3 provides a simplified overview of the paper’s main points. When the internal integrity of a blood vessel is disrupted, collagen within the basement membrane of the vessel wall, and tissue factor within the medial/adventitial layers, are exposed to blood. This leads to the accumulation and activation of platelets and the generation of thrombin, which converts soluble fibrinogen to insoluble fibrin. Platelet aggregation mainly occurs when platelet-bound glycoprotein VI and glycoprotein Ib-V-IX bind directly to collagen, or indirectly to von Willebrand factor bound to collagen, respectively. Platelet integrin α2β1 also plays a role in the aggregation of platelets to collagen. Although tissue factor does not directly cause thrombus formation at a site of injury, it does increase thrombin through a cascade of protein-protein interactions. In turn, thrombus formation stimulates platelets to release platelet-activating molecules, which occurs through a conformational change in integrin αIIbβ3 and subsequent fibrinogen and von Willebrand factor interactions (Furie et al., 2008).
In a new window | Download PPT
Figure 3: Simplified flow chart of the coagulation cascade following vessel wall damage. Items in green represent common elements of the collagen and tissue factor pathways. Items in orange represent elements from the tissue factor pathway, while items in blue represent elements in the collagen pathway.
Very few studies have investigated how BFR influences vascular endothelial damage. To date there are mixed results regarding the effect of BFR, either passive or with exercise, on endothelial injury (Jenkins et al., 2013; Shimizu et al., 2016). In a systematic review by da Cunha Nascimento et al., (2019) all studies that were cited indicated that in response to acute or chronic BFR exercise, (i) prothrombin time did not change, (ii) prothrombotic factors did not increase, and (iii) thrombolytic factors either increased or did not change significantly (da Cunha Nascimento et al., 2019). However, these findings should be interpreted with caution, because no solid conclusions can be drawn due to the small number of identified studies, and the poor quality of the study methodologies. Although not conclusive, these findings suggest that BFR may not have significant negative implications for thrombus formation.
With the use of BFR, few cases of thrombus formation have been reported. In a review of the literature, Loenneke et al., (2011) found only one study that reported thrombotic events resulting from BFR exercise (Loenneke et al., 2011). In this study (Nakajima et al., 2006), the incidence of thrombotic events during BFR training sessions was lower (0.055%) than that of the general population (~0.2% to 0.26%) (Loenneke et al., 2011; Nakajima et al., 2006). A more recent study identified no cases of thrombotic events in a diverse population (clinical and healthy) of 12,642 people who performed BFR training (Yasuda et al., 2017). Although these are encouraging results, further research should be conducted to confirm these findings.
Safety of BFR training in a stroke population
BFR training has the potential to improve muscular strength and size, cardiovascular fitness, walking speed, and functional scores, and may also reduce the muscle wastage in healthy populations (Conceição et al., 2019; Cook et al., 2017). Based on the evidence provided in this review, for all healthy young and elderly participants undertaking resistance or aerobic BFR exercise, BP does not increase above the recommended maximal BP of 250/115 mmHg (American College of Sports Medicine, 2018). Additionally, smaller BP increases occur in response to aerobic BFR exercise. In regard to a hypertensive population, all three papers reported that participants exceeded the safe BP recommended by the ACSM for hypertensive participants in BFR resistance exercise conditions, and two papers identified a similarly inappropriate BP in the HL (65% 1 RM) condition. However, if the ESSA position statement on hypertension is followed, exercise did not result in an inappropriate BP increase in the BFR conditions, or in any of the other exercise conditions (American College of Sports Medicine, 2018; Sharman et al., 2019). Taken together, it is clear that BFR exercise is unlikely to raise BP above safe levels, or to increase the risk of clotting in healthy young and elderly participants. By contrast, in a hypertensive population, BFR resistance exercise could be considered a less appropriate training modality due to the high BP’s that can be reached (although it can be identified as safe depending on the guidelines that are used). However, based on the limited number of studies currently available, it appears that BP responses are lower in response to aerobic exercise with BFR compared with resistance exercise with BFR. Aerobic exercise with BFR exercise may therefore be more appropriate for a hypertensive population, but further research into this area is required. This evidence implies that in a normotensive stroke population, which is roughly 25% of the total stroke population, both resistance and aerobic exercise with BFR may be an appropriate training modality. In a stroke population that is hypertensive, however, aerobic exercise with BFR may be more suitable than resistance exercise with BFR. Overall, clinician discretion and appropriate supervision is required on a case-by-case basis to determine the suitability of any exercise prescription for individual participants, and BFR training is no exception.
In conclusion, BFR training may be an appropriate training modality in some stroke populations. However, more research is needed to investigate the factors that affect changes in BP in response to BFR training in stroke populations. This information will ultimately help to inform the safety and benefits of this training modality in clinical populations.
Acknowledgments
The authors have no acknowledgment to make.
Funding
There are no funding sources to declare.
Conflicts of interest and any disclosures
The authors have no conflicts of interest or disclosures to make. Dr. Gregory Bix is both an author of this manuscript and an Editorial Board Member of Conditioning Medicine. Dr. Bix did not participate, at any level, in the editorial review of this manuscript.
References
Luke J. Schmidt1
1Queensland University of Technology, Tissue Repair and Translational Physiology Group, School of Biomedical Sciences, Faculty of Health, Brisbane, Queensland, Australia.
Ann M. Stowe2,3
2Department of Neurology, University of Kentucky, Lexington, Kentucky, USA. 3Department of Neurology and Neurotherapeutics, Peter O’Donnell Jr. Brain Institute, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Gregory J. Bix4,5
4Clinical Neuroscience Research Center, Departments of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, Louisiana, USA. 5Tulane Brain Institute, Tulane University, New Orleans, Louisiana, USA.
Jonathan M. Peake1
1Queensland University of Technology, Tissue Repair and Translational Physiology Group, School of Biomedical Sciences, Faculty of Health, Brisbane, Queensland, Australia.
Tony J. Parker1
1Queensland University of Technology, Tissue Repair and Translational Physiology Group, School of Biomedical Sciences, Faculty of Health, Brisbane, Queensland, Australia.
Corresponding author:
Luke Schmidt
Email: l2.schmidt@qut.edu.au

In a new window | Download PPT
Figure 1: Hypothetical mechanisms behind the exercise pressor reflex associated with BFR that may explain an increase in BP beyond what would be expected by a similar load without BFR.

In a new window | Download PPT
Figure 2: Absolute BP values compiled from resistance and aerobic exercise trials (mean ± SD) (Bazgir et al., 2016; Downs et al., 2014; May et al., 2017; Picón et al., 2018; Staunton et al., 2015; Sugawara et al., 2015; Takano et al., 2005). This figure presents the means of all the peak absolute values for the HL/HI and BFR conditions presented in Table 1. When there was only one study that provided absolute values for an exercise condition, there are no SD bars included. Additionally, when no absolute data were presented in any of the studies contained in Table 1 for a particular exercise condition, there were no data included in this figure. HI = High-intensity aerobic training; HL = High load resistance training; LL-BFR-C = continuous blood flow restriction with low-load resistance training; LL-BFR-I = intermittent blood flow restriction with low-load resistance training; LI-BFR-C = continuous blood flow restriction with low-intensity aerobic training; LI-BFR-I = intermittent blood flow restriction with low-intensity aerobic training.

In a new window | Download PPT
Figure 3: Simplified flow chart of the coagulation cascade following vessel wall damage. Items in green represent common elements of the collagen and tissue factor pathways. Items in orange represent elements from the tissue factor pathway, while items in blue represent elements in the collagen pathway.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7029 | 110 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA