International bi-monthly journal of cell signaling, tissue protection, and translational research.
Remote Ischemic Conditioning induced miRNA responses in humans
Melanie Lunding1, Rolf Ankerlund Blauenfeldt2,3, Jesper Just1,4, Kim Ryun Drasbek1,5
Author Affiliations


- 1Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, DK-8000, Aarhus, Denmark.
- 2Department of Neurology, Aarhus University Hospital, DK-8200, Aarhus, Denmark.
- 3Department of Clinical Medicine, Aarhus University, DK-8200, Aarhus, Denmark.
- 4Department of Molecular Medicine, Aarhus University Hospital, DK-8200, Aarhus, Denmark.
- 5Sino-Danish Center for Education and Research, University of Chinese Academy Sciences, Beijing, China/Aarhus, Denmark.
Abstract
Remote ischemic conditioning (RIC) is a non-invasive, non-pharmacological treatment where short cycles of reversible ischemia and reperfusion are applied to a tissue, organ, or limb to attenuate ischemia reperfusion injury (IRI) in a remote organ. The underlying mechanism is believed to involve humoral and neurological pathways not yet fully understood. Short strands of non-coding RNA, miRNAs, released and carried in the blood stream have been suggested as a pivotal humoral factor for protection. This review sought to assess which miRNAs may mediate the protective effect of RIC in humans. For this purpose, 734 studies were screened, out of which 721 were excluded while the remaining 13 formed the basis of this review. Enrichment analysis was conducted to analyze target genes, diseases, and pathways for the most frequently and consistently up- or downregulated miRNAs. The miRNA response profile to RIC differed between studies. This may be attributed to small sample sizes and interstudy differences. In a minimum of 2 studies, miR-16-5p, miR-21, miR-24, miR-144, and miR-150 were significantly upregulated, whereas miR-1 was downregulated. Interestingly, ischemic diseases and pathways related to cellular senescence were significantly enriched with these miRNAs. Inhibition of cellular senescence may be a pivotal mechanism by which RIC can attenuate IRI. However, knowledge of miRNAs induced by RIC is limited and large-scale randomized controlled trials are warranted.
Keywords: Remote ischemic conditioning, Ischemia, miRNA, Human studies
Abstract
Remote ischemic conditioning (RIC) is a non-invasive, non-pharmacological treatment where short cycles of reversible ischemia and reperfusion are applied to a tissue, organ, or limb to attenuate ischemia reperfusion injury (IRI) in a remote organ. The underlying mechanism is believed to involve humoral and neurological pathways not yet fully understood. Short strands of non-coding RNA, miRNAs, released and carried in the blood stream have been suggested as a pivotal humoral factor for protection. This review sought to assess which miRNAs may mediate the protective effect of RIC in humans. For this purpose, 734 studies were screened, out of which 721 were excluded while the remaining 13 formed the basis of this review. Enrichment analysis was conducted to analyze target genes, diseases, and pathways for the most frequently and consistently up- or downregulated miRNAs. The miRNA response profile to RIC differed between studies. This may be attributed to small sample sizes and interstudy differences. In a minimum of 2 studies, miR-16-5p, miR-21, miR-24, miR-144, and miR-150 were significantly upregulated, whereas miR-1 was downregulated. Interestingly, ischemic diseases and pathways related to cellular senescence were significantly enriched with these miRNAs. Inhibition of cellular senescence may be a pivotal mechanism by which RIC can attenuate IRI. However, knowledge of miRNAs induced by RIC is limited and large-scale randomized controlled trials are warranted.
Keywords: Remote ischemic conditioning, Ischemia, miRNA, Human studies
Introduction
Biomarkers of the remote ischemic conditioning (RIC) response are needed to improve our understanding of the underlying molecular mechanisms that activate protective biological pathways, and to understand the difference between RIC responders and non-responders, to facilitate its translation into the clinic.
The protective effects of RIC are thought to be mediated by both humoral and neurological pathways that may to some extent be interdependent (Pickard et al., 2016). Several humoral factors are in part secreted into the extracellular space as cargoes in ectosomes, exosomes, and other small vesicles that all fall within the term extracellular vesicles (EVs). These EVs are nanometer- to micrometer-sized lipid bilayer-coated particles able to enter the circulation and facilitate both local and distant cell to cell communication (Rufino-Ramos et al., 2017). Several RIC induced humoral factors have been suggested as reviewed by Tsibulnikov et al. (2019) including microscopic ribonucleic acids (miRNA) (Li et al., 2014), which are of special interest in this review. MiRNAs are small noncoding RNA strands of 18-24 nucleotides that regulate gene expression and protein translation by silencing messenger RNA (mRNA) molecules (Rehwinkel et al., 2005; Wakiyama et al., 2007) (Figure 1). Interestingly, miRNAs have the ability to target several different mRNAs, possibly by affecting multiple pathways simultaneously in a complex way. With increasing evidence supporting the hypothesis that the effects of RIC are, in part, mediated by miRNAs released to the blood that then attenuates ischemic damage through multiple pathways (Chen et al., 2018; Hess et al., 2015), we here review the literature to pinpoint common miRNAs, across different diseases and in healthy individuals, which may mediate the protective effect of RIC in humans. This analysis could provide directions for future research.
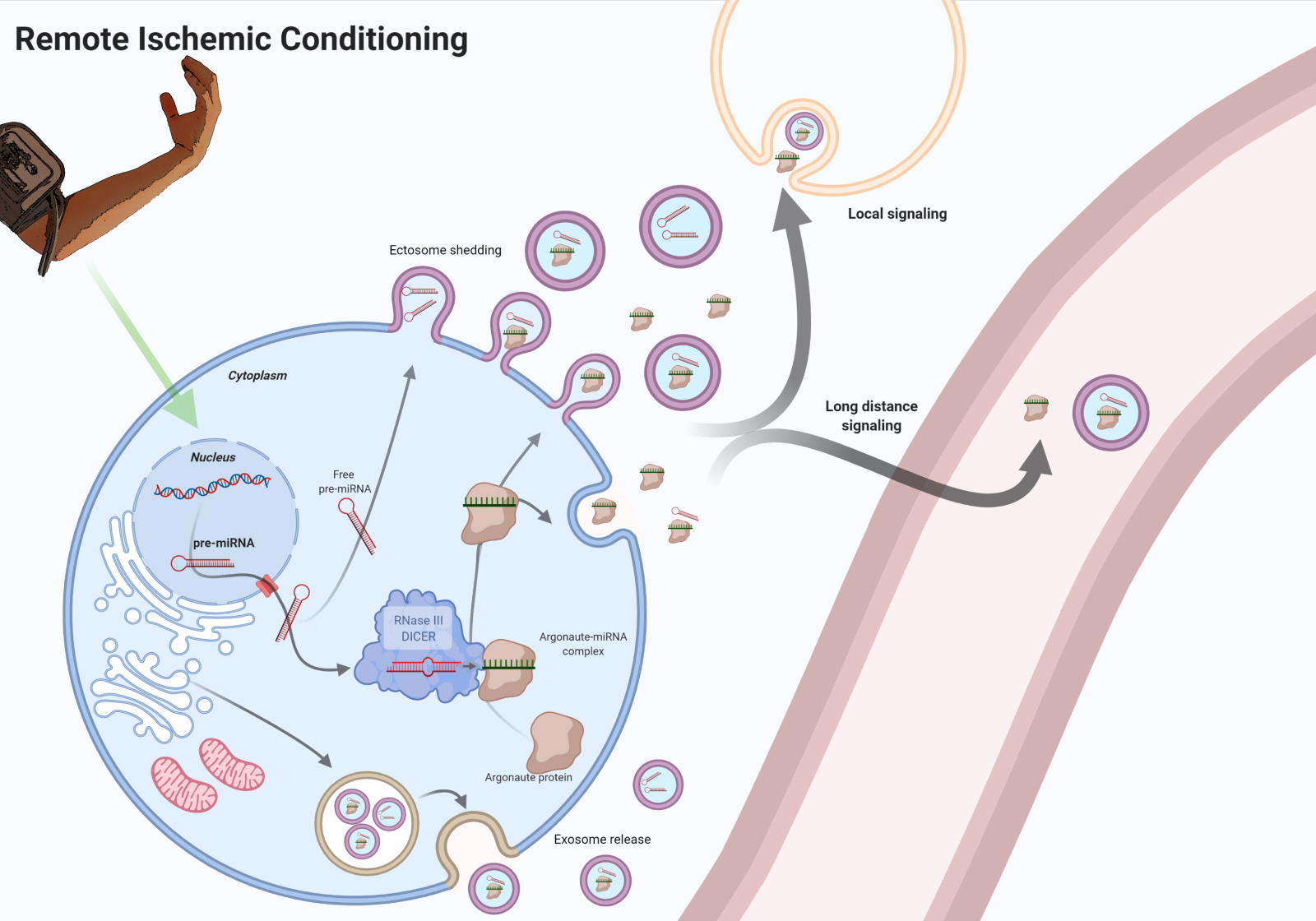
In a new window | Download PPT
Figure 1: The protective humoral mediators of RIC are released from the affected cells into the circulation, including miRNAs in EVs and protein complexes. RIC of an arm releases protective signals in the blood. These molecular signals could be transmitted through the release of miRNA from cells that are affected by RIC. MiRNAs are transcribed as primary miRNAs (pri-miRNAs) (Lee Y, 2004) mostly from introns (Lin SL, 2006) in the nucleus. The 5’ and 3’ extension of pri-miRNA are removed to form a precursor miRNA (pre-miRNA) (Denli AM, 2004), which is transported to the cytoplasm via a nuclear export factor (Yi R, 2003). Here, the RNase III enzyme, Dicer, binds and cleaves the pre-miRNA yielding a mature miRNA duplex (Hutvágner G, 2001) consisting of a 5’ strand and a 3’ strand. After binding of Argonaute, one of the strands in the miRNA duplex is retained as the seed sequence while the other passenger strand is degraded. As part of the RISC (RNA-induced silencing complex), the Argonaute-miRNA complex binds mRNA targets with complementary sequences to the seed strand, resulting in reduced levels of the target protein via inhibition of protein translation or mRNA degradation. (Rand TA, 2005). Created with BioRender.com.
Objectives
Our objective was to review the effect of RIC on the miRNA profile in humans and further provide functional insight on the most frequently regulated miRNAs. Finally, we touch upon ongoing clinical trials and provide directions for future research.
Methods
Literature search
This narrative review was based on a systematic literature search performed on PubMed 08.07.2021 using the search string (post-conditioning OR per-conditioning OR pre-conditioning OR preconditioning OR perconditioning OR postconditioning OR ischemic conditioning OR RIC) AND (shRNA OR miRNA OR miR OR microRNA OR snoRNA OR pirna OR non coding rna OR non coding rnas OR ncRNA). The search had no limitations on year of publication. Studies investigating the effect of RIC on miRNA levels in humans were included and underwent full text screening for eligibility. Exclusion criteria were 1) Reviews/Systemic reviews/Meta-analyses/Case reports/Conference abstracts/Protocol papers and 2) Animal/Tissue/Cell studies, and 3) Interventions other than RIC. Two reviewers independently performed both the full text screen and the title and abstract screen ensuring each rejection of an article was validated by a second reviewer. The systematic review was performed using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). The studies were then divided into two groups. The first group included studies where RIC was applied on healthy participants. The second group included studies where RIC was applied on patients. The latter was further divided into pre-, per-, and postconditioning studies. Extracted data included: author, year of publication, study population, RIC protocol, target organ, sample type, miRNA purification and quantification method, time between the RIC procedure and sample collection, miRNA results, and primary outcome results. The p-values of miRNA-fold changes were used as a measure of miRNA responses to RIC. P-values < 0.05 were accepted as statistically significant.
Pathway enrichment analysis
MiRNAs with a statistically significant p-value and consistent up- or downregulation in a minimum of 2 of the 13 studies were further investigated (miR-1, miR-16-5p, miR-21, miR-144, miR-24, and miR-150). These miRNAs were then used as input for gene target prediction and enrichment analysis to investigate the miRNAs putative protective effects in the RIC response. For this, the miRNet platform (Xia lab, McGill University, Montreal, Quebec, Canada) (Chang et al., 2020) was applied. This platform utilizes well annotated, high-quality miRNA-interaction-data obtained from experimental technologies (Fan et al., 2016). Not all articles clearly stated whether the 5’ or 3’ miRNA strand was measured, and some used primers that quantified both. Consequently, miRNet was set to include both the 5’ and 3’ strand of each miRNA (except for miR-16-5p where strand-information was available). In addition to the gene target prediction, miRNA disease associations and biological processes were analyzed using the Gene Ontology Biological Process (GOBP) Classes (Ashburner et al., 2000; "The Gene Ontology resource: enriching a GOld mine," 2021). For REACTOME (Gillespie et al., 2022) pathway analysis, miRNA target genes, shared by a minimum of 2 miRNAs, were used as input. An adjusted p-value of < 0.05 was accepted as statistically significant.
Results
The initial PubMed search yielded a total of 734 studies for title and abstract screening using the search terms as described above. Out of these, 718 were excluded during the abstract and title screen for not addressing RIC induced miRNA responses in humans, leaving 16 studies for full-text screening. Among these, two studies were excluded for analyzing differentially expressed genes (DEG) or long noncoding RNA (lncRNA) rather than miRNA. A third study was excluded for analyzing transient ischemic attack (natural ischemic preconditioning) rather than RIC. Finally, 13 studies were included in the review (Figure 2). The included studies analyzed miRNA expression in 948 participants (out of 1150 participants) spanning different disease entities (acute myocardial infarction, chronic heart diseases, acute kidney injury, and inflammatory bowel disease) and healthy subjects. A total of three studies analyzed healthy participants. The remaining ten studies analyzed RIC patients. Out of these, five focused on pre-conditioning, three on per-conditioning and two on post-conditioning. Only one study analyzed patients with a non-cardiac disease, which was active ulcerative colitis.
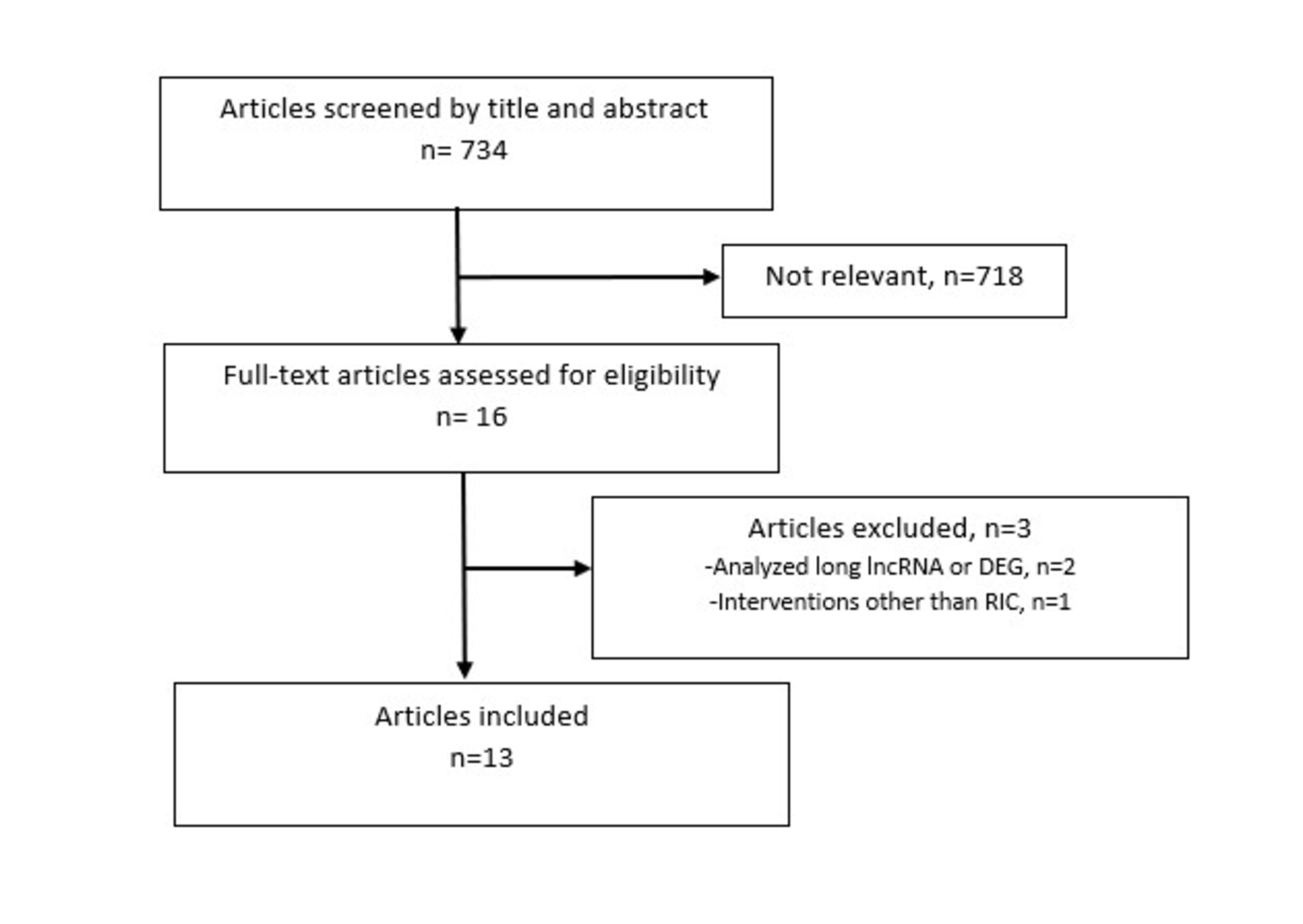
In a new window | Download PPT
Figure 2: Schematic of the inclusion and exclusion of articles for the review. Inclusion criteria: 1) RIC protocol, 2) MiRNA quantification, and 3) Human study. Exclusion criteria: 1) Reviews/Systemic reviews/Meta-analyses/Case reports/Conference abstracts/Protocol papers, 2) Animal/tissue/cell studies, and 3) Interventions other than RIC.
RIC induced miRNA responses in healthy participants
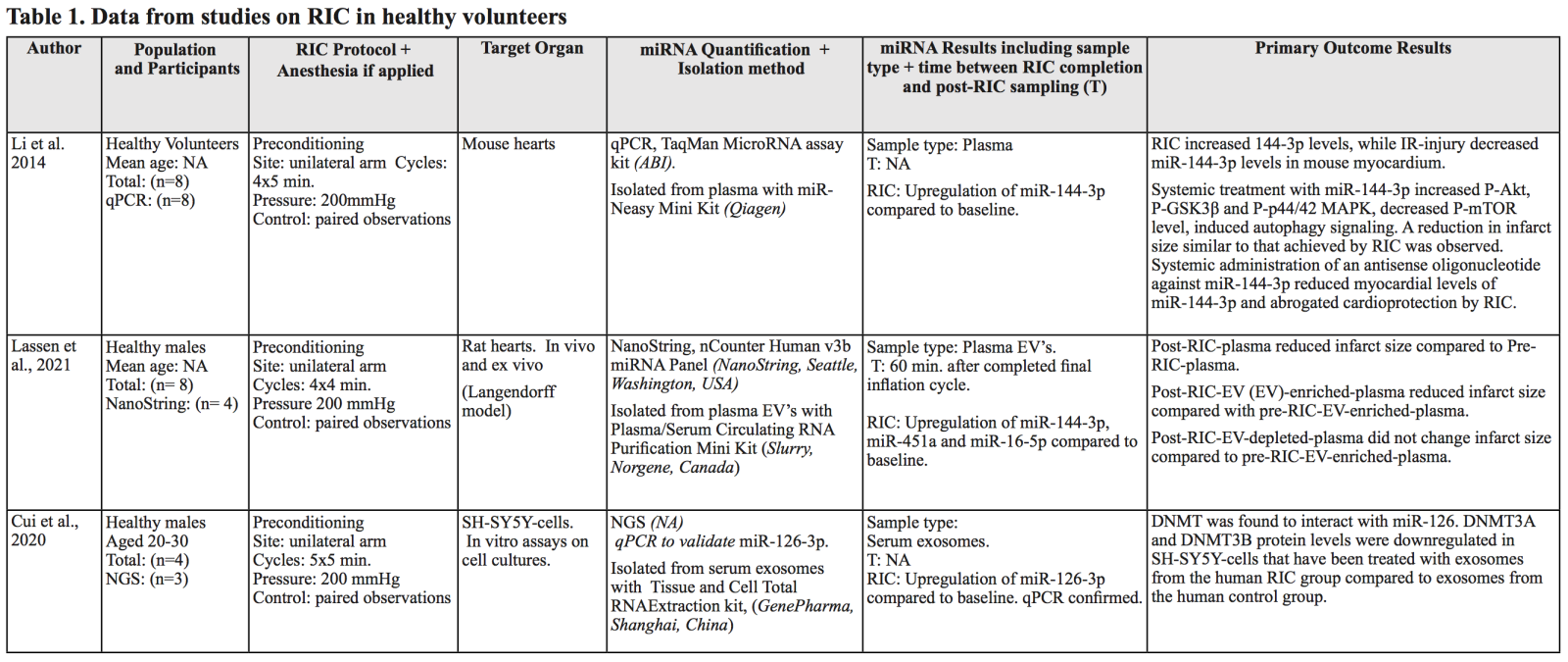
The miRNA response of remote ischemic preconditioning has been studied in healthy participants (Table 1) using a RIC-protocol with four or five cycles of 5/5 minute ischemia/reperfusion (I/R). First, Li et al. (2014) showed that miR-144-3p was upregulated 15 minutes after RIC in human volunteers undergoing four cycles of 5/5 minute I/R as well as in RIC treated C57/BL6 male mice (8-10 weeks old). In addition, they found miR-144-3p to be cardioprotective in a mouse Langendorff infarct/reperfusion model to a similar extent as RIC itself. Furthermore, increased levels of miR-144-3p were found in both EVs and in Argonaute complexes in plasma. In a recent study, Lassen et al. (2021) studied the EV miRNA response one hour after four cycles of RIC in healthy young males using NanoString technology (nCounter Human v3b miRNA panel). They also observed a significant upregulation of miR-144-3p, in addition to miR-451a and miR-16-5p, in post-RIC plasma EVs. Furthermore, perfusing with an EV-enriched post-RIC plasma fraction in a rat heart Langendorff-model (250-280g male Sprague Dawley rats) lead to a significant reduction in infarct size following global ischemia while no effect was seen with pre-RIC EVs or EV-depleted post-RIC plasma. This reduction corresponded with the reduction seen when perfusing with unfractionated post-RIC plasma, suggesting that a substantial protective effect of RIC is transported by EVs. By applying five cycles of RIC on three healthy males, Cui et al. (2020) reported several miRNAs to be differentially expressed in EVs collected immediately after RIC using Next Generation Sequencing (NGS). Fifty of these miRNAs were presented in a heatmap without p-values. However, none of these correlated with the miRNAs found by Lassen et al. (2021). This could be attributed to the small sample size and the difference in blood sampling time point. Cui et al. (2020) solely focused on the effect of miR-126-3p and analyzed its effect in human neuroblastoma SH-SY5Y cells, which they found to have an impact on DNA methyltransferase 3 alpha and -beta (DNMT3A and DNMT3B) protein levels, which was also observed after incubation with the human post-RIC serum EVs.
RIC induced miRNA responses in patients
Pre-conditioning
The cardioprotective effect of remote ischemic preconditioning in patients undergoing elective isolated first-time coronary artery bypass graft (CABG) surgery was the focus of four studies using identical RIC protocols of three cycles of 5/5 minute I/R on the upper arm (Table 2). In a double blinded single center study, by Krogstad et al. (2015), RIC did not reduce the incidence of postoperative atrial fibrillation. Using a rigorous qRT-PCR protocol and the miRCURY LNA Universal RT miRNA PCR Human panel I and II covering 730 human miRNAs on randomly selected patients (n = 10 in each group), Krogstad et al. (2015) did not find significant differences in miRNA levels between RIC and sham patients or pre- and post-RIC (30 minutes) samples. Interestingly, using a similar protocol, but a different miRNA microarray, Frey et al. (2019) found different miRNA profiles at different timepoints in serum EVs from 7 CABG patients receiving either three cycles of 5/5 minute I/R on the upper arm in the RIC group (n = 3) or 30 minutes with an uninflated cuff in the sham group (n = 4). However, only a subgroup (7 out of 58 patients) was selected based on their EV concentration for the miRNA analysis. RIC patients with a large increase in EVs and sham patients with low EV counts were selected. Blood was drawn before RIC/sham and induction of anesthesia, and then again 5 and 60 minutes after RIC. Using the 384 TaqMan Human MicroArray Card, Array A, they found the largest number of differentially expressed miRNAs 5 minutes after RIC (24 miRNAs), while only 6 differentially expressed miRNAs were present 60 minutes after RIC, indicating that miRNA expression changes quickly, mirroring the current physiological state. Only miR-28, miR-320, miR-92a, and miR-21 were significantly upregulated at both five and 60 minutes after RIC. Unfortunately, a correlation between miRNA levels and the myocardial protection found in the RIC group was not performed.
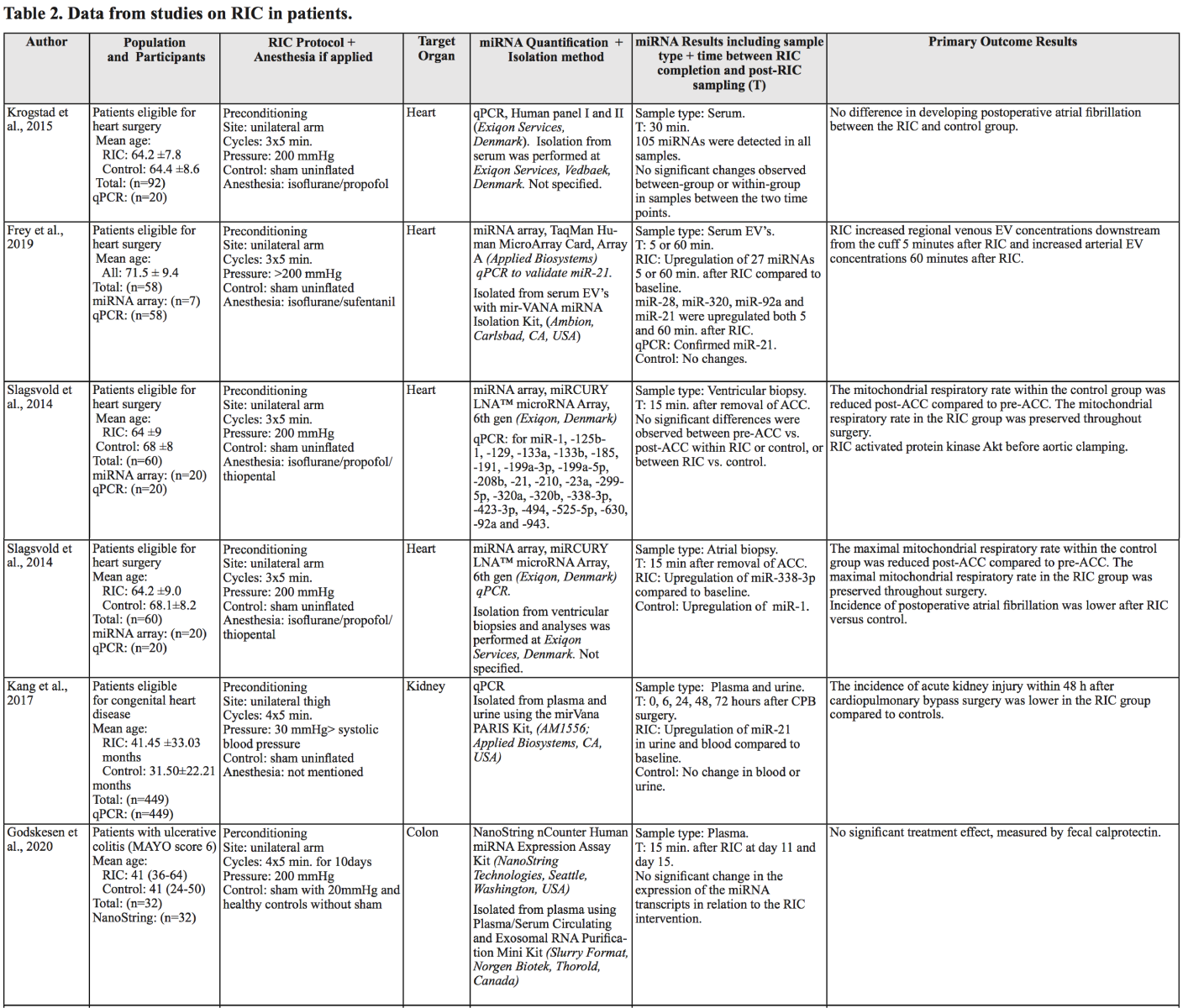
In 2014, Slagsvold et al. (2014a, 2014b) published two articles (Slagsvold, Moreira, et al., 2014; Slagsvold, Rognmo, et al., 2014) studying miRNA changes in tissue biopsies obtained from patients during surgery. These studies were, presumably, conducted on the same patient cohort. The difference between the studies, in relation to miRNA analysis, was the origin of tissue used for miRNA analysis. They quantified the miRNA levels in the right atrial appendage and ventricular biopsies in the first and second study respectively from a subset consisting of 10 RIC and 10 control patients for each study. Following labeling of all miRNAs in each sample, they were analyzed by hybridization on the miRCURY LNA microRNA Array (sixth generation) covering 1223 human mature miRNAs. Only the analysis of miRNA expression in the atrial appendage biopsies showed differences, with miR-338-3p being upregulated after aortic cross clamping (ACC) in the RIC group and miR-1 in the control group. In addition, atrial miR-133a and miR-133b were increased in both the control and the RIC group after ACC. The negative result of the second study was attributed to the short time interval between collection of pre- and post-ACC biopsies (35–70 minutes) and obstacles impeding the collection of ventricular biopsies. Even though they did not observe major differences in miRNA expression in myocardial tissue following RIC, in both studies, they observed beneficial effects of RIC in the CABG patients. This could be due to a delayed tissue miRNA response in contrast to the miRNA changes found in blood. Lastly, a fifth preconditioning study conducted by Kang et al. (2018) applied a RIC protocol with four cycles of 5/5 minute I/R on a lower limb 12h before elective cardiopulmonary bypass (CPB) surgery in 449 children with congenital heart disease. Blood and urine samples were collected before and after CPB in both RIC and a sham control group. They focused on miR-21, and they too, found it to be upregulated in plasma but also in urine compared to baseline in the RIC group using qPCR. Interestingly, the rate of acute kidney injury within 48h after CPB surgery in the RIC group was significantly lower than in the control group.
Per-conditioning
Three studies analyzed the miRNA profile after ischemic perconditioning (Table 2). Godskesen et al. (2020) was the only study to investigate RIC in a noncardiac-patient group as they randomized 22 patients with active ulcerative colitis to RIC or a sham protocol where the cuff pressure was 20 mmHg at inflation max. They applied a RIC protocol with four cycles of 5/5 minute I/R on the upper arm daily for 10 days. Plasma samples were taken at different timepoints: 1 day before RIC, day 1, 15 minutes after RIC, and at day 11. An additional ten healthy control (HC) plasma samples were obtained and used for comparison. They profiled 798 human miRNAs with the NanoString platform. No significant miRNA response to RIC was observed and treatment effect measured by fecal calprotectin was not significantly altered by RIC, which as the authors noted, may reflect how chronic inflammation affects the response to RIC.
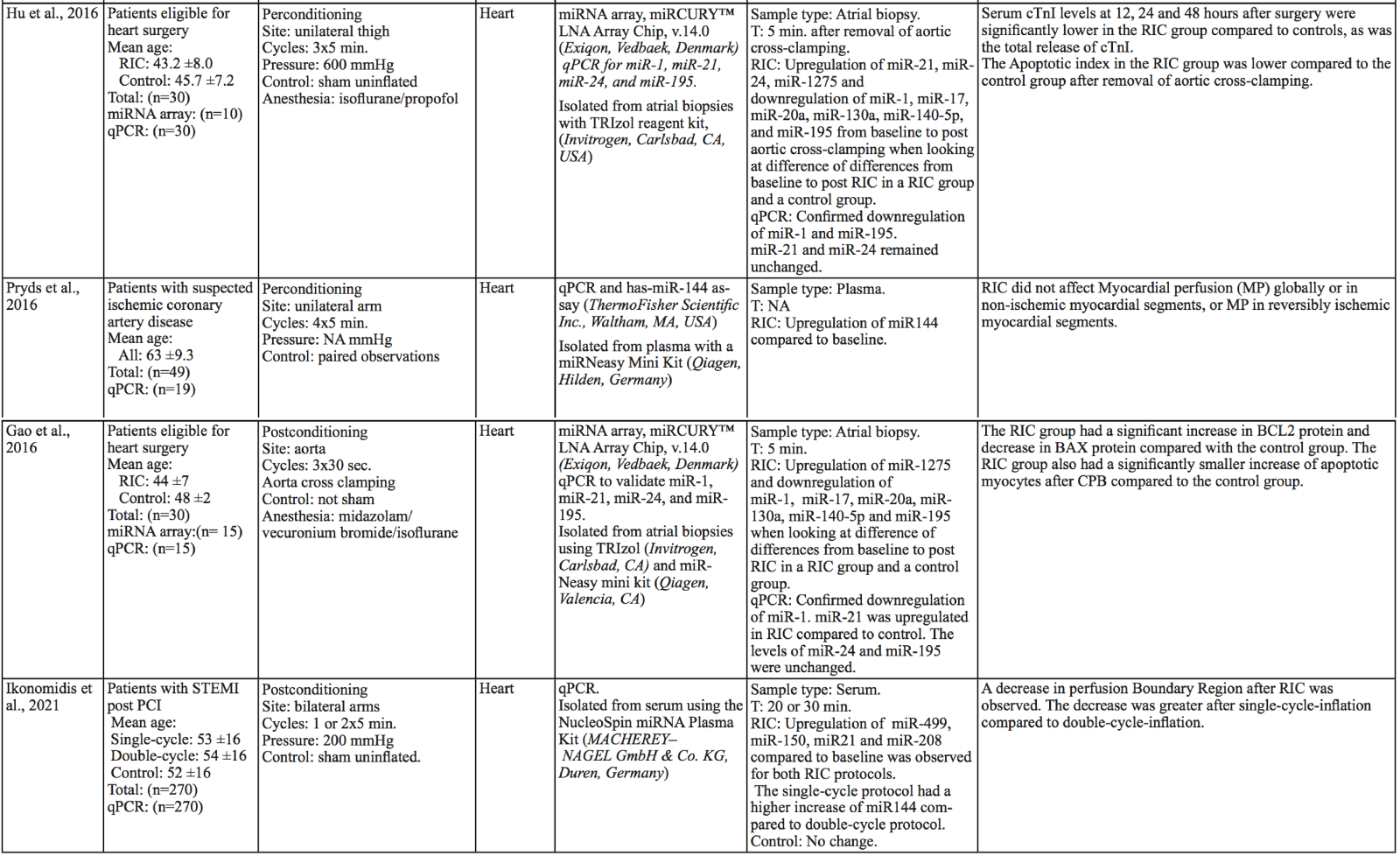
Hu et al. (2016) used a similar RIC protocol as already described in the preconditioning studies (three cycles of 5/5minute I/R) but applied it on a lower limb during aortic valve replacement surgery. They also analyzed right atrial appendage biopsies using a hybridization miRNA array (miRCURY LNA Array Chip v.14.0) on a subset of the included patients. Comparing five RIC and five sham patients they found miR-21, miR-24, and miR-1275 upregulated in the RIC group while miR-1, miR-17, miR-20a, miR-130a, miR-140-5p, and miR-195 were downregulated. In a qPCR validation analysis of miR-1, -21, -24, and -195, only miR-1 and miR-195 were significantly reduced in the RIC group. Unfortunately, they were not able to quantify the miR 3p or 5p subtypes that in many instances have different mRNA targets, and thus, different functional effects. They report a significant association between RIC and reduced cardiac troponin I levels and apoptotic index but did not directly link these to miRNA changes.
As a follow-up on the miR-144-3p discovery in healthy subjects, Pryds et al. (2018) quantified this miRNA in 19 patients with a suspected diagnosis of stable ischemic coronary artery disease, who were treated with four cycles of 5/5 minute I/R on the upper arm. This was done in a prospective outcome-assessor-blinded study based on paired observations before and after RIC, with blood sampling before and after RIC. Using targeted TagMan qPCR on plasma samples, they found an increase in miR-144-3p after RIC compared to baseline, however RIC did not affect myocardial perfusion and miR-144-3p was not correlated with post-RIC myocardial perfusion changes.
Post-conditioning
Only two studies on post conditioning were included (Table 2). In contrast to the other studies, Gao et al. (2016) applied ischemic conditioning locally instead of on a remote limb. This was performed in a double blinded randomized study including 30 rheumatic heart valve disease patients. Thirty seconds after aortic cross declamping, at the end of aortic valve replacement surgery, they performed three cycles of 30 seconds aorta clamping followed by 30 seconds declamping, resulting in global myocardial ischemic conditioning. A microarray analysis on right atrial muscle biopsies showed changes in miRNAs following the surgical procedure, and interestingly, also showed differences when comparing post-conditioning and -control samples. qPCR validation showed significant atrial miR-1 downregulation and miR-21 upregulation following RIC, while miR-195 and miR-24 could not be verified.
In a recent study, Ikonomidis et al. (2021) showed that one cycle of 5/5 minute I/R on both arms (RIC1) had a greater decrease in perfusion boundary region than two cycles (RIC2). Two hundred seventy patients with STEMI (ST-elevation myocardial infarction) after primary percutaneous coronary intervention (PCI) were randomized to RIC1, RIC2, or sham (no pressure cuff inflation). They analyzed serum miRNA changes 40 minutes after intervention using qPCR. All analyzed miRNAs (21, 144, 150, 208, and 499) were increased after RIC. Intriguingly, miR-144 levels were higher following one RIC cycle compared to two cycles. Furthermore, Serum miR-144 and carotid-femoral pulse wave velocity changes post-RIC were correlated and associated with left ventricular end-systolic volume reduction at 12-month follow-up in the RIC1 group.
RIC induced miRNA pathways
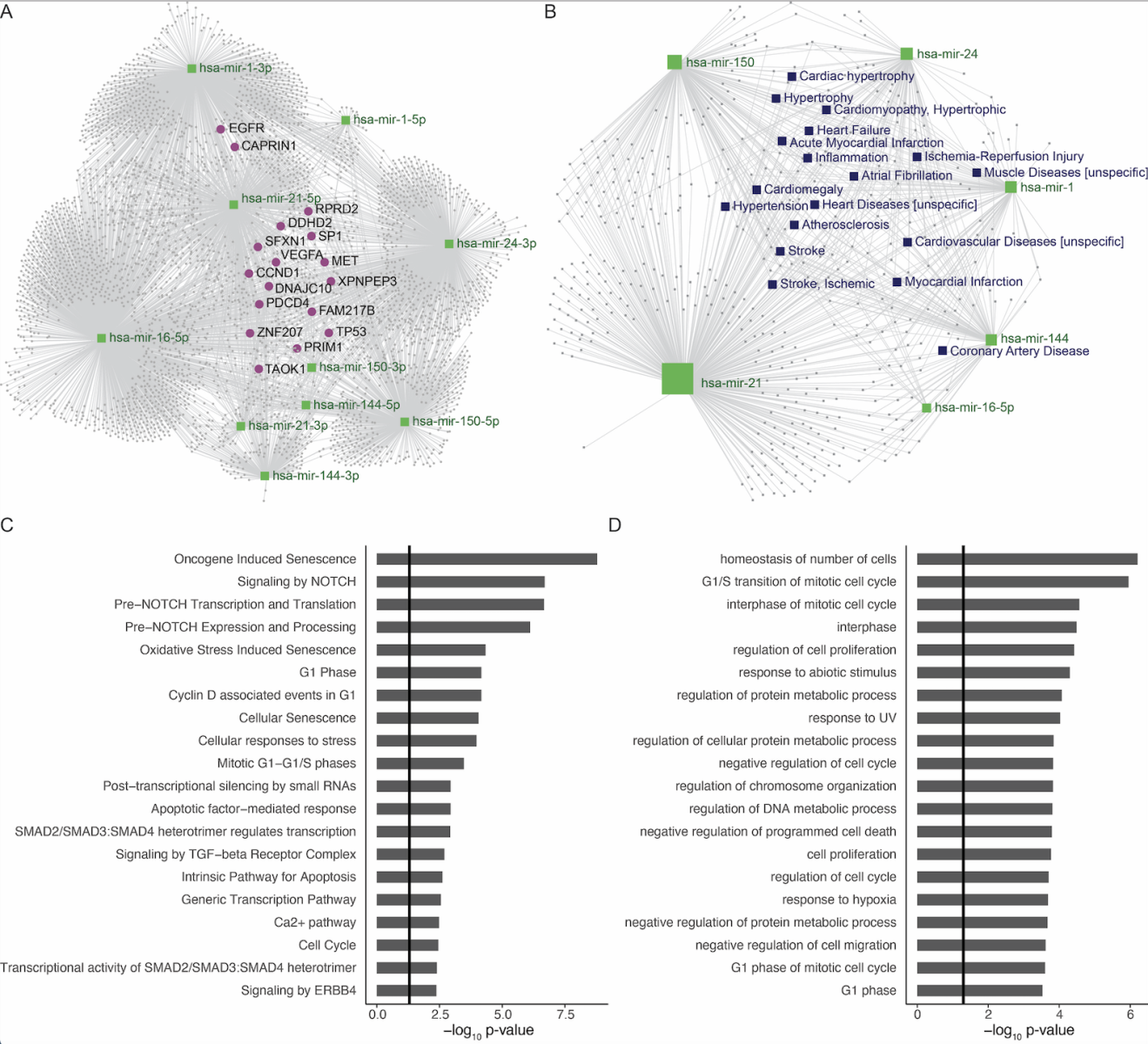
Among the miRNAs studied in the 13 articles, a subset of six miRNAs were statistically significant, and consistently up- or downregulated in a minimum of two studies: miR-16-5p, miR-21, miR-24, miR-144, and miR-150 were significantly increased after RIC compared to before RIC, whereas miR-1 was significantly decreased. Experimentally validated gene targets of all miRNAs were identified, and genes targeted by three or more of our selected miRNAs were highlighted. A number of these genes were associated to proliferation and cell cycle control (EGFR, SP1, TP53) and mitosis (MET, ZNF207, TOAK1) (Figure 3a). As most of the selected miRNAs were upregulated, we speculate that targeting of these genes will negatively impact cell proliferation. Furthermore, ischemia reperfusion pathologies like acute myocardial infarction and ischemic stroke were found to be among significantly enriched diseases (Figure 3b). In line with the target genes identified above and decreased proliferation, the top 20 enriched pathways (REACTOME) and biological processes (GOBP) for these miRNAs’ gene targets were in particular related to mitotic cell cycle, proliferation, and cellular senescence (Figure 3c and 3d).
In a new window | Download PPT
Figure 3: Gene targets, disease associations and enriched biological pathways of RIC miRNAs. A: Gene targets: Network of gene targets for miRNAs that were consistently significantly regulated in minimum 2 studies: The 3’ and 5’ strand for miR-21, miR-24, miR-144, and miR-150 as well as the 5’strand for miR-16 in green, genes in grey. (Genes targeted by minimum 3 miRNAs were highlighted in pink). B: Disease associations: Network of diseases associated with the miRNAs in green squares. Diseases in grey squares (relevant diseases highlighted in purple squares). C: Pathways (REACTOME): Top 20 pathways found to be enriched for gene targets shared by a minimum of two miRNAs ranked according to highest adj. p-value. D: Biological process (GOBP): Top 20 biological processes found to be enriched for gene targets shared by a minimum of two miRNAs ranked according to highest adjusted p-value.
Discussion
Which miRNAs mediate the protective effect of RIC in humans?
Based on the hypothesis that the protective effect of RIC is partly mediated by miRNAs that are released to the blood and then attenuate ischemic damage, this review sought to answer which miRNAs may mediate the protective effect of RIC in humans. Several miRNAs were found to be up- or down-regulated by RIC in 13 studies. Yet, the hope to find a subset of dominant miRNAs regulated by RIC consistently throughout the current literature was not met. However, miR-16-5p, miR-21, miR-24, miR-144, miR-150, and miR-1 were differentially expressed in a minimum of two studies. Of these, three (miR-1, -21, -144) have earlier been identified in a systematic review including cell and animal studies, as well as four clinical studies (Kohns et al., 2019). As there were only three studies analyzing healthy participants and one study analyzing non-cardiac patients, we were unable to disclose disease or organ specific RIC induced miRNA changes. But interestingly miR-144 was reported to be upregulated after RIC in both healthy participants and cardiac patients.
Discrepancy in study design and demographics
The inconsistency in findings of the 13 studies may in part be explained by interstudy differences and small sample sizes. First and foremost, most studies analyzed the miRNA expression in a subgroup of patient samples, as a secondary outcome (Table 1-2), increasing the margin of error. Results of such small sample sizes could possibly reflect human heterogenicity and comorbid conditions rather than treatment effect. Thus, the heterogenicity in pre-RIC samples would be interesting to assess. Furthermore, not all studies selected the subgroups at random (4 out of 13 studies did not, a fifth failed to state the selection process), and their characteristics were not accessible, increasing the risk of selection bias and confounding.
Unfortunately, not all studies stated or discriminated between the 3’ and 5’ miR-strands in their analyses. This is rather crucial, as two miR-strands of the same precursor have been reported to be inversely expressed and have opposite functional impacts (Griffiths-Jones et al., 2011; Zhang et al., 2019). In two studies, only predefined specific miRNAs were analyzed using qPCR analysis, while the rest screened a broader panel. Most studies used qPCR to confirm screened findings, and not all were reproducible (Hu et al., 2016) - some were even contradicting (Gao et al., 2016).
Ideally, observed changes in the circulating miRNA profile should only be a result of physiological or pathological conditions. However, pre-analytical procedures such as sample collection and handling have also been shown to substantially influence the circulating miRNA profile (Kim et al., 2021; Binderup et al., 2018; Glinge et al., 2017). This pre-analytical variability has been shown to derive from the choice of blood collection tube and anticoagulant (Kim et al., 2012), storage conditions, and repeated freeze-thaw cycles (Glinge et al., 2017), as well as hemolysis (Kirschner et al., 2011) that interestingly has been shown to increase expression of miR-144 (Wu et al., 2017). Several of the 13 studies reviewed here failed to describe pre-analytical procedures and only two assessed hemolysis in the samples. These studies clearly emphasize the need for standardized pre-analytical protocols. Furthermore, the isolation of “circulating miRNAs” will include both “free” protein-miRNA complexes (eg. miRNA-HDL, miRNA-Ago2) and miRNAs encapsulated in EVs. The profile of free miRNAs and EV miRNAs have been shown to be differentially affected under different conditions (Nik Mohamed Kamal & Shahidan, 2019), and as such, ought to be analyzed separately if characterization of the biological mechanisms behind a physiological or pathological response is the aim. Unfortunately, none of the included studies examined both “free” and EV encapsulated miRNAs. Taken together, consistency and standardization, and agreement upon the use of standardized operating procedures, will be important in future studies in order to capture true differences in circulating miRNAs during physiological or pathological conditions and to be able to compare the results from different studies.
Furthermore, differences in sample type (tissue/serum/plasma/urine), site, and timepoint of collection may also give rise to different miRNA expressions as miRNA expression is known to be tissue specific, which makes comparison of results from different biopsies challenging (Ludwig et al., 2016). Yet, for these 13 studies the discrepancy still prevails when subdividing results with respect to sample type. However, the sample type of choice may also affect the optimal sample collection timepoint. Such a timepoint has not been established, though one study suggested the cardioprotective effect is fully established within 30-60 minutes (Hildebrandt et al., 2016). On the contrary, Kang et al. (2018) reported a peak serum level of plasma miR-21 24h after RIC and Frey et al. (2019) demonstrated different miRNA expression profiles in serum EVs at 5 minutes vs. 60 minutes after RIC, suggesting different expression time intervals for different miRNAs. Hence, the results may be highly affected by the timepoint of sample collection. Different RIC protocols have also been reported to contribute to different results (Ikonomidis et al., 2021; Johnsen et al., 2016). Looking at all the RIC protocols, they varied from one to 10 ischemic cycles lasting 30 seconds to 5 minutes each. Both remote upper and lower limbs were used, as well as direct conditioning (intermittent clamping of the aorta) - and they included pre-, per-, and postconditioning with different time intervals between RIC and ischemia.
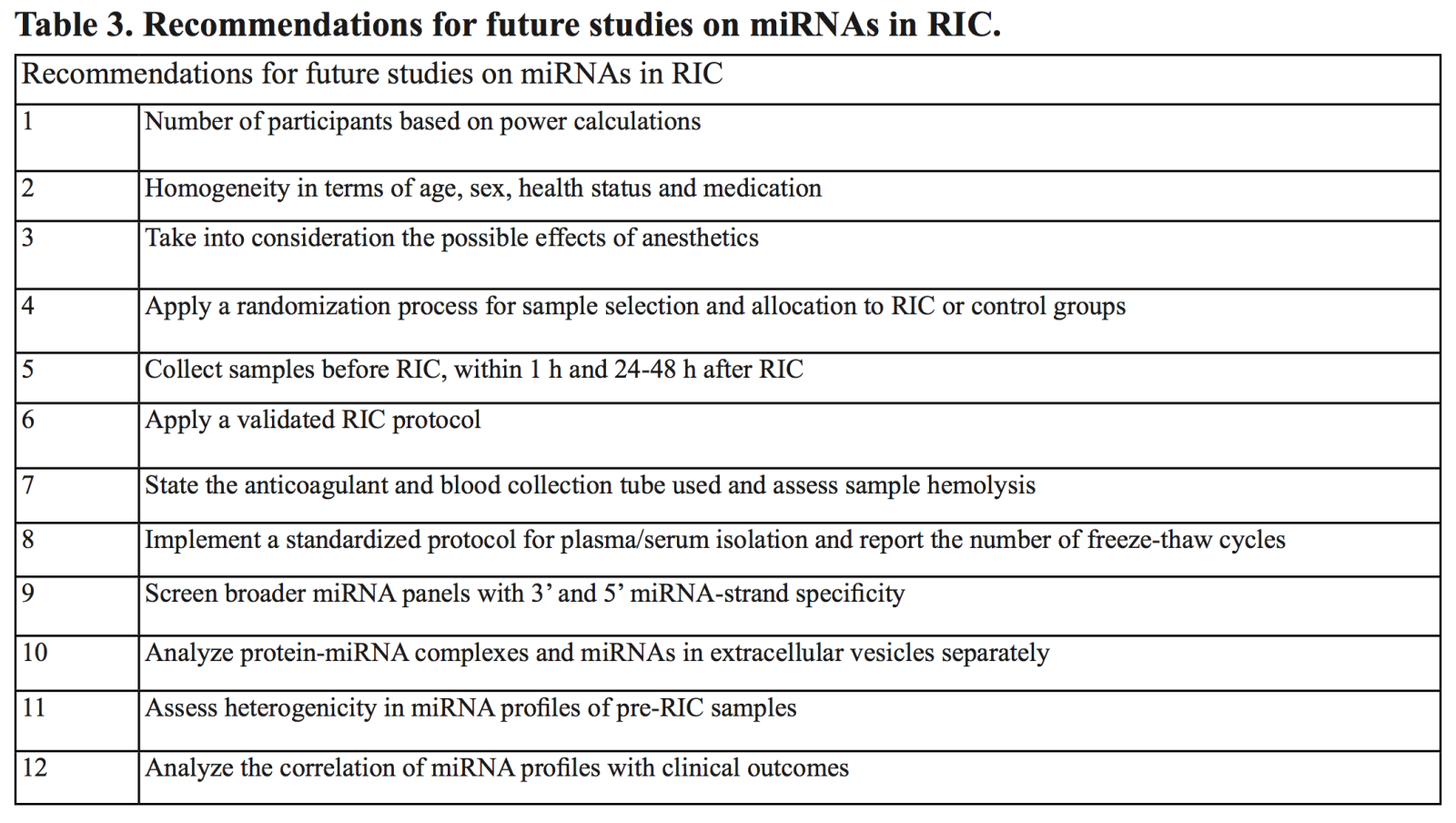
The study populations differed from young to old, healthy to co-morbid, and in gender as well. Age, gender, and comorbidity may give rise to different miRNA expression profiles (Giordano et al., 2018; Heinen et al., 2018), potentially acting as confounding factors. This includes metabolic disorders such as obesity (Silveira et al., 2022), diabetes (Kim & Zhang, 2019), and hypertension (Matshazi et al., 2021). However, it is currently unknown how comorbidity affects the RIC mediated miRNA response in humans. Furthermore medication, and in particular, beta-blockers, propofol, and sevoflurane, with the latter administered in many of the reviewed studies (Table 1-2) have been found to interfere with the protective effect of RIC (Behmenburg et al., 2018; Cho et al., 2019). This could explain why Krogstad et al. (2015) did not observe an effect after the use of propofol, in contrast to Hu et al. (2016). Thus, drug interference needs further clarification. In an attempt to avoid interstudy differences and pitfalls as mentioned above, we here present recommendations for future studies on miRNAs (Table 3).
The underlying biological pathways targeted by RIC miRNAs
The plethora of miRNAs reported to be affected by RIC in the 13 studies may also reflect the many different pathways through which RIC operates. The significantly altered expressions of miR-1, miR-16-5p, miR-21, miR-24, miR-144, and miR-150 after RIC emphasize their possible role in RIC. Although contradicting results exist, miR-21, miR-24, miR-144, and miR-150 have been reported to alleviate IRI in previous preclinical studies (Dong et al., 2020; Minghua et al., 2018; Ou et al., 2020; Yang et al., 2021), whereas miR-1 and miR-16-5p both have been reported to aggravate infarction (Pan et al., 2012; Wang et al., 2020). The putative protective pathways and associated diseases of these six miRNAs were therefore further analyzed. We found IRI, ischemic stroke, and myocardial infarction to be significantly enriched diseases for these miRNAs (Figure 3b), thus underlining their putative role in IRI. Our pathway analysis pointed towards three areas of considerable relevance: oxidative stress induced senescence, cell cycle, and cell signaling and intrinsic pathway for apoptosis (Figure. 3c). These results strongly suggest that these miRNAs may partly mediate their effect by regulating cell senescence. The senescence phenomenon has been investigated in both physiological and pathological contexts and is attributed to the physiological result of telomere erosion in the aging cell. However, stressors such as hypoxia and reactive oxygen species can induce the same response under ischemic events. Senescent cells are arrested in the G1 phase of the cell cycle initiated by the p53/p21 and p16/RB pathway (Jacobs & de Lange, 2004). This is consistent with our analysis that found p53 to be an enriched target gene and G1/s transition of mitotic cells to be an enriched pathway among the six miRNAs (Figure 3d).
Although senescence protects against apoptosis, it comes at a price. Senescent cells are characterized by a senescence-associated-secretory phenotype (SASP) that promotes chemotaxis and inflammation with detrimental effects (Oubaha et al., 2016). The detrimental effects have been reported in ischemic mouse models. One study reported increased levels of p21 and p16 in the myocardium of C57BL/6 male mice (3-4 months of age) exposed to myocardial infarction compared with controls. They found senolytic treatment (selectively killing of senescent cells) to attenuate SASP-induced cytokines and reduce infarct size (Dookun et al., 2020). Similar observations have been reported in a murine stroke model of transient middle cerebral artery occlusion (male CD1 mice, 12 weeks of age). This study found an increased expression of p21 and p16 in the infarct area with a positive correlation with induction of pro-inflammatory cytokines (Torres-Querol et al., 2021). The activity of the senescence-pathways as well as the SASP is controlled by the relative levels of their constituents and regulators. MiRNAs strongly regulate the levels of these constituents. Suppression of global miR expression is reported to induce p53/p21 signaling and senescence (Mudhasani et al., 2008). This is consistent with studies reporting specific proteins of this pathway to be targets of miRNAs (Jones & Lal, 2012). To summarize, our findings suggest regulation of cellular senescence as a pivotal part of RIC in humans. RIC-induced miRNAs may modulate cell cycle, and senescence, in cells of ischemic tissues, and may alleviate the detrimental inflammatory effects of the SASP.
Current prospects
If we can unravel the role of RIC induced miRNAs in different organs in terms of origin, pathways, and influence, then potential molecules for clinical administration could emerge. It may be molecules with a direct protective effect or molecules targeting disease pathways. Both agomirs and antagomirs, which mimic and inhibit miRNA effects respectively, hold potential, but an even faster way of utilizing the miRNAs may be as biomarkers after the induction of RIC to assess patients for whom RIC is beneficial.
To translate the experimental results, large scaled randomized clinical trials are warranted to exclude human heterogenicity and selection biases. Pre-, per-, and post RIC miRNA expression profiles should be analyzed in bigger study groups, including different patient groups. As RIC is currently being investigated in clinical trials of cardiac and stroke patients, these patient groups would be natural first study populations, though other patient groups warrant further investigation too. There are currently 3 reported ongoing studies on ClincalTrials.gov; ENOS: NCT04266639, RESIST: NCT03481777 and NCT03984123) analyzing miRNAs role in RIC. RESIST is a randomized, patient-assessor blinded, sham-controlled trial. In this study, RIC is applied in the ambulance on patients with acute stroke and continued in the in-hospital phase. Blood-samples are taken in the ambulance, upon arrival at the stroke center and after 24 hours. Among other aims, this study will perform a miRNA and EV characterization to elucidate the RIC treatment profile (Blauenfeldt et al., 2020). Notably, for validating causal relations, knockouts or antagomirs have shown great results in abolishing documented effects (Song et al., 2018). However, interventional inhibition of protecting pathways will probably never be an option in human studies. For this we still rely on experimental studies.
Conclusion
This study reviewed the current literature on RIC induced miRNA responses in humans. We found 13 relevant studies investigating this. Several different miRNAs were reported to be affected by RIC, possibly in part due to small sample sizes and study heterogenicity. MiR-16-5p, miR-21, miR-24, miR-144, and miR-150 were significantly upregulated and miR-1 downregulated in a minimum of two of the 13 studies. Diseases with ischemia reperfusion pathology were significantly enriched for these miRNAs, and so were pathways of cell cycle and senescence for the miRNA target genes, suggesting that modulation of cellular senescence may be one of the protective effects of RIC in humans. Elucidating the protective pathways affected by RIC miRNAs could be conveyed into RIC biomarkers or IRI attenuating therapeutics. Future studies should aim to establish an optimal RIC protocol before identifying circulating pre and post RIC miRNA profiles with 3’ or 5’ miRNA specificity in both non medicated healthy participants and IRI patient groups with sham controls. The association of IRI attenuation with RIC induced miRNA profiles should be assessed. Samples should be collected prior to RIC, within the first hour, and preferentially 24-48 hours after. Finally, group homogeneity in terms of age, sex, health status, and medication, is crucial. With the globally increasing frequency of ischemic diseases, translating the protective mechanisms of RIC into the clinic may bring great socioeconomic advantages.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
Melanie Lunding1
1Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, DK-8000, Aarhus, Denmark.
Rolf Ankerlund Blauenfeldt2,3
2Department of Neurology, Aarhus University Hospital, DK-8200, Aarhus, Denmark. 3Department of Clinical Medicine, Aarhus University, DK-8200, Aarhus, Denmark.
Jesper Just1,4
1Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, DK-8000, Aarhus, Denmark. 4Department of Molecular Medicine, Aarhus University Hospital, DK-8200, Aarhus, Denmark.
Kim Ryun Drasbek1,5*
1Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, DK-8000, Aarhus, Denmark. 5Sino-Danish Center for Education and Research, University of Chinese Academy Sciences, Beijing, China/Aarhus, Denmark.
Corresponding author:
Kim Ryun Drasbek
Email: ryun@cfin.au.dk

In a new window | Download PPT
Figure 1: The protective humoral mediators of RIC are released from the affected cells into the circulation, including miRNAs in EVs and protein complexes. RIC of an arm releases protective signals in the blood. These molecular signals could be transmitted through the release of miRNA from cells that are affected by RIC. MiRNAs are transcribed as primary miRNAs (pri-miRNAs) (Lee Y, 2004) mostly from introns (Lin SL, 2006) in the nucleus. The 5’ and 3’ extension of pri-miRNA are removed to form a precursor miRNA (pre-miRNA) (Denli AM, 2004), which is transported to the cytoplasm via a nuclear export factor (Yi R, 2003). Here, the RNase III enzyme, Dicer, binds and cleaves the pre-miRNA yielding a mature miRNA duplex (Hutvágner G, 2001) consisting of a 5’ strand and a 3’ strand. After binding of Argonaute, one of the strands in the miRNA duplex is retained as the seed sequence while the other passenger strand is degraded. As part of the RISC (RNA-induced silencing complex), the Argonaute-miRNA complex binds mRNA targets with complementary sequences to the seed strand, resulting in reduced levels of the target protein via inhibition of protein translation or mRNA degradation. (Rand TA, 2005). Created with BioRender.com.

In a new window | Download PPT
Figure 2: Schematic of the inclusion and exclusion of articles for the review. Inclusion criteria: 1) RIC protocol, 2) MiRNA quantification, and 3) Human study. Exclusion criteria: 1) Reviews/Systemic reviews/Meta-analyses/Case reports/Conference abstracts/Protocol papers, 2) Animal/tissue/cell studies, and 3) Interventions other than RIC.

In a new window | Download PPT
Figure 3: Gene targets, disease associations and enriched biological pathways of RIC miRNAs. A: Gene targets: Network of gene targets for miRNAs that were consistently significantly regulated in minimum 2 studies: The 3’ and 5’ strand for miR-21, miR-24, miR-144, and miR-150 as well as the 5’strand for miR-16 in green, genes in grey. (Genes targeted by minimum 3 miRNAs were highlighted in pink). B: Disease associations: Network of diseases associated with the miRNAs in green squares. Diseases in grey squares (relevant diseases highlighted in purple squares). C: Pathways (REACTOME): Top 20 pathways found to be enriched for gene targets shared by a minimum of two miRNAs ranked according to highest adj. p-value. D: Biological process (GOBP): Top 20 biological processes found to be enriched for gene targets shared by a minimum of two miRNAs ranked according to highest adjusted p-value.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7215 | 32 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA