Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Post-stroke treatment with microRNA-7 inhibitor protects against long-term neural dysfunction after experimental ischemic stroke
Time:2023-02-21
Number:5274
Ding-Fang Shi1,2, Xu Zhang1, Jing Xia2, Xiao-Yu Hou2
Author Affiliations


- 1Research Center for Biochemistry and Molecular Biology, Jiangsu Key Laboratory of Brain Disease Bioinformation, Xuzhou Medical University, Xuzhou, Jiangsu 221004, China.
- 2State Key Laboratory of Natural Medicines, School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu 211198, China.
Conditioning Medicine 2022. 5(5): 186-191.
Abstract
Acute ischemic stroke (AIS) is the leading cause of long-term physical disabilities in adults. The peri-infarct tissue remains the focus of neuroprotective therapies for AIS. Previously, we found that brief ischemic postconditioning confers cerebroprotection by downregulating microRNA-7a/b-5p (miR-7) levels after global ischemia. However, the dynamic expression of miR-7 in the peri-infarct tissue and the therapeutic significance of targeting miR-7 on long-term neurological impairments after AIS remain unknown. Rats were subjected to transient middle cerebral artery occlusion followed by reperfusion and posttreatment with miR-7 inhibitor (Anti-7) three hours after occlusion. Quantitative polymerase chain reaction was performed to detect the expression of miR-7. Staining with 2,3,5-triphenyl tetrazolium chloride was used to detect infarct volume. We also performed the rotarod test, cylinder test, and corner test to assess sensorimotor behavioral recovery in rats. We showed that miR-7 levels increased significantly in the peri-infarct cortex after experimental ischemic stroke. Posttreatment with Anti-7 reduced infarct volumes and attenuated neurological impairments. Anti-7 posttreatment did not affect the overall survival rate in rats suffering from ischemic stroke. More importantly, analysis of the modified neurological severity score and behavioral tests showed that Anti-7 posttreatment was beneficial towards long-term neurofunctional recovery after ischemic stroke. Our findings reveal that inhibiting miR-7 mimics the effects of postconditioning on AIS and this inhibition may underlie the long-term improvement in neurological outcomes after ischemic stroke observed in response to ischemic postconditioning. Therefore, inhibiting miR-7 may be a potential neuroprotective strategy for post-stroke treatment of AIS.
Keywords: Acute ischemic stroke, Long-term disability, MicroRNA-7, Neuroprotective therapy, Sensorimotor function; survival rate
Abstract
Acute ischemic stroke (AIS) is the leading cause of long-term physical disabilities in adults. The peri-infarct tissue remains the focus of neuroprotective therapies for AIS. Previously, we found that brief ischemic postconditioning confers cerebroprotection by downregulating microRNA-7a/b-5p (miR-7) levels after global ischemia. However, the dynamic expression of miR-7 in the peri-infarct tissue and the therapeutic significance of targeting miR-7 on long-term neurological impairments after AIS remain unknown. Rats were subjected to transient middle cerebral artery occlusion followed by reperfusion and posttreatment with miR-7 inhibitor (Anti-7) three hours after occlusion. Quantitative polymerase chain reaction was performed to detect the expression of miR-7. Staining with 2,3,5-triphenyl tetrazolium chloride was used to detect infarct volume. We also performed the rotarod test, cylinder test, and corner test to assess sensorimotor behavioral recovery in rats. We showed that miR-7 levels increased significantly in the peri-infarct cortex after experimental ischemic stroke. Posttreatment with Anti-7 reduced infarct volumes and attenuated neurological impairments. Anti-7 posttreatment did not affect the overall survival rate in rats suffering from ischemic stroke. More importantly, analysis of the modified neurological severity score and behavioral tests showed that Anti-7 posttreatment was beneficial towards long-term neurofunctional recovery after ischemic stroke. Our findings reveal that inhibiting miR-7 mimics the effects of postconditioning on AIS and this inhibition may underlie the long-term improvement in neurological outcomes after ischemic stroke observed in response to ischemic postconditioning. Therefore, inhibiting miR-7 may be a potential neuroprotective strategy for post-stroke treatment of AIS.
Keywords: Acute ischemic stroke, Long-term disability, MicroRNA-7, Neuroprotective therapy, Sensorimotor function; survival rate
Introduction
Stroke is the second cause of death worldwide and continues to be the leading cause of chronic physical disabilities in adults. Acute ischemic stroke (AIS) accounts for nearly 78% of all stroke cases in China (Wang et al., 2017). Early thrombolysis or thrombectomy to achieve reperfusion is the only widely approved clinical treatment for AIS and these treatments have improved the overall survival rate (Kaye et al., 2011, Ganesh and Goyal 2018). However, reperfusion often causes additional delayed damage to the peri-infarct (ischemic penumbra) tissue, further aggravating cerebral infarction, which contributes to poor functional outcomes and neuropsychiatric symptoms in stroke survivors (Ramos-Cabrer et al., 2011, Ku et al., 2013, Sarraj et al., 2021, Yang and Liu 2021). Therefore, there is an urgent need to develop novel neuroprotective strategies to mitigate damage to peri-infarct tissue post-stroke to improve long-term outcomes.
Increasing evidence suggests that ischemic postconditioning protects against neuronal loss, improves neurological functions, and extends the therapeutic time window of thrombolysis after cerebral ischemia (Zhao et al., 2006, Ren et al., 2008, Liu et al., 2013, Esmaeeli-Nadimi et al., 2015). Understanding the cellular and molecular events underlying postconditioning-induced cerebral protection will provide potential therapeutic targets for AIS. We found that ischemic postconditioning confers mitochondrial protection by downregulating microRNA-7a/b-5p (miR-7) levels and stabilizes voltage-dependent anion channels, thereby maintaining neuronal bioenergy and calcium homeostasis in the hippocampus after global cerebral ischemia (Yao et al., 2018). In mammals, members of the conserved miR-7 family are expressed in neurons in the brain, especially in the cerebral cortex, hypothalamus, and pituitary (Sanek and Young 2012, Piwecka et al., 2017, Zhao et al., 2020). Physiologically, miR-7 is involved in neurodevelopment, while abnormal miR-7 expression has been implicated in the progression of neuropsychiatric disorders including neurodegenerative diseases and schizophrenia (Choi et al., 2015, Zhang et al., 2015, Zhao et al., 2020). More interestingly, we found that patients with AIS have significantly elevated levels of miR-7 in peripheral blood compared with blood samples from healthy age-matched controls (Yao et al., 2018). Decreased miR-7 expression has been found in infarct regions after experimental ischemic stroke (Kim et al., 2018, Mehta et al., 2022). However, the dynamic changes of miR-7 levels in the peri-infarct cerebral cortex and its contribution to long-term neurological dysfunction after focal ischemic stroke remain unknown.
In the present study, we examined the levels of miR-7 in the peri-infarct region of the cerebral cortex after experimental ischemic stroke in rats subjected to transient middle cerebral artery occlusion (MCAO). We also evaluated the therapeutic effects of the miR-7 inhibitor, Anti-7, on chronic functional recovery, which would provide a potential drug target for AIS treatment.
Materials and Methods
Experimental animals
Adult male Sprague-Dawley (SD) rats, weighing 240-300 g, were kept on a 12 h light/dark cycle with ad libitum access to food and water. All experiments were approved by the local Animal Care and Use Committee (Approval ID: SYXK (SU) 2016-0028).
Experimental ischemic stroke
Experimental ischemic stroke was induced by intraluminal occlusion of the left MCA for 60 minutes using a suture (Beijing Cinontech Co., Ltd, Beijing, China) in SD rats under anesthesia as mentioned previously (Liu et al., 2013). Briefly, rats were anesthetized with 5% isoflurane for induction and 1.5%-2% isoflurane for maintenance. A two cm incision was made in the midline neck and the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed. The CCA and ECA were then ligated. A nylon monofilament with a diameter matched to the weight of rats was inserted slowly through the CCA and advanced to the ICA until a slight resistance remained (approximately 18 mm to the bifurcation of the CCA). After 60 minutes of occlusion, the suture was gently removed. Rectal temperature was maintained in the normal range (36.5℃ to 37.5℃) with a heating pad during the operation. The Longa method (Longa et al., 1989) was used to evaluate the success of the model: 0, no neurological deficit; 1, failure to stretch the contralateral (right) forepaw fully; 2, contralateral circling ; 3, failing to the contralateral; and 4, no spontaneous movement. Animals with scores between 2-3 were included in the experiments.
Drug treatment
Anti-7 (4 nmol; GenePharma, location) was delivered into the rat right cerebral lateral ventricle 3 h after MCAO. The coordinates used for intracerebroventricular injection were anteroposterior, 0.8 mm; lateral, 1.5 mm, and depth, 3.5 mm from bregma. Anti-7 (with 2′-OMe modification): 5′ ACAACAAAAUCACUAGUCUUCCA 3′.
Quantitative real-time PCR (qPCR) for miR-7 detection
The peri-infarct cortex from rats subjected to MCAO were harvested and rapidly frozen in liquid nitrogen. Total RNA was extracted with Trizol® reagent (Invitrogen Life Science, Carlsbad, CA, USA). For the assessment of miR-7 expression, quantitative PCR (qPCR) was performed using a StepOne™ Plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) and the reactions were carried out with SYBR Premix Ex Taq II (TaKaRa, Dalian, China). U6 served as an internal control. The 2-ΔΔCT method was used to calculate the relative expression levels.
Reverse transcription primers for miR-7: 5′GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAACAA3′; U6: 5′AAAATATGGAACGCTTCACGAATTTG3′.
QPCR primers for miR-7: forward 5′CTGGAGTGGAAGACTAGTGATT3′, reverse 5′GTGCAGGGTCCGAGGT3′; U6: forward 5′CTCGCTTCGGCAGCACATATACT3′, reverse 5′ACGCTTCACGAATTTGCGTGTC3′.
Infarction analysis
After 24 h of recovery, rat brains were cut into coronal sections (2 mm) and then stained with 2,3,5-triphenyl tetrazolium chloride (TTC; 2% in 0.1 M phosphate buffer) (Beijing Cinontech Co., Ltd, Beijing, China) for 30 minutes at 37 °C. Image-Pro Plus software was used to estimate the infarct volume. Infarct volume (%) = (the volume of contralateral hemisphere - the non-infarcted volume of ipsilateral hemisphere) / (2 x the volume of contralateral hemisphere).
Survival rate evaluation and weight assessment
The rats were kept in individually ventilated cage systems with ad libitum access to food and water, and the 28-day survival rates in rats were evaluated after MCAO. The body weight of rats was measured before MCAO and 1, 4, 7, 14, 21, and 28 days after MCAO.
Behavioral tests
For sensorimotor functional assessment after MCAO with or without Anti-7 treatment, rats were subjected to behavioral tests, including neurological score, rotarod test, cylinder test, and corner test. Rats were trained for three days before MCAO and behavioral tests were performed at 1, 4, 7, 14, 21, and 28 days after MCAO.
Neurological score
The modified neurological severity score (mNSS), a composite of motor, sensory, reflex, and balance evaluation graded on a scale from 0-18 (normal score 0, maximal deficit score 18) was used to assess neurological deficits. A higher score indicates more severe neurological dysfunction.
Rotarod test
The rats were placed on a rotating rod and the rotating speed was increased from 4 rpm to 40 rpm during a 300 second testing trial (Doeppner et al., 2014). The latency to fall was recorded as the time before the rat fell off the rotating rod. The mean duration (seconds) was recorded as the average value of three trials.
Cylinder test
The cylinder test was performed to measure asymmetries in forelimb use between the non-impaired (left, L) and impaired forelimbs (right, R). The rats were placed in a transparent cylinder (diameter: 20 cm; height: 30 cm) and videotaped during the tests. Twenty contacts on the cylinder wall were recorded involving the L, R, or both (B) forelimbs and the final score was calculated as: (L-R) / (L + R + B) × 100% (Schallert et al., 2000).
Corner test
The corner test was carried out to evaluate the sensory and postural asymmetries. Each rat was placed between the two angled (30°) boards (30 × 20 × 1 cm3) facing the corner before the test. When reaching the corner, the rats subjected to MCAO usually turned to the side of the brain injury. Twenty trials were performed for each rat and the percentage of left turns was recorded.
Statistical analysis
The behavioral data are presented as means ± standard error of the mean (SEM), whereas other data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 6 (GraphPad software, San Diego, CA). Behavioral tests and weight were analyzed using two-way ANOVA. The level of miR-7 was analyzed using One-way ANOVA. Infarct volumes were analyzed by two-tailed Student’s t-test. Survival curves were computed by the Log-rank test. P < 0.05 was considered statistically significant.
Results
The levels of miR-7 increase in the peri-infarction cortex after MCAO
Previously, we found a significant rise of miR-7 levels in peripheral venous blood from both AIS patients and rats subjected to experimental ischemic stroke. Here, we used qPCR analyses to examine dynamic changes of miR-7 levels in the peri-infract cortex after 3, 6, 12, and 24 h of reperfusion following experiment ischemic stroke. As shown in Fig. 1, the levels of miR-7 increased markedly in the peri-infract tissue after 12 and 24 h of reperfusion. These results imply that miR-7 plays an important role in neurological injury after ischemic stroke.
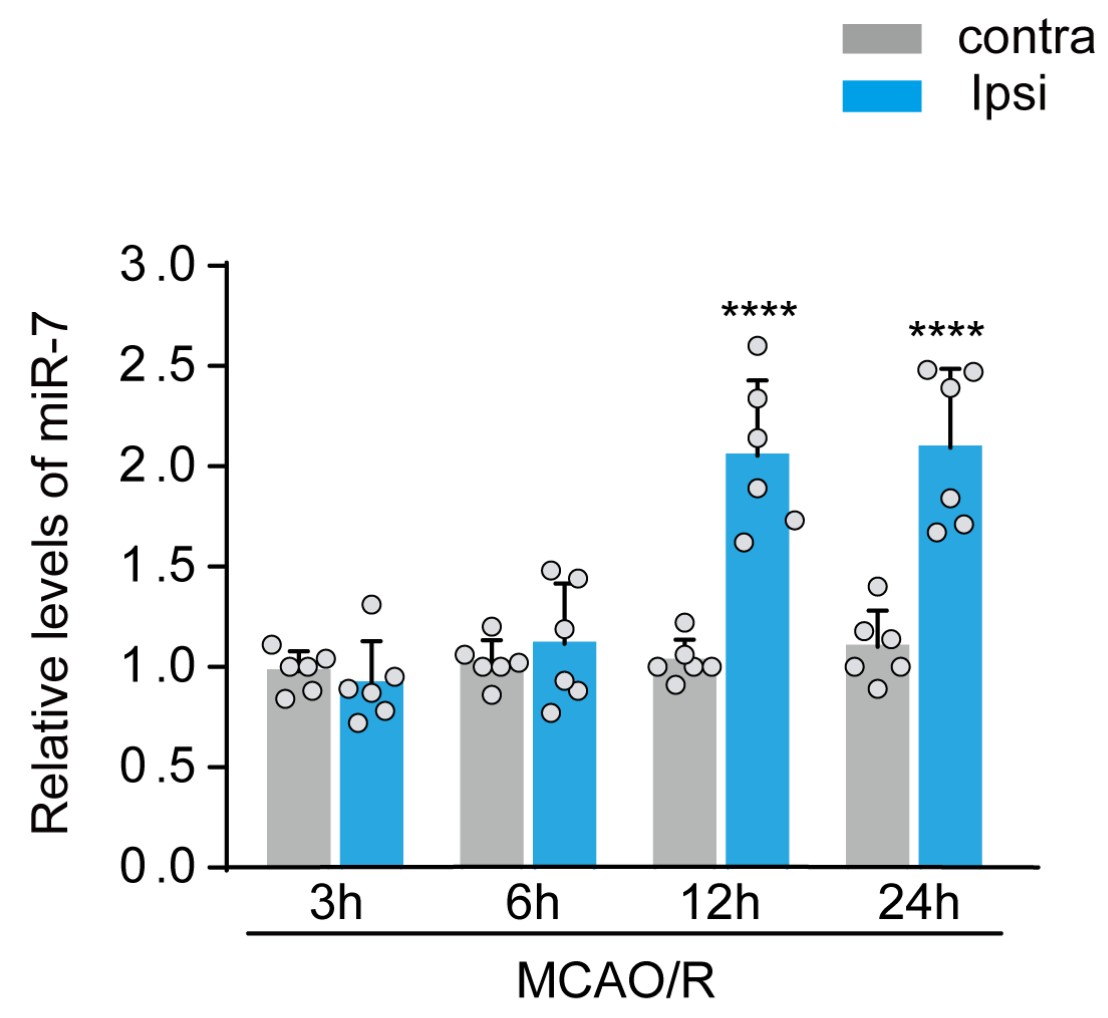
In a new window | Download PPT
Figure 1: The increase in miR-7 levels in rat peri-infarct tissue after ischemic stroke. qPCR analysis of miR-7 levels in the peri-infarct region 3 h, 6 h, 12 h, and 24 h after MCAO. Relative levels of miR-7 in ischemic ipsilateral regions were normalized to respective contralateral regions. Data are presented as the mean ± SD (n = 6 rats); F (7,40) = 23.09, ****P < 0.0001 versus contralateral regions; One-way ANOVA with Tukey’s method.
Postconditioning with Anti-7 attenuates infarct volumes in rats after MCAO
To determine whether Anti-7, a previously proven effective inhibitor of miR-7, has potential therapeutic effects, we delivered the drug into the right lateral cerebral ventricles 3 h after MCAO. Infarct volumes were assessed 24 h after stroke by TTC staining, and representative images are presented in Fig. 2A. The data showed the increase in infarct volumes after MCAO. However, infarct volumes in the Anti-7 treatment group were reduced significantly compared with the MCAO group (Fig. 2B). The results suggest that Anti-7 postconditioning has a neuroprotective effect after MCAO.
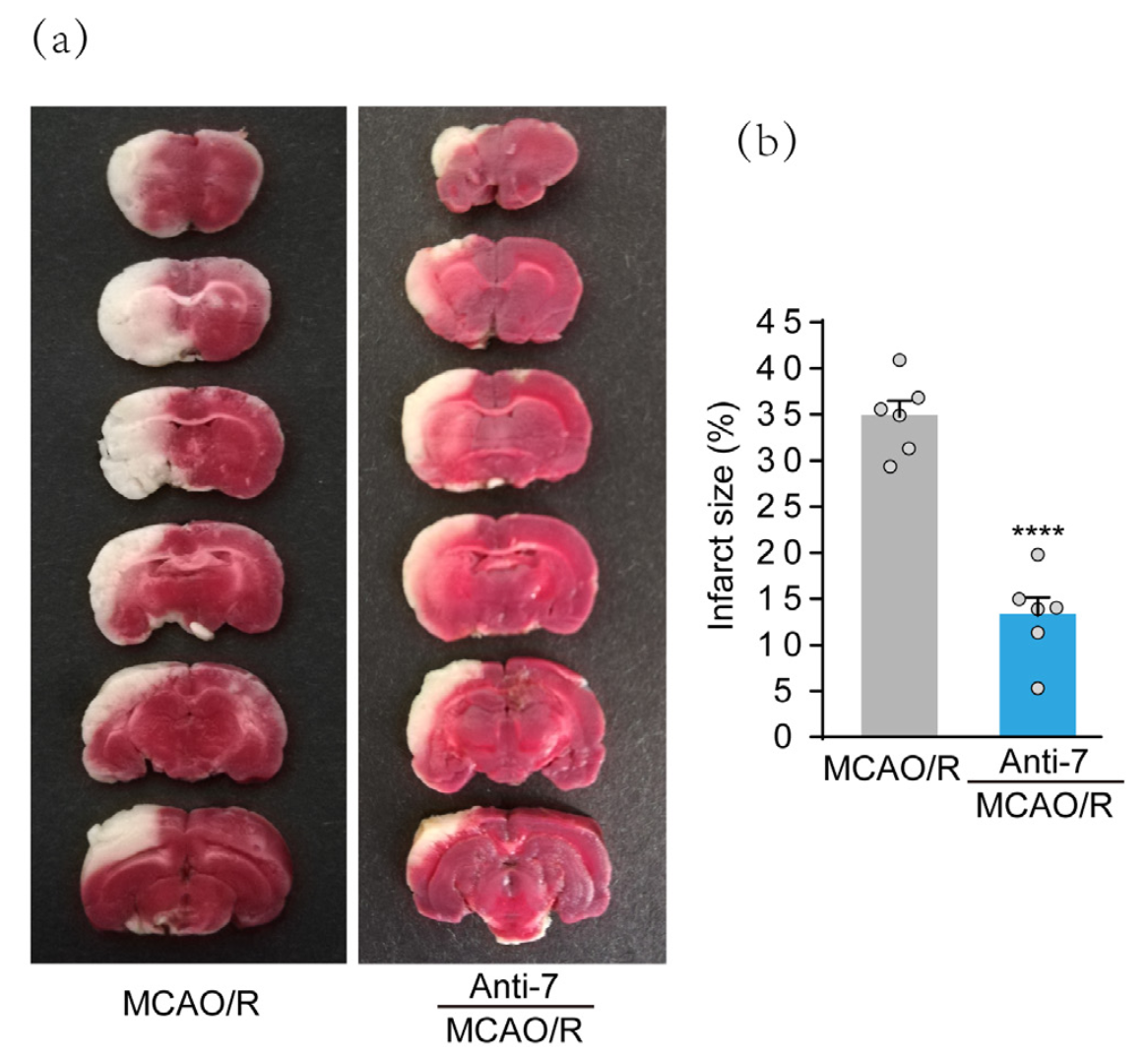
In a new window | Download PPT
Figure 2: Post-stroke treatment with Anti-7 attenuates infarct volumes in rats after ischemic stroke. (a) The representative image of rat brain sections by TTC staining. Rats were injected with Anti-7 into the lateral cerebral ventricles 3 h after MCAO. (b) Quantification analysis of infarct size. Data are presented as the mean ± SD (n = 6 rats); ****P < 0.0001 versus MCAO groups; Student’s t-test.
Anti-7 does not affect body weight and survival rate of rats after MCAO
Cerebral ischemia leads to short-term body weight loss in rats (Ran et al., 2018). Thus, we evaluated weight change between the MCAO and Anti-7 posttreatment groups. As shown in Fig. 3A, there was no statistical difference between Anti-7 posttreatment group and the MCAO group. In addition, we evaluated the effect of Anti-7 on survival rate after MCAO and the data showed that the 28-day survival rate was higher in the Anti-7-treated group (82.35%) than in the MCAO group (65.39%). However, the difference was not statistically significant (Fig. 3B). The results suggest that miR-7 inhibitor does not markedly affect body weight change and survival rate after MCAO in rats.

In a new window | Download PPT
Figure 3: Post-stroke treatment with Anti-7 does not affect weight and survival rate in rats after ischemic stroke. (a) The body weight was measured at pre-operation and 1 d, 4 d, 7 d, 14 d, 21 d, and 28 d after MCAO. Data are presented as the mean ± SD (n = 6 rats); F (1,14) = 2.285, P = 0.1529, n.s., no significance versus MCAO groups; Two-way repeated measures ANOVA with Bonferroni post hoc test. (b) Survival rate was assessed after MCAO (n = 17 for Anti-7-treated group; n = 26 for MCAO group). P = 0.2638, n.s., no significance versus MCAO group. Log-rank (Mantel-Cox) test.
Postconditioning with Anti-7 improves long-term neurological outcomes in rats after MCAO
To investigate the effect of Anti-7 on long-term functional recovery after MCAO, neurofunctional deficits were evaluated by mNSS, rotarod test, corner test, and cylinder test after Anti-7 treatment. Modified NSS analysis showed that neurological function was impaired after MCAO, but was attenuated by Anti-7 posttreatment (Fig. 4A). Moreover, the rotarod, corner, and cylinder tests showed that sensorimotor functions were damaged after MCAO and treatment with Anti-7 improved sensorimotor functions significantly (Fig. 4B-D). These results demonstrate that Anti-7 improves neurological impairment and delays functional recovery after MCAO.
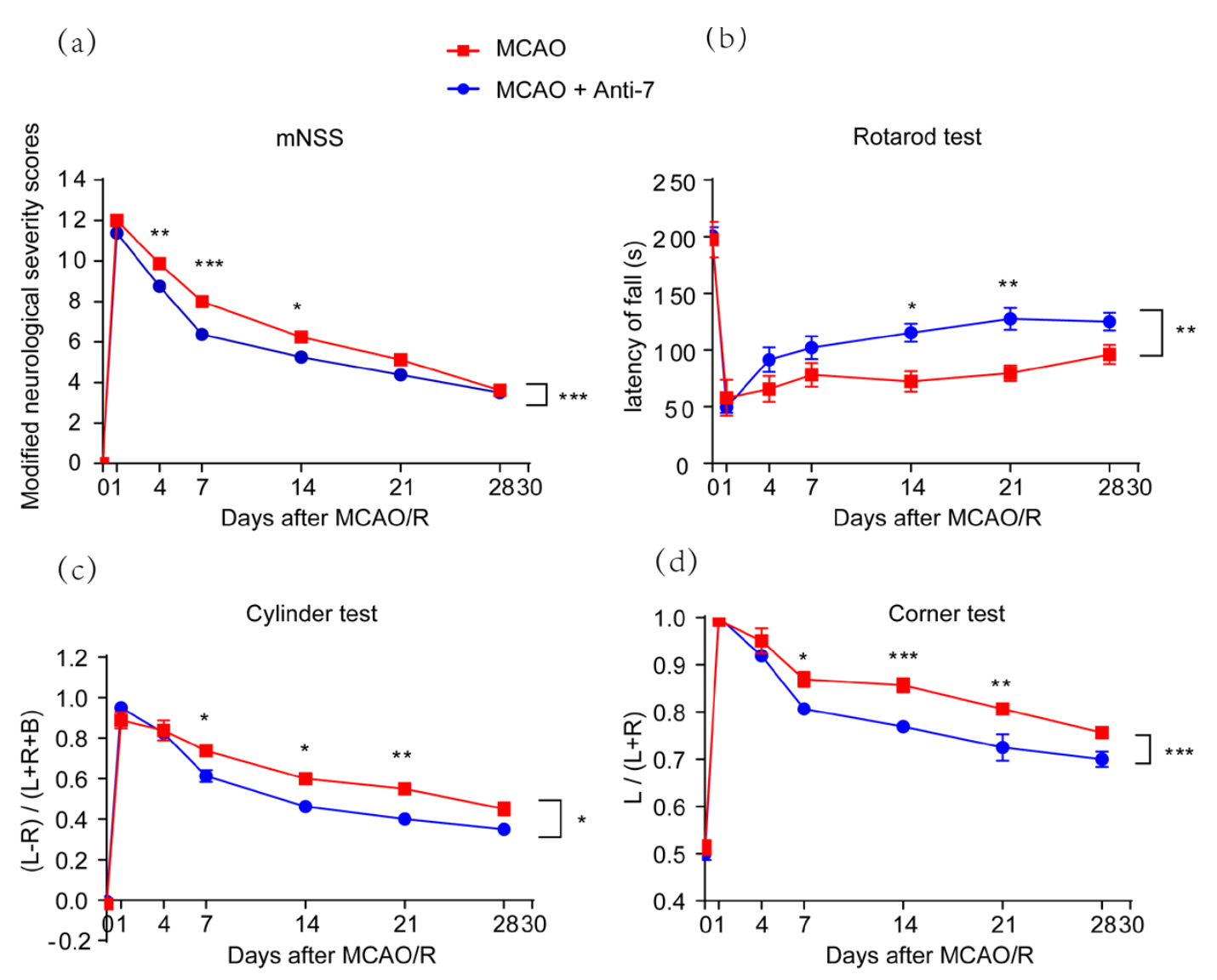
In a new window | Download PPT
Figure 4: Post-stroke treatment with Anti-7 facilitates long-term functional recovery in rats after ischemic stroke. (a) The neurological dysfunctions were evaluated by modified neurological severity score (mNSS), F (1,14) = 24.6, P = 0.0002. (b) The rotarod, F (1,14) = 15.2, P = 0.0016, (c) the cylinder, F (1,14) = 7.272, P = 0.0174, and (d) the corner tests were used to evaluate sensorimotor functional deficits. L, left; R, right; B, both forepaws. F (1,14) = 17.3, P = 0.0010. Data are represented as the mean ± SEM (n = 6 rats); ***P < 0.001, **P < 0.01, *P < 0.05 versus MCAO groups; Two-way repeated measures ANOVA with Bonferroni post hoc test.
Discussion
The economic burden of stroke treatment and care is increasing with an aging population (Heron 2021, Mei et al., 2022). Early recanalization therapy with intravenous thrombolysis or mechanical thrombectomy after AIS efficiently improves the prognosis of patients and has decreased the rate of stroke-related motility (Liu et al., 2018, Pang et al., 2019). Much attention is required to develop novel neuroprotective drugs for promoting long-term recovery from functional outcomes and neuropsychiatric symptoms in patients suffering from AIS. In this study, we provided evidence that reperfusion following experimental ischemic stroke induces a sustained upregulation of miR-7 levels in the peri-infarct cortex. More importantly, a miR-7 inhibitor applied after stroke not only alleviates cerebral infarction but also ameliorates long-term sensorimotor functions in rats.
In the previous studies, we focused on the underlying molecular mechanisms of postconditioning-mediated neuroprotection (Liu et al., 2013, Yao et al., 2018, Li et al., 2019). We found that ischemic postconditioning maintains bioenergy homeostasis and thereafter neuronal survival by preventing miR-7-voltage-dependent anion channel activation after acute hypoxic ischemia. This study extends these findings by providing evidence that miR-7 is associated with neurological damage and severe chronic functional deficits after focal ischemia. The clinical studies indicate that the occurrence of stroke in men is nearly 30% higher than that in women (Appelros et al., 2009). As gender differences exist during acute and chronic stages of ischemia (Fukuda et al., 2009), a limitation of the current study is not taking the intrinsic difference between male and female rats in to account.
The molecular mechanisms underlying the miR-7-mediated poor outcomes might be related to mitochondrial disorders and synaptic plasticity impairments by targeting voltage-dependent anion channels in neurons. Interestingly, miR-7 acts on endothelial cells and is a potential agent for anti-angiogenic therapy in solid tumors (Cui et al., 2017). Therefore, we proposed that Anti-7-mediated angiogenesis might be associated with long-term recovery after stroke. Additionally, astroglia, microglia, and endothelial cells are significantly activated in peri-infarction regions at least 24 h after stroke (Haley and Lawrence 2017, Yang et al., 2017, Yoon et al., 2018, Guo et al., 2021). However, specific cell types associated with increased miR-7 expression and its targeted signaling events require further investigation, which would help to define the underlying mechanisms of Anti-7-induced acute and chronic effects.
Recently, miR-7 was found to target NLR family pyrin domain containing 3 (NLRP3) and toll-like receptor 4 (TLR4) and to alleviate microglial inflammation in a mice model of Parkinson's disease and cerebral hemorrhage (Zhou et al., 2016, Zhang et al., 2018). Also, miR-7 targets Bax and poly [ADP-ribose] synthase 1 (PARP-1) (Li et al., 2016, Luo et al., 2018). However, these substrate proteins are upregulated after ischemic stroke (Jiao and Li, 2021, Xu et al., 2022, Zhu et al., 2022). It would be interesting to know why miR-7 only targets a few specific proteins in various cell types after ischemic stroke.
MiR-7 is also involved in various neuropsychological disorders, and thus it is expected to become a clinical therapeutic target. Abnormal oligomerization or accumulation of α-synuclein in specific brain regions contribute to a group of neurodegenerative disorders known as α-synucleinopathies. MiR-7 overexpression down-regulates α-synuclein levels by post-transcriptional mechanism and autophagy-mediated degradation (Junn et al., 2009, Choi et al., 2018). A previous study showed reduced miR-7 levels in the infarct region after MCAO. Pre-treatment with a miR-7 mimic improved motor function recovery, while posttreatment with a miR-7 mimic at two hours of reperfusion dis not induce significant protection (Kim et al., 2018). These findings suggest that miR-7 is involved in different signaling events in the infarct area in comparison to peri-infarct tissue. Therefore, miR-7 is a potential target for reperfusion therapy, but not a prophylactic program for AIS. In addition, increased miR-7 expression has been implicated in the pathophysiology of schizophrenia, as proposed by targeting SH3 and multiple ankyrin repeat domains 3 (SHANK3) (Zhang et al., 2015). Therefore, it will be interesting to determine miR-7 targets and subsequent molecular consequences under different pathological conditions.
Recent reports reveal that circular RNA Cdr1as and long non-coding RNA Cyrano are the two RNAs that most frequently cross-link to miR-7 in human and mouse brain (Piwecka et al., 2017, Kleaveland et al., 2018). Cdr1as has many sites to miR-7 (130 and 73 sites in mouse and human Cdr1as, respectively) and regulates miR-7 stability or transport in neurons. Cyrano uses an extensively paired site to miR-7 to trigger destruction of this microRNA and loss of Cyrano causes increased miR-7 target repression. Whether Cdr1as and Cyrano regulate miR-7 levels in neurons after ischemic stroke remains to be evaluated.
Taken together, postconditioning with Anti-7 alleviates neurological damage and long-term sensorimotor dysfunction after ischemic stroke. MiR-7 is a promising target for reperfusion therapy to improve long-term functional outcomes, providing a novel and feasible therapeutic strategy for AIS.
Disclosure of statement
The authors declare there are no conflicts of interest.
Acknowledgment and Sources of Funding
Acquisition and analysis of data, Writing-Original Draft. Xu Zhang: Acquisition and analysis of data, Writing-Original Draft. Jing Xia: Acquisition and analysis of data, Writing-Original Draft. Xiao-Yu Hou: Research conception, Writing - Review & Editing, Funding acquisition. This work was supported by grants from the National Natural Science Foundation of China (81673418 and 82173801) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA310007).
References
Ding-Fang Shi1,2
1Research Center for Biochemistry and Molecular Biology, Jiangsu Key Laboratory of Brain Disease Bioinformation, Xuzhou Medical University, Xuzhou, Jiangsu 221004, China. 2State Key Laboratory of Natural Medicines, School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu 211198, China.
Xu Zhang1
1Research Center for Biochemistry and Molecular Biology, Jiangsu Key Laboratory of Brain Disease Bioinformation, Xuzhou Medical University, Xuzhou, Jiangsu 221004, China.
Jing Xia2
2State Key Laboratory of Natural Medicines, School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu 211198, China.
Xiao-Yu Hou2
2State Key Laboratory of Natural Medicines, School of Life Science and Technology, China Pharmaceutical University, Nanjing, Jiangsu 211198, China.
Ding-Fang Shi and Xu Zhang contributed equally to this article.
Corresponding author: Xiao-Yu Hou
Email: xyhou@cpu.edu.cn
Correspondence should be addressed to Xiao-Yu Hou, PhD (xyhou@cpu.edu.cn).

In a new window | Download PPT
Figure 1: The increase in miR-7 levels in rat peri-infarct tissue after ischemic stroke. qPCR analysis of miR-7 levels in the peri-infarct region 3 h, 6 h, 12 h, and 24 h after MCAO. Relative levels of miR-7 in ischemic ipsilateral regions were normalized to respective contralateral regions. Data are presented as the mean ± SD (n = 6 rats); F (7,40) = 23.09, ****P < 0.0001 versus contralateral regions; One-way ANOVA with Tukey’s method.

In a new window | Download PPT
Figure 2: Post-stroke treatment with Anti-7 attenuates infarct volumes in rats after ischemic stroke. (a) The representative image of rat brain sections by TTC staining. Rats were injected with Anti-7 into the lateral cerebral ventricles 3 h after MCAO. (b) Quantification analysis of infarct size. Data are presented as the mean ± SD (n = 6 rats); ****P < 0.0001 versus MCAO groups; Student’s t-test.

In a new window | Download PPT
Figure 3: Post-stroke treatment with Anti-7 does not affect weight and survival rate in rats after ischemic stroke. (a) The body weight was measured at pre-operation and 1 d, 4 d, 7 d, 14 d, 21 d, and 28 d after MCAO. Data are presented as the mean ± SD (n = 6 rats); F (1,14) = 2.285, P = 0.1529, n.s., no significance versus MCAO groups; Two-way repeated measures ANOVA with Bonferroni post hoc test. (b) Survival rate was assessed after MCAO (n = 17 for Anti-7-treated group; n = 26 for MCAO group). P = 0.2638, n.s., no significance versus MCAO group. Log-rank (Mantel-Cox) test.

In a new window | Download PPT
Figure 4: Post-stroke treatment with Anti-7 facilitates long-term functional recovery in rats after ischemic stroke. (a) The neurological dysfunctions were evaluated by modified neurological severity score (mNSS), F (1,14) = 24.6, P = 0.0002. (b) The rotarod, F (1,14) = 15.2, P = 0.0016, (c) the cylinder, F (1,14) = 7.272, P = 0.0174, and (d) the corner tests were used to evaluate sensorimotor functional deficits. L, left; R, right; B, both forepaws. F (1,14) = 17.3, P = 0.0010. Data are represented as the mean ± SEM (n = 6 rats); ***P < 0.001, **P < 0.01, *P < 0.05 versus MCAO groups; Two-way repeated measures ANOVA with Bonferroni post hoc test.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5274 | 13 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA