Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Alternating the electric fields therapy and brain tumor immune microenvironment for glioma
Time:2023-02-21
Number:8423
Wentao Hu1,2, Hongyu Liu1, Yuyang Liu1, Jialin Liu1, Ling Chen1
Author Affiliations


- 1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
- 2School of Medicine, Nankai University, Tianjin 300071, China.
Conditioning Medicine 2022. 5(5): 155-164.
Abstract
Tumor-treatment fields (TTFields) consist of low-intensity, intermediate-frequency, and alternating electric fields that are delivered to the tumor region by noninvasive arrays. TTFields have revolutionized the treatment modality of tumors, particularly glioblastomas (GBMs) and mesotheliomas. TTFields can induce a number of biological responses, including mitotic catastrophe, to kill cancer cells by targeting polarizable intracellular molecules. Notably, exposure of cancer cells to TTFields has been shown to result in immunogenic cell death, which is reflected by damage-associated molecular patterns and the formation of micronuclei that further activate DNA sensor-related inflammatory pathways, indicating the immunomodulatory role for TTFields. These results prompt further exploration to determine whether TTFields can “heat up” a “cold” tumor immune environment and can work synergistically with immunotherapy. Thus, we review emerging advances in the understanding of the mechanisms underlying the anticancer effects of TTFields, with particular attention to how TTFields shape tumor immune microenvironments and any potential implications for their application in combination with immunotherapy.
Keywords: TTFields, Tumor immune microenvironments, Antitumor immunity, Review, Glioma
Abstract
Tumor-treatment fields (TTFields) consist of low-intensity, intermediate-frequency, and alternating electric fields that are delivered to the tumor region by noninvasive arrays. TTFields have revolutionized the treatment modality of tumors, particularly glioblastomas (GBMs) and mesotheliomas. TTFields can induce a number of biological responses, including mitotic catastrophe, to kill cancer cells by targeting polarizable intracellular molecules. Notably, exposure of cancer cells to TTFields has been shown to result in immunogenic cell death, which is reflected by damage-associated molecular patterns and the formation of micronuclei that further activate DNA sensor-related inflammatory pathways, indicating the immunomodulatory role for TTFields. These results prompt further exploration to determine whether TTFields can “heat up” a “cold” tumor immune environment and can work synergistically with immunotherapy. Thus, we review emerging advances in the understanding of the mechanisms underlying the anticancer effects of TTFields, with particular attention to how TTFields shape tumor immune microenvironments and any potential implications for their application in combination with immunotherapy.
Keywords: TTFields, Tumor immune microenvironments, Antitumor immunity, Review, Glioma
Introduction
Tumor-treatment fields (TTFields), regarded as the fourth modality in cancer management, leverage alternating electric fields at intermediate frequencies (100–300 kHz) and low intensities (1–3V/cm) to exert a biophysical force targeted at charged or polarizable molecules (Mun et al., 2018). TTFields are delivered in a noninvasive manner via cutaneous transducer arrays placed around the anatomic region where tumors are localized. EF-11, a phase III trial assessing the efficacy of NovoTTFields versus chemotherapy alone for recurrent glioblastoma (GBM), found comparable efficacy between the two modalities but less toxicity in the TTFields group, leading to the approval of TTFields by the Food and Drug Administration (FDA) for use in recurrent GBM therapy in 2011 (Stupp et al., 2012). EF-14, a landmark trial that first revealed an increase in overall survival (OS) for newly diagnosed GBM, a decade after the application of temozolomide (TMZ) for GBM therapy, led to FDA approval of TTFields for newly diagnosed GBM in 2015 (Stupp et al., 2015). Additionally, TTFields were approved by the FDA in 2019 for malignant pleural mesothelioma based on the results of the STELLAR study (Ceresoli et al., 2019). Although TTFields have been shown to possess favorable anticancer efficiency and less systemic toxicity in certain malignancies, the usage of TTFields in real-world practice remains uncommon (3–12% in newly diagnosed GBM and 0–16% in recurrent GBM), mainly because of its high cost and less well-defined mechanisms of action (Wick, 2016; Connock et al., 2019; Lassman et al., 2020).
The efficacy of TTFields depends on various factors including, but not limited to, intensity, frequency, exposure time, exposure direction, cell division rate, and patient compliance. Within the range of low intensities, the proliferation inhibition effect of TTFields increased as the intensity increased, and the lowest intensity for the complete proliferation arrest of F-98 cells (rat glioma cell lines) was 2.25 V/cm (Kirson et al., 2004; Kirson et al., 2007). An optimal frequency of ~200 kHz has been widely reported for glioma cells beyond which the effect of TTFields decreased with increased frequency (Kirson et al., 2004; Kirson et al., 2007; Li et al., 2021). The exposure time has been shown to increase the efficacy of TTFields (Giladi et al., 2015). Given the mechanisms of action of TTFields, the angle between the field and the axis of cell division is crucial to TTField effects (Kirson et al., 2007). Thus, it is recommended to apply two perpendicular fields considering the random orientation of the axis of division. It has also been shown that TTFields selectively act on dividing cells, leaving quiescent cells intact (Kirson et al., 2004). Moreover, some studies have revealed that TTField-induced cell death inversely correlates with the cell-division rate or cell-doubling time (Giladi et al., 2015; Shahaf et al., 2018). Compliance, defined as the relative device on-time (%) in the total treatment period, is an independent prognostic factor for patients with GBM receiving TTFields, with higher compliance (>50% or >75%) being associated with prolonged survival time (Kanner et al., 2014; Toms et al., 2019).
Immunotherapy, which kills tumor cells by modulating the immune response, is regarded as a breakthrough in the field of cancer treatment (Couzin-Frankel, 2013). However, to date, immunotherapy has shown clinical benefits only in a minority of cases and in some types of tumors (Hu et al., 2022). Therefore, there is an urgent need to develop more effective immune-based therapies. It has been recognized that different tumor immune microenvironment (TIME) classes dictate the responsiveness of tumors to immunotherapy. Infiltrated-excluded (I-E) TIMEs, characterized by a high immune cell population and relatively reduced cytotoxic lymphocytes (CTLs) in the tumor core, regarded as “cold,” are hypothesized to be more resistant to immunotherapy. Conversely, infiltrated-inflamed (I-I) TIMEs, characterized by high CTLs and high expression of immune checkpoint molecules, considered “hot,” are postulated to be more responsive to immunotherapy (Binnewies et al., 2018). However, further studies are warranted to understand how to “heat” the “cold” TIME to a “hot” TIME.
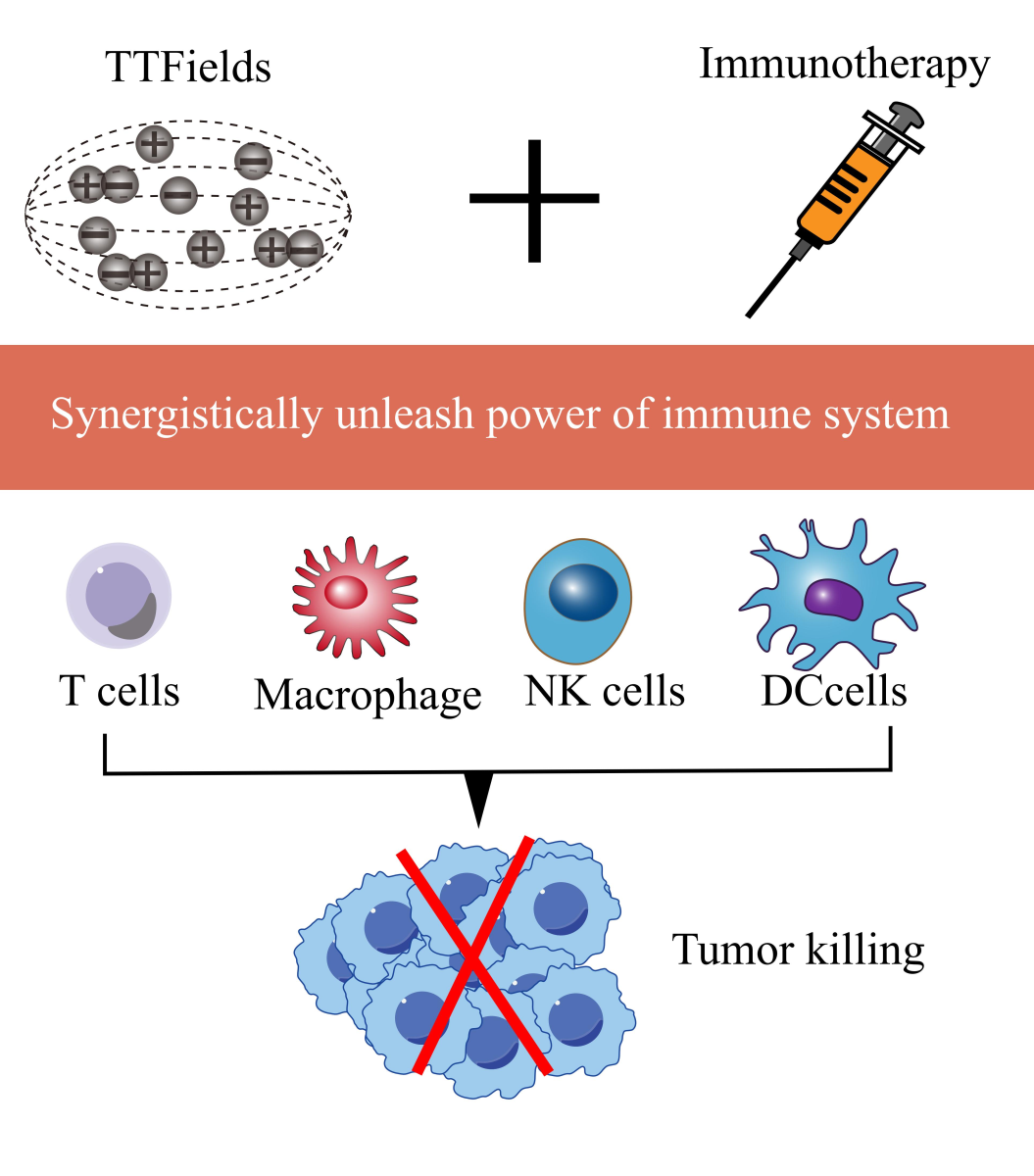
In this review, we summarize the non-immunological mechanisms underlying the anticancer effects of TTFields, including aberrant mitosis, reduced DNA repair capacity, increased replication stress, autophagy upregulation, enhanced cell membrane permeability, blood-brain barrier (BBB) disruption, cell metabolism alteration, and suppression of invasion and migration. In addition, we review the emerging evidence to unveil the role of TTFields in the tumor immune microenvironment and provide rationales for its combined use with immunotherapy (Figure 1).
In a new window | Download PPT
Figure 1: The synergistic role of TTFields with immunotherapy in augmenting antitumor immune responses.
Mechanisms of the direct anticancer action of TTFields and their relationships with the TIME
Aberrant mitosis
Interference of TTFields with the process of mitosis followed by cell cycle arrest and cell death was considered to be the commonly documented and the first mechanism of action identified to explain the anticancer effects of TTFields. The mitotic spindle that helps to separate sister chromatids during mitosis consists of microtubules formed by the polymerization of tubulin dimers (Kline-Smith and Walczak, 2004). Septin, which is localized to the metaphase plate during mitosis, serves as a mitotic scaffold for cytokinetic effectors, such as mitotic checkpoint regulators, and is responsible for chromosome segregation and spindle elongation (Spiliotis et al., 2005). Both tubulin dimers and septin are critical for the coordination of cytokinesis and exhibit relatively high dipole moments. Several studies have shown that exposure of cancer cells to TTFields could lead to abnormal spindle assembly, tubulin depolymerization, and septin localization, which impairs the normal function of these intracellular macromolecules and organelles, further resulting in aberrant mitotic exit, aneuploidy, and cell death (Kirson et al., 2004; Gera et al., 2015; Giladi et al., 2015; Timmons et al., 2018).
Regarding the mechanisms by which TTFields exert electric-field effects on dividing cells, it was proposed that TTFields generate electric field forces, and the field intensity was postulated to increase at the cleavage furrow as the “hourglass” structure is formed following anaphases. This effect is also termed dielectrophoretic (DEP), in which polar and/or charged macromolecules such as tubulin and septin are forced to change their original orientation and move towards the furrow (Kirson et al., 2007; Pethig, 2010; Li et al., 2020). Notably, the combination of TTFields with mitotic checkpoint inhibitors has been demonstrated to decrease cell proliferation and increase the apoptotic rate earlier and longer in glioblastoma cells compared to either treatment alone (Kessler et al., 2018).
Reduced DNA repair capacity and increased replication stress
The synergistic antitumor effects of combining TTFields with other modalities, such as chemotherapy and radiotherapy, which kill cancer cells primarily by inducing DNA damage and replication stress have been observed in several studies (Schneiderman et al., 2010; Giladi et al., 2014; Giladi et al., 2017; Karanam et al., 2017; Lee et al., 2019; Jo et al., 2020; Lazaridis et al., 2020). Remarkably, Schneiderman et al. (2010) reported that TTFields in combination with chemotherapeutic agents led to a similar decrease in cell viability of wild-type and multidrug-resistant (MDR) cells, with the latter overexpressing ATP-binding cassette transporters, indicating the ability of TTFields to sensitize MDR cancer cells to chemotherapies (Schneiderman et al., 2010; Silginer et al., 2017). These results suggest TTFields potentially interfere with DNA replication and damage repair.
Giladi et al. (2017) demonstrated that the number of γH2AX foci, a marker of DNA double-strand breaks (DSBs), was increased in the group treated with radiotherapy and TTFields compared to that in either treatment alone, similar to the formation of Rad51 foci, which reflect homologous recombination repair (HRR) efficiency (Thacker, 2005; Giladi et al., 2017). These results indicate that TTFields make cancer cells conditionally vulnerable to radiotherapy by inducing the accumulation of irradiation-induced DNA damage, at least in part, via impaired HRR. In agreement, Karanam et al. (2017) found that BRCA1 pathway genes involved in the HRR and the Fanconi anemia pathways (i.e. BRCA1, FANCD2, and FANCA) were downregulated following TTField exposure using differential gene expression analysis, and that the ability to repair DNA DSBs was also impaired after exposure to TTFields and ionizing radiation. In addition, Karanam et al. (2020) detected the presence of several markers of DNA replication stress under TTField exposure, such as reduced new DNA fiber length and increased R-loop formation, indicating that TTField exposure could also increase cancer cell replication stress, impair replication fork maintenance, and cause DNA DSBs. Taken together, the established role of TTFields in DNA damage response and replication stress pathways theoretically contributes to its use in future combination treatment strategies.
Autophagy upregulation
Several studies have indicated the induction of autophagy following TTFields application. Shteingauz et al. (2018) demonstrated an increase in cellular granularity, which was assumed to be attributable to lysosomal accumulation and associated with autophagy in cell lines treated with TTFields Additionally, a marked elevation in a variety of autophagic structures, including the appearance of double-membraned autophagosomes, mitochondria with swollen matrices, expanded endoplasmic reticulum (ER), and vacuoles or vesicles containing cytosolic material, was observed using electron microscopy in TTfield-exposed cells (Silginer et al., 2017; Shteingauz et al., 2018; Kim et al., 2019). In addition to alterations in the morphology of TTField-exposed cells, an increase in the expression of microtubule-associated protein light-chain 3 (LC3-II), a marker used to monitor autophagy, was also observed in TTField-treated cells, suggesting that TTFields could potentially enhance the activation of autophagy (Tanida et al., 2008; Silginer et al., 2017; Shteingauz et al., 2018; Kim et al., 2019).
TTFields are speculated to drive the activation of autophagy by aberrant mitosis and suppression of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/ mammalian target of rapamycin (mTOR) signaling pathway. Shteingauz et al. (2018) reported that LC3-green fluorescent protein puncta were increased in the TTField treatment group relative to that in untreated control, among cells that underwent mitosis, but not among non-dividing cells, indicating that TTField-induced autophagy is dependent on TTField-related abnormal mitosis (Inoue et al., 2014; Shteingauz et al., 2018). Furthermore, mitotic aberration may drive elevated proteotoxic stress reflected by increased expression of the ER stress marker GRP78 and low-energy (adenosine triphosphate, ATP) levels, leading to activation of AMP-activated protein kinase (AMPK) that is believed to inhibit the effects of mTOR on autophagy (Garcia and Shaw, 2017; Shteingauz et al., 2018; Rominiyi et al., 2021). Kim et al. (2019) found that TTFields may induce miR-29b to negatively regulate Akt2, leading to suppression of the Akt2/mTOR/p70S6K signaling pathway, which is known to deactivate autophagy (Paquette et al., 2018; Kim et al., 2019). The role of autophagy upregulation in TTField-induced cell death is controversial, as contradictory results have been reported (Garcia and Shaw, 2017; Silginer et al., 2017; Kim et al., 2019). Thus, whether activation of autophagy could render cells more sensitive or more resistant to TTField treatment is unclear and warrants further study.
Enhanced cell membrane permeability and blood-brain barrier (BBB) disruption
Electroporation, defined as an increase in cell membrane permeability by a high-pulsed electric field, is a well-established technique used to deliver DNA or chemotherapeutic drugs into cells (Chen et al., 2006; Yarmush et al., 2014). In contrast, TTFields, as alternating electric fields with far lower intensities, seem to have a similar effect on cell membrane permeability. For example, Chang et al. (2017, 2018) demonstrated that TTFields render cancer cells more permeable to substances as large as 20 kDa, but not greater than 50 kDa, with increased uptake of luciferase substrates (D-luciferin and coelenterazine), membrane-penetrating reagents (dextran-FITC and ethidium D), and tumor fluorescent biomarkers (5-ALA) into GBM cell lines upon TTFields treatment compared to no TTFields treatment. These data provide a rationale for future combinatorial treatment of TTFields with anticancer agents to increase intracellular concentration (Colditz and Jeffree, 2012; Chang et al., 2017; Chang et al., 2018). Furthermore, an increase in the number and size of holes in GBM cell membranes upon TTField application was observed. Notably, the enhanced membrane permeability exerted by TTFields on cells is reversible and specific to cancer cells (Chang et al., 2018). In addition, a theoretical study revealed that TTFields may modify ion channels by causing changes in cell membrane potential (Li et al., 2020). In line with this, Neuhaus et al. (2019) reported that TTFields activated Cav1.2-mediated Ca2+ entry, thus affecting Ca2+signaling and ion channel activity, which is associated with glioma progression (Neuhaus et al., 2019; Pei et al., 2020).
In addition to affecting cell membrane permeability and ion channels, TTFields have also been reported to modulate BBB integrity. Some studies have shown that BBB disruption induced by pulsed electrical fields may circumvent the hurdles of anticancer drug delivery into the tumor core (Li et al., 2018; Sharabi et al., 2019). Similarly, emerging studies have reported a significant reversible increase in enhancement by dynamic contrast-enhanced magnetic resonance imaging in brain tissues of rats administered gadolinium following TTField application, pointing toward the ability of TTFields to transiently open the BBB (Kessler et al., 2019; Salvador et al., 2020). Moreover, these data also revealed delocalized tight junction proteins (such as claudin-5) and a disturbed blood vessel structure in brain cryosections of rats upon TTField application, which may partly explain the disruption of the BBB (Kessler et al., 2019; Salvador et al., 2020). Considering that the BBB is the main barrier preventing the entry of the vast majority of neurotherapeutic drugs into the brain, the capacity of TTFields to open the BBB will pave the way for the development of effective drug delivery systems for numerous central nervous system (CNS) disorders (Pardridge, 2005; Daneman and Prat, 2015).
Cell metabolism alteration
Reprogramming of cellular metabolism is one of the hallmarks of malignancy, first recognized a century ago by Otto Warburg, provides an aberrant metabolic milieu to support tumor initiation and progression (Warburg, 1956; Faubert et al., 2020). Recently, several studies have shown that the application of TTFields may elicit cell metabolic responses, such as diminished uptake of tyrosine in patients with high-grade glioma after TTField treatment, increased mitochondrial respiration and glutaminolysis pathway in cells exposed to TTFields, and decreased culture media acidification (Bosnyák et al., 2018; Ceccon et al., 2018; Wong et al., 2018). Notably, the change in glucose metabolism is an instrumental part of cancer-related metabolic aberrations. Elevated expression of pyruvate kinase M2 (PKM2), an isoform of the glycolytic enzyme PK, is reportedly linked to enhanced anabolic glucose metabolism and contributes to tumorigenesis (Christofk et al., 2008; Dayton et al., 2016). Patel et al. (2021) reported that TTField exposure induced a significant reduction in PKM2 expression in human GBM cells, as confirmed by western blotting, immunofluorescence, and a novel radiotracer [18F] DASA-23. These results indicate that TTFields exposure might downregulate cell expression of PKM2, thereby impairing the pro-tumoral metabolic niche and inhibiting cancer cell growth.
Suppression of invasion and migration
Activation of invasion and metastasis are hallmarks of cancer, which enables tumors to circumvent complete resection and antitumor modalities, thus presenting a major hurdle for a cure (Hanahan and Weinberg, 2011). Kirson et al. (2009) found a significant inhibition of tumor metastasis to the lungs in two animal models treated with TTFields compared to sham control. Furthermore, using wound healing and transwell assays, several studies have shown that exposure to TTFields can suppress the migration and invasion of multiple cancer cell lines, such as GBM cells, glioma-initiating cells, and osteosarcoma cells (Kim et al., 2016; Silginer et al., 2017; Oh et al., 2020). These studies also propounded a multitude of underlying mechanisms to explain the inhibitory effects of TTFields on cancer invasion and migration, such as preventing epithelial-mesenchymal transition, downregulating matrix metalloproteinases 2 and 9, and suppressing angiogenesis (Kim et al., 2016; Oh et al., 2020). Notably, Voloshin et al. (2020a) showed that TTFields exposure might interfere with the directionality and robustness of cancer migration by inducing changes in cytoskeleton dynamics, including microtubule disruption and actin reorganization.
The impact of direct anticancer effects of TTFields on the tumor immune environment
Here, we partly discuss the impact of the direct anticancer effects of TTFields based on our understanding of TTField-induced antitumor immune response. Aberrant mitosis, increased replication stress, and dampened DNA repair capacity could result in lagging chromosomal DNA and chromatin bridges, which could eventually form micronuclei that further activate cytosolic DNA sensor-related inflammatory pathways, such as cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)/stimulator of interferon gene (STING)-dependent inflammation pathways (Mackenzie et al., 2017), as discussed below in more detail. Moreover, in terms of the relationship between immune responses and autophagy, it is believed that autophagy ensued by cell death could elicit a pro-inflammatory immune response. For instance, autophagy is regarded to favor the release of cytokines or damage-associated molecular patterns (DAMPs) (including high mobility group protein B1 [HMGB1], ATP, and calreticulin [CRT]), which are associated with immunogenic cell death (Ma et al., 2013), as discussed below in detail. In addition, it is widely accepted that the BBB is intimately involved in regulating interactions between the immune system and CNS, such as cytokine transport and immune cell trafficking across the BBB, and is historically deemed to make the CNS an immune-privileged organ with a paucity of circulating immune cell infiltration (Carson et al., 2006; Banks and Erickson, 2010; Louveau et al., 2015). In multiple sclerosis patients, disruption of the BBB is regarded as a defining and early feature to promote immune cell infiltration and to induce an autoimmune response (Lengfeld et al., 2017). Likewise, as mentioned above, the application of TTFields reversibly enhanced BBB permeability and disrupted BBB integrity, suggesting the potential role of TTFields in potentiating circulating immune cell infiltration into brain tumors. The abscopal effect, a phenomenon where regression of distant or metastatic tumors occurs beyond the scope of the localized treatment site, particularly refers to the effect of radiation and is thought to be mediated by the systemic antitumor immune response (Postow et al., 2012; Zhang et al., 2022). Analogously, TTField exposure is believed to inhibit tumor invasion and reduce tumor metastasis. For example, Kirson et al. (2009) reported that lung metastases were significantly decreased in TTField-treated rabbits implanted with tumors within the kidney capsule, and prominent immune cell infiltration was also noted around and within the metastasis upon TTField application. These results suggest that TTField application appears to have a similar abscopal effect by inducing a systemic antitumor immune response.
The antitumor immunity induced by TTFields
In addition to the direct inhibition of tumor cell growth, as previously indicated, TTFields also induce a multitude of biological effects that are referred to as immune-mediated antitumor effects, indicating an immune-modulating effect of TTFields in tumors, and providing a rationale for its combinatorial treatment with immunotherapy. The effects discussed below, including increased immune cell infiltration into metastases and dependence of tumor-killing ability on patient immunocompetence, seem to confirm the immunomodulatory role of TTFields in cancer treatment. In addition, some in silico analyses of differentially expressed genes (DEGs) for comparing samples from cancer cell lines, samples from animal models treated with TTFields, and samples receiving no TTFields have suggested that immune response-related pathways were prominently upregulated in the TTField-treated group (Lee et al., 2020; Wainer-Katsir et al., 2022).
The anticancer effects of TTFields are dependent on the immunocompetence of patients
Steroids has been routinely employed for decades in the treatment of CNS tumors and is among the most powerful agents for relieving tumor-related edema and alleviating immune-related adverse events (irAEs) associated with immunotherapy use (Dietrich et al., 2011). However, owing to the well-known immunosuppressive effect, including but not limited to T cell suppression, the concomitant use of steroids and some anticancer therapies are contraindicated (Zhang et al., 2006; Roth et al., 2015; Giles et al., 2018). Wong et al. (2014) evaluated the characteristics of responders and non-responders in a phase III clinical trial of TTFields versus chemotherapy for recurrent GBM and found that the mean cumulative dexamethasone dose was significantly lower in responders than in non-responders in the TTField cohort (35.9 mg vs. 485.6 mg; p < 0.0001) A threshold of 4.1 mg dexamethasone was noted to affect survival outcomes of patients treated with TTFields (Wong et al., 2015). A significantly superior OS was detected in patients receiving dexamethasone ≤ 4.1 mg per day versus those receiving > 4.1 mg (11.0 months vs. 4.8 months; p < 0.0001). These results strongly indicate the involvement of an immunological component in TTField-mediated anticancer effects. Furthermore, the authors also found that TTFields-treated patients with higher proportions of CD3+, CD4+, and CD8+ T lymphocytes had a relatively favorable median OS, indicating that immunocompetence of patients influence the anticancer effects exerted by TTFields (Wong et al., 2015). Taken together, these findings suggest that therapeutic TTFields act at least in part by eliciting anti-tumor immune responses.
Immunomodulatory effects of TTFields on cancer cells
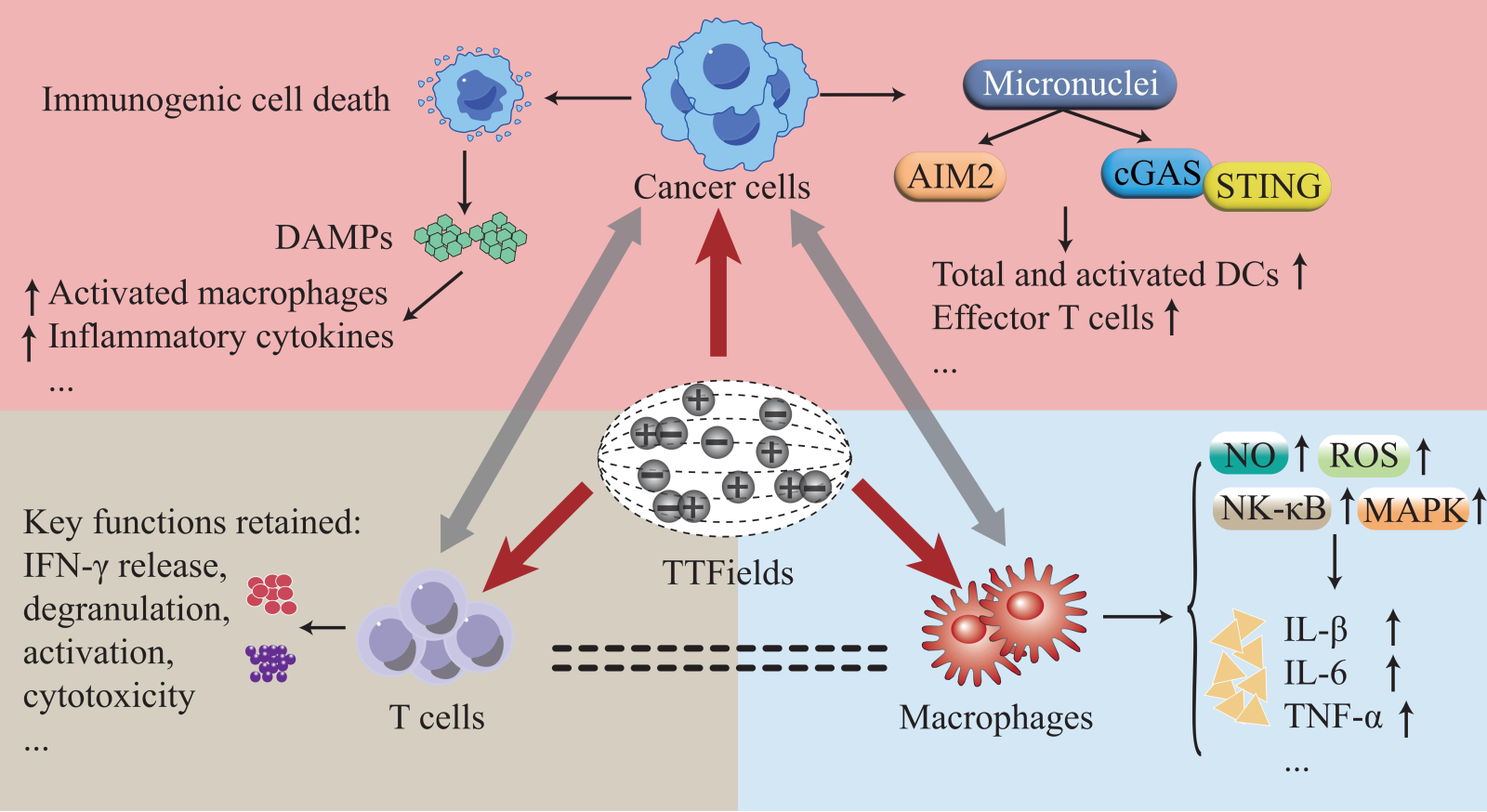
In addition to direct damage to cancer cells, it has been demonstrated that several biological events triggered by TTFields in cancer cells are involved in the transformation of the tumor immune environment. On the one hand, TTFields promote immunogenic cell death (ICD) characterized by an increased release of damage-associated molecular patterns (DAMPs) that act on diverse immune cells and exert immunomodulatory effects. On the other hand, the formation of micronuclei, and thus activated DNA sensor-related pathways driven by TTFields, elicits multiple cellular responses leading to changes in the immunogenicity of exposed cells (Figure 2).
TTFields induce ICD of cancer cells, which can lead to potent anticancer immunity. TTField-mediated cell death has been proven by several studies to occur in both caspase-dependent (the trait of apoptosis) and caspase-independent ways, which may be due to the different cancer types or diverse genetic contexts (Gera et al., 2015; Giladi et al., 2015; Silginer et al., 2017; Kim et al., 2019; Voloshin et al., 2020b). Necrosis was initially shown to be linked to increased emission of DAMPs and augmented ICD (Garg et al., 2010). In contrast, apoptosis is widely regarded as an immunologically silent cell death type; however, emerging data have revealed that apoptotic cells also emit DAMPs and elicit antitumor immunity (Voll et al., 1997; Krysko et al., 2006; Boozari et al., 2010; Krysko and Vandenabeele, 2010; Garg et al., 2012b). More recently, a new concept of cell immunogenic death, deemed to be mediated by DAMPs, has emerged, which could trigger efficient antitumor immunity (Kroemer et al., 2013). Reactive oxygen species (ROS) and ER stress are believed to facilitate trafficking of DAMPs, which comprise extracellular ATP, HMGB1, and the cell surface-expressed protein CRT (Zitvogel et al., 2010; Garg et al., 2012a; Krysko et al., 2012). Remarkably, several studies have demonstrated that upon exposure of cancer cells to TTFields, prominent extracellular secretion of HMGB1 and ATP, and enhanced surface expression of CRT were observed (Holtzman and Talia, 2016; Wong et al., 2018; Voloshin et al., 2020b). Moreover, the phosphorylation level of eIF2α, a quintessential marker of ER stress, was significantly elevated in cancer cells following TTFields application, suggesting that TTFields may trigger ICD by augmenting ER stress in cancer cells (Bezu et al., 2018; Voloshin et al., 2020b).
Cell mitosis following DNA DSBs leads to the formation of micronuclei that contain DNA, which can be induced by TTFields and change the immunogenicity of cells (Harding et al., 2017). Homologous recombination (HR) and non-homologous end-joining repair are two major reciprocal mechanisms responsible for repairing DNA DSBs (Khanna and Jackson, 2001). As mentioned above, TTField-treated cancer cells have been reported to possess impaired HRR and elevated DNA DSBs, indicating the micronuclei formation potential of cancer cells after TTFields (Giladi et al., 2017). Indeed, Chen et al. (2022) identified a significantly higher frequency of large clusters of micronuclei transported from the nucleus into the cytoplasm through focal disruption of the nuclear envelope in cancer cells treated with TTFields (Chen et al., 2022). Cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS), a cytosolic DNA sensor that can activate the transcription factor interferon regulatory factor 2 (IRF3) and stimulator of interferon genes (STING) to activate type I interferon (IFN), was found to be prominently localize in the micronuclei (Sun et al., 2013; Harding et al., 2017). Absent in melanoma 2 (AIM2), an innate pattern recognition receptor, can mediate inflammasome assembly to drive caspase-1 activation (Lamkanfi and Dixit, 2014). In addition, as a DNA sensor, cytosolic DNA induces the formation of the AIM2 inflammasome (Fernandes-Alnemri et al., 2009; Hu et al., 2016). Chen et al. (2022) reported that cytosolic micronuclei clusters triggered by TTField application comprehensively recruited and activated cGAS and AIM2; thus, their downstream signaling was triggered to increase the production of proinflammatory cytokines (PICs), type 1 IFNs (T1IFNs), and T1IFN-responsive genes. These results indicate that TTFields could induce the formation of micronuclei (cytosolic DNA) and thus activate DNA sensors (such as cGAS and AIM2)-associated signaling pathways, leading to the formation of an inflammatory microenvironment.
Effects of TTFields on immune cells
Emerging evidence has shown the potential of TTFields in the recruitment and activation of immune cells, which could potentiate the immunogenicity of the tumor microenvironment. After intraperitoneal injection of TTField-treated cells into mice, a marked recruitment of leukocytes (CD45+) was observed (Voloshin et al., 2020b). Moreover, by comparing the T cell infiltration rates of patients with GBM before and after TTField treatment, immunohistochemical staining significantly increased CD3+ and CD8+ T cell infiltration in some patients following TTFields (Diamant et al., 2021). Moreover, upon culturing splenocytes with conditioned media from TTField-exposed KR158 cells (GBM cells), Chen et al. (2022) found that total and activated (CD80/CD86+) dendritic cells (DCs) and activated effector (CD44+CD62L-) T cells were markedly increased in a STING or AIM2 dependent manner. In addition, by coculturing bone marrow-derived DCs (BMDCs) with a TTField-administered LLC-1 cell (lung carcinoma cell line) suspension, researchers found that DC maturation and activation markers, including major histocompatibility complex II, CD40, and CD80, were markedly upregulated in these BMDCs (Voloshin et al., 2020b).
The direct effects of TTFields on immune cells (such as T cells and macrophages, as discussed below) resulted in decreased cell viability and enhanced pro-inflammatory effects of immune cells, but had little or no impact on immune cell functions. The compatibility of TTFields with pivotal functions of tumor-infiltrating T cells is a prerequisite for combining TTFields modality and immunotherapy. After T cells derived from peripheral blood or GBM samples were directly exposed to TTFields, researchers found that except for reduced viability of dividing T cells, other key functions, such as IFN-γ release, degranulation, activation, and cytotoxicity, were retained (Simchony et al., 2019; Diamant et al., 2021). Co-culture of bone marrow-derived macrophages with CT26 cells (colorectal carcinoma cell lines) exposed to TTFields led to macrophage activation in an HMGB1-dependent manner; elevated pro-inflammatory cytokines, and decreased anti-inflammatory cytokines were also detected in supernatants from these co-cultures (Wong et al., 2018). Intriguingly, the co-culture of TTFields-treated RAW 264.7 mouse macrophages with 4T1 cells (murine mammary carcinoma cells) resulted in a marked elevation of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α, and IL-6 (Baugh and Bucala, 2001; Park et al., 2019). Moreover, the direct exposure of RAW 264.7 cells to TTFields led to reduced cell viability but increased production of nitric oxide and ROS, which represent the proinflammatory activation of macrophages (Boscá et al., 2005; Park et al., 2019). Nuclear factor kappa B (NF-κB), consisting of several family members including the p65 subunit, generally localized to the cytoplasm by its inhibitor I kappa B (IκB), is a crucial regulator of gene expression involved in numerous biological responses, such as immune responses and inflammation (Nabel and Verma, 1993; Scott et al., 1993; Dolcet et al., 2005). Mitogen-activated protein (MAP) kinases, including extracellular signal regulated kinases, c-Jun N-terminal kinases, and p38 MAPKs, are regulated by a phosphorylated cascade. They enable cells to exert orchestrated responses to diverse extracellular stimuli and play an important role in regulating inflammatory responses (Huang et al., 2010; Kyriakis and Avruch, 2012). Strikingly, the phosphorylation levels of p38 MAPK, IκB-α, and p65 were significantly elevated in TTField-administered RAW 264.7 cells, indicating that the NF-κB and MAPK pathways are activated in macrophages upon TTFields treatment to regulate the immunomodulatory responses (Park et al., 2019).
Conclusion and future perspectives
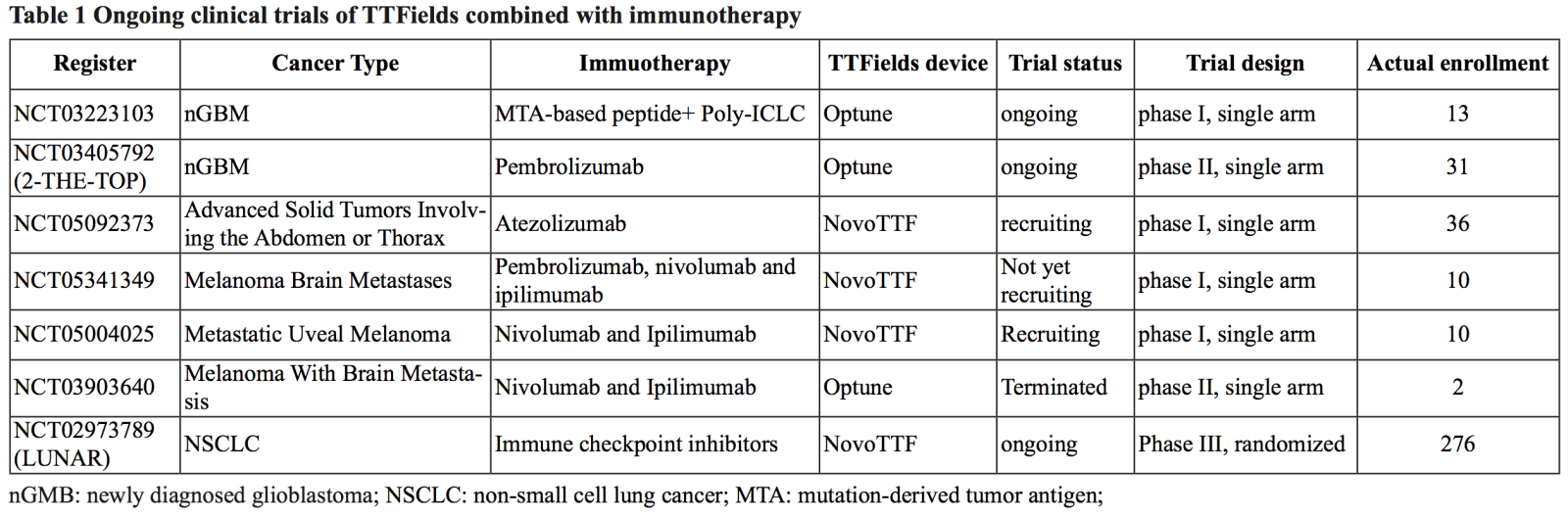
As pre-clinical evidence concerning the effects of TTFields on TIME has continuously emerged, studies assessing the efficacy of combining TTFields with immunotherapy are ongoing. Voloshin et al. (2020b) demonstrated enhanced antitumor efficacy of TTFields when combined with immune checkpoint inhibitors in vivo. They observed a marked decrease in tumor volume in mice orthotopically implanted with LLC-1 cells with combinatorial treatment of TTFields and anti-PD-1 relative to that observed in either treatment alone. For more specific application details, mice were inoculated with orthotopic lung cancer cells, and the TTFields were delivered to the mouse lungs six days afterward and maintained for seven days. For combined use, anti_PD-1 (250 mg/mouse) was administered one day after TTField initiation and maintained for six days. In addition, prominent increases in IFN-γ secretion in cytotoxic CD8+ tumor-infiltrating T cells and leukocyte infiltration occurred in tumors following combined treatment, indicating that the concomitant use of the two therapies could potentiate antitumor immunity. Although numerous preclinical studies have focused on the cellular and molecular levels for the proposed mechanisms of action of TTFields, more studies focusing on the biological effects of TTFields on normal and cancerous cells are needed to further support the clinical application of TTFields in combination treatment with existing modalities. However, to date, no completed clinical trials have evaluated the efficacy of concurrent application of TTFields with immunotherapy. Nevertheless, emerging registered clinical trials are currently active that may substantiate the synergistic action of TTFields and immunotherapy in the treatment of malignancies (Table 1). More clinical trials are warranted to evaluate the synergistic effects of combined TTFields and immunotherapy.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgements
This study was funded by National Natural Science Foundation of China, No. 82172680.
References
Wentao Hu1,2
1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China. 2School of Medicine, Nankai University, Tianjin 300071, China.
Hongyu Liu1
1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Yuyang Liu1
1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Jialin Liu1
1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Ling Chen1
1Department of Neurosurgery, First Medical Center of Chinese PLA General Hospital, Beijing 100853, China.
Corresponding author:
Ling Chen
Email: chen_ling301@163.com)
and
Jialin Liu
Email: liu_00174@163.com
Wentao Hu, Hongyu Liu and Yuyang Liu contributed equally to this article.

In a new window | Download PPT
Figure 1: The synergistic role of TTFields with immunotherapy in augmenting antitumor immune responses.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8423 | 21 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA