Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Circulating Factors that Promote Brain Health during Aging
Time:2023-04-22
Number:4409
Muyassar Mamtilahun1, Yaohui Tang1, Guo-Yuan Yang1
Author Affiliations


- 1Med-X Research Institute and School of Biomedical Engineering, Shanghai Jiao Tong University Shanghai 200000, China.
Conditioning Medicine 2022. 5(6): 205-212.
Abstract
With the increase in lifespan, aging-associated neurodegenerative diseases and stroke have become a primary threat to human health. However, therapeutic options for aging-induced neurological disorders are still limited, which places economic and psychological burdens on society and families. Aging is the most fundamental and unadjustable risk factor for neurodegenerative diseases and stroke. Accumulating evidence has shown that circulating factors in young blood promotes brain health during aging, casting new light on finding novel therapeutic approaches for the treatment of stroke and neurodegenerative diseases. Here, we review the current studies on systematic circulation and brain aging, neurodegenerative diseases, and stroke, summarizing the current knowledge on how circulating factors mediate brain function during health and disease conditions, and discuss the limitation and future hopes of circulating factors from bench to bed translation.
Keywords: Brain aging, Circulating factors, Growth factor, Neurodegenerative disease, Parabiosis, Stroke
Abstract
With the increase in lifespan, aging-associated neurodegenerative diseases and stroke have become a primary threat to human health. However, therapeutic options for aging-induced neurological disorders are still limited, which places economic and psychological burdens on society and families. Aging is the most fundamental and unadjustable risk factor for neurodegenerative diseases and stroke. Accumulating evidence has shown that circulating factors in young blood promotes brain health during aging, casting new light on finding novel therapeutic approaches for the treatment of stroke and neurodegenerative diseases. Here, we review the current studies on systematic circulation and brain aging, neurodegenerative diseases, and stroke, summarizing the current knowledge on how circulating factors mediate brain function during health and disease conditions, and discuss the limitation and future hopes of circulating factors from bench to bed translation.
Keywords: Brain aging, Circulating factors, Growth factor, Neurodegenerative disease, Parabiosis, Stroke
Introduction
With the increase in lifespan around the world, aging-induced neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson’s disease (PD), and stroke have become a significant threat to human health (Katan and Luft, 2018; Association, 2022). Aging is the most prominent and unadjustable risk factor for AD, PD, and stroke (Reeve et al., 2014; Yousufuddin and Young, 2019; Association, 2022). As a major part of the central nervous system (CNS), the brain modulates body functions. It is separated from peripheral blood circulation by the blood-brain barrier (BBB). The BBB forms a strict screen, protecting the brain from xenobiotics, microorganisms, and circulating toxic agents, and maintains brain parenchyma homeostasis and brain function (Abbott et al., 2010; Sifat et al., 2017). Accumulating evidence has revealed that plasma proteins circulating in blood changes with age, and the plasma composition of the young systemic circulation was very different from that of old systemic circulation in humans and rodents (Conboy et al., 2005; Villeda and Wyss-Coray, 2013; Drew, 2017). Furthermore, studies showed that plasma proteins could pass through the BBB and participate in brain function modulation. BBB permeability increases with age (Montagne et al., 2015; Yang et al., 2020), indicating that the interaction between the brain and systematic circulation is much more profound than once thought. A series of studies showed that young systemic factors had positive effects on the function of the aging brain, while circulating factors from aging individuals had negative effects on the young brain (Villeda et al., 2011; Villeda and Wyss-Coray, 2013). Parabiosis or plasma infusion, exercise, and caloric restriction improved brain function by changing the components of the systemic circulation in preclinical studies, providing new hope for finding a cure against aging-induced neurodegenerative diseases and stroke. However, more studies are needed to translate the significant treatment effects observed in preclinical studies into the clinical setting.
In this review, we summarize current research on the therapeutic effects of young blood and circulating factors on age-related cerebral diseases. We also discuss controversies, limitations, and future perspectives. We hope that this review is useful for developing a better understanding of the role of young blood and its circulating factors with respect to age-related neurodegenerative diseases and stroke.
The interaction between the brain and systematic circulation
Circulating blood integrates signals from all organs and provides means for communication between peripheral tissues and the brain. Most parts of the brain, except the circumventricular organs (CVO) including the midline of the ventricular system, posterior pituitary gland, pineal gland, the median eminence of the hypothalamus, and area postrema, are separated from blood circulation by the BBB, which is characterized by low permeability and high selectivity (Broadwell et al., 1983; Abbott et al., 2010). Since the 1900s, scientists believed that only lipid-soluble small molecules and gas could cross the BBB, and most large molecules could not cross the BBB, which included antigens, antibodies, and contents of the plasma (Saunders et al., 2014). Currently we know that there are multiple mechanisms that govern large molecule transportation through the BBB, including passive diffusion, active efflux paracellular diffusional pathways, carrier-mediated transports, and receptor-mediated transcytosis (Kadry et al., 2020). Functionally, the brain not only integrates sensory signals from external environments and directs motor responses, but also responds to changes in other organs of the body by interacting with systematic circulation, including regulation of growth, metabolism, reproduction, and lifespan (Libert and Pletcher, 2007; Jeong et al., 2012; Alcedo et al., 2013; Zhang et al., 2013). Recently, Yang et al. (2020) found that plasma proteins readily permeate the healthy brain parenchyma, with transport maintained by BBB-specific transcriptional programs. Unlike immunoglobulin G antibodies, plasma protein uptake decreased in the aged brain, caused by an age-related change in ligand-specific receptor-mediated transportation to nonspecific caveolar transcytosis (Yang et al., 2020). High-resolution magnetic resonance imaging analysis of regional BBB permeability in the living human brain showed age-dependent BBB breakdown in the hippocampus, a critical region for learning and memory, worsens with mild cognitive impairment and correlates with injury to pericytes (Montagne et al., 2015). This age-related change occurs along with a specific loss of pericyte coverage (Montagne et al., 2015; Yang et al., 2020). Those findings confirmed that the brain not only modulated the functions of the body and distant organs by secreting hormones into blood circulation, but also was affected by changes in blood circulation that occurred with aging and peripheral inflammations. Parabiosis provides a tool to investigate the effects of blood and plasma-derived factors on brain aging and aging-associated neurological diseases.
Aging is a systematic event that gradually leads to a decline in function and regeneration capacity of the major body systems. The characteristics of the aging brain such as decreased neuro-regeneration, altered vasculature, increased neuroinflammation, and impairments in synaptic plasticity lead to neurological dysfunction (Wyss-Coray, 2016). Historically, there have been many attempts to use blood to restore subjects' youth and health (Myhre, 1990; Learoyd, 2012). Parabiosis, first introduced by Paul Bert in 1864, is a technique that connects the blood circulation of two animals by a joint body to explore the effects of circulating blood on the organs of the two animals (Conboy et al., 2013). Heterochronic parabiosis studies found that young blood circulation expands the lifespan of aged mice and promotes stem cell regeneration in the heart, muscle, liver, and brain (Conboy et al., 2005). Villeda et al. (2014) found that exposure of aged mice to young blood circulation by heterochronic parabiosis reverses the effects of aging on the brain of the older animal at molecular, structural, functional, and cognitive levels. In contrast, exposure to old blood or old blood-derived chemokines C-C Motif Chemokine Ligand 11 (CCL11) impaired the cognitive function of young mice by decreasing synaptic plasticity (Villeda et al., 2011). In heterochronic blood exchange, aged-mice blood induced cell and tissue senescence in young animals after one single exchange, and this induction of senescence was abolished if old animals were treated with senolytic drugs before blood exchange (Jeon et al., 2022). Another study found that young blood induced vascular remodeling and increased neurogenesis in aged-mice brain (Katsimpardi et al., 2014). They also showed that growth differentiation factor 11 (GDF11) alone had the same effect as young blood on the aged brain (Katsimpardi et al., 2014). Human cord-blood plasma or treatment with cord-blood-derived factor tissue inhibitor of metalloproteinases 2 (TIMP2) was shown to rejuvenate the aged hippocampus and improve cognitive function in aging mice (Castellano et al., 2017). Kuroda et al. (2017) showed that peripheral blood-derived circulating fibroblast growth factor 21 (FGF21) promotes the proliferation and remyelination of oligodendrocyte precursor cells (OPCs), emphasizing the novel concept – the peripheral milieu controls CNS regeneration (Kuroda et al., 2017). A recent study by Gan and Südhof (2019) revealed that thrombospondin-4 (THBS4) and SPARC-like protein 1 (SPARCL1) in young blood could directly promote synaptic function in aged mice. Another study reported that up-regulation of vascular adhesion molecule 1 (VCAM1) in the plasma of aged humans and mice accelerated brain aging, and systemic administration of anti-VCAM1 antibody or deletion of the Vcam1 gene in brain endothelial cells (BECs) rescued cognitive functions in aged mice and reversed brain aging characteristics, including decreased microglial reactivity and lower neural precursor cell activity (Yousef et al., 2019). Using tools such as parabiosis to search for circulating rejuvenation factors remains an area that attracts scientists’ immense interest.
Besides the young blood and young blood factors, exercise has shown to be another means to promote neurogenesis and hippocampal function of the aging brain by increasing cerebral blood flow and changing the components of the peripheral circulation. A meta-analysis study found that physical exercise invariably resulted in structural and functional changes in the ippocampus/parahippocampus area and a cluster within the cerebellum, indicating that exercise was crucially relevant to preserve cognitive functions in elder adults (Ji et al., 2021). In young adults, exercise was reported to increase brain-derived neurotrophic factor (BDNF) concentration in peripheral blood circulation and enhance BDNF release from the brain (Zoladz et al., 2008; Seifert et al., 2010). Several studies showed that exercise could promote neuroplasticity and decrease neuroinflammation by modulating the composition of peripheral blood circulating factors (Gleeson et al., 2011; Spielman et al., 2016; Lin et al., 2018). Horowitz et al. (2020) found that exercise increased plasma concentrations of glycosylphosphatidylinositol (GPI)-specific phospholipase D1 (GPLD1), a liver-derived GPI-degrading enzyme, and improved cognitive function in aged mice by altering signaling cascades after GPI-anchored substrate cleavage. Their study also found that the peripheral concentration of GPLD1 increased in active and healthy elderly humans (Horowitz et al., 2020). Meanwhile, physical exercise was shown to reduce the expression of neuroinflammatory genes and improve cognitive function by increasing complement cascade inhibitors such as clusterin (CLU) in the circulating blood of the animal model and of patients with cognitive impairment (De Miguel et al., 2021). Collectively, the above evidence indicated that young blood plasma or young blood factors have positive effects on age-related brain function (Table 1). Understanding its underlying mechanisms could provide novel approaches and ideas to combat age-related brain degenerative diseases and stroke.
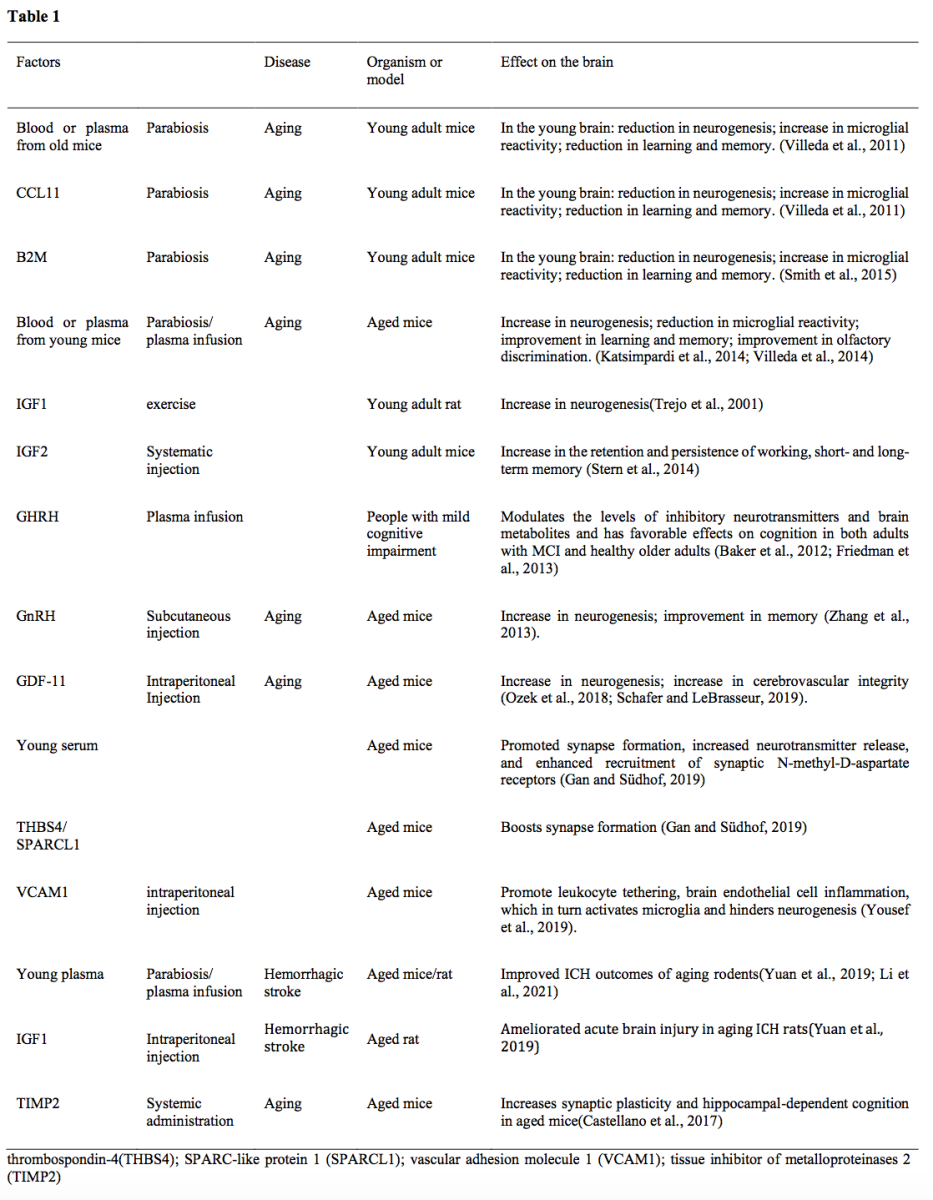
Baker et al., 2012; Friedman et al., 2013; Ozek et al., 2018; Schafer et al., 2019; Smith et al., 2015; Stern et al., 2014; Trejo et al., 2001.
Changes in systemic environment of age-related brain diseases
Neurodegenerative diseases
With the growth of the aged population, neurodegenerative diseases become more prevalent and lack an effective cure, bringing enormous economic and psychological burden to the patient's families and society (Reeve et al., 2014; Association, 2022). AD and PD are the most common neurodegenerative diseases. Studies have shown that aging blood circulation and aging blood factors induce BBB disruption in the hippocampus, reduce neurogenesis, increase microglial reactivity, and affect cognitive function in rodents and humans (Montagne et al., 2015; Yousef et al., 2019; Yang et al., 2020). Exposure to young blood or young blood factors alleviated cognitive deficits of aging-brain by improving vascular function and by promoting synaptic plasticity and hippocampal gene expression networks related to learning and memory (Zhang et al., 2019). The role of blood circulation in cognitive function of the aging brain is gaining increasing interest as new therapeutic approaches to neurodegenerative diseases.
Alzheimer’s disease
AD is the most common neurodegenerative disease with a particular onset and course of cognitive and functional decline associated with age and ultimately results in death (Association, 2022). In a preclinical study, heterochronic parabiosis or young plasma infusion improved neuron cell function at the molecular level and cognitive function of elderly AD mice, but did not show a reduction in the amyloid beta (Aβ) burden (Middeldorp et al., 2016). Morales et al. (2020) found that infusion of whole blood or plasma from elderly animals with extensive Aβ deposition in their brains or intravenous injection of purified Aβ developed significantly higher levels of brain amyloidosis and neuroinflammation in young mice, indicating the role of peripheral amyloid-dependent or -independent factors associated with AD development. Another study presented that systemic administration of young plasma reduced neuroinflammation and the deposition of Aβ, decreased tau hyperphosphorylation level, and reversed the cognitive impairment in aged 3×Tg-AD mice (Zhao et al., 2020). Kim et al. (2020) found that young plasma infusion showed partial trend-level improvements in hippocampal glycogen synthase kinase beta (GSKβ)/Tau expression, neuroplasticity, and mitochondrial function in AD mice. Infusion of the plasma from exercised mice showed general improvement accompanied by positive effects on cognitive function by increasing blood BDNF concentration (Kim et al., 2020). In a mouse model of cerebral amyloid angiopathy (CAA), a major pathological feature of AD, young plasma improved cognition, learning and memory impairment, and anxiety, while preventing neuronal apoptosis, enhancing neurogenesis, and reducting cerebral hemorrhage in CAA mice (Li et al., 2021). However, Aβ in the cortex and hippocampus was not reduced (Li et al., 2021).
Studies have reported that circulating factors, including growth differentiation factor 11 (GDF11) and C-C Motif chemokine Ligand 11 (CCL11), could be involved in aging-associated neurodegenerative disease progression. Daily intravenous injection of young-blood circulating factor GDF11 decreased neuroinflammation, increased vascularization, and improved cognitive function of AD mice (Zhang et al., 2018), casting light on anti-neurodegenerative drug development. However, studies also found that age-induced cognitive-impaired adults showed no difference in circulating GDF11 level from healthy adults (Yang et al., 2017). The controversial effects of circulating GDF11 on brain vasculature and cognitive function could be a result of age-related changes in BBB permeability. Circulating CCL11, a member of the eotaxin family that activates C-C chemokine receptor 3 (CCR3), has also been reported to increase with age in plasma and cerebrospinal fluid (CSF) of mice and humans (Villeda et al., 2011; Hoefer et al., 2017; Huber et al., 2018). Circulating CCL11 plays a crucial role in cerebral physiological function (Mendelsohn and Larrick, 2011). CCL11 induced neuroinflammation, oxidative stress, tau phosphorylation, and the production of β-amyloid, indicating it is a potential risk factor for AD (Zhu et al., 2017; Huber et al., 2018). However, it is still unclear whether CCL11 is increased in the brain of AD patients (Cherry et al., 2017). Similar to GDF11, there were controversial results about the function of CCL11 in the CNS. Recombinant CCL11 promotes the migration and proliferation of mouse neural progenitor cells (Wang et al., 2017).
Infection, especially Gram-negative bacteria-induced infection, correlated with the early development of AD by increasing lipopolysaccharide (LPS) concentration in the blood of humans and mice. Regular usage of anti-inflammatory drugs was associated with a reduction in AD development (Vlad et al., 2008; Zhan et al., 2018). LPS in the blood of AD patients is three-fold higher than that in the control group (Zhao et al., 2017). Increased blood LPS can disrupt BBB and enter the brain parenchyma, inducing myelin injury and myelin basic protein degradation, and neuroinflammation, ultimately leading to cognitive impairment (Zhan et al., 2018). A series of data showed that LPS could act on leukocyte and microglial toll-like receptor (TLR)4- cluster of differentiation 14 (CD14)/TLR2 receptors, increase nuclear factor kappa B -mediated cytokines, including interleukin (IL) 1, IL6, and tumor necrosis factor (TNF) that lead to upregulated Aβ levels, damagde oligodendrocytes, and white matter injury in AD brain (Ikeda et al., 1999; Rossol et al., 2011; Enkhbaatar et al., 2015; Kayagaki et al., 2015). Taken together, the blood LPS, gram-negative bacteria, and inflammation may provide new insights for AD prevention and treatment.
Clinical and preclinical studies have shown that exercise improves memory deficits by improving hippocampal neurogenesis and plasticity and changing molecular biomarkers and brain volumes in dementia (Liu et al., 2020; Castells-Sánchez et al., 2022; El-Domiaty et al., 2022). Studies have reported that exercise increases the peripheral concentration of growth factors, including BDNF, myokine cathepsin B (CTSB), and klotho, which are associated with improved cognition and synaptic function, decreased β-amyloid, and resilience to neurodegenerative diseases (Duzel et al., 2016; Gaitán et al., 2021). Aerobic exercise training induced neurogenesis by increasing BDNF, reducing CCL11 and oxidative stress in blood circulation (Cho and Roh, 2016). Exercise-induced plasma changes in BDNF and increased plasma CTSB concentration were positively associated with cognitive changes in middle-aged adults who are at risk for dementia (Gaitán et al., 2021).
Parkinson’s disease
Parkinson's disease (PD) is the second most common neurodegenerative disease that damages the motor and cognitive function of patients (Tolosa et al., 2021). PD patients showed elevated plasma levels of chemokine concentration, including CCL2/MCP-1, CCL11/eotaxin, CCL24/eotaxin-2, and CXCL10/IP-10 (Rocha et al., 2014). Neutralization of circulating CCL11 suppressed neuroinflammation, prevented loss of dopaminergic neurons, normalized striatal neurotransmitters, and improved motor functions in PD mice (Chandra et al., 2016). Studies have shown that blood circulation may be the transporter for α-synuclein, which is strongly associated with dopaminergic neuron damage, to the brain in PD. Four-month heterochronic parabiosis of wild-type mice and transgenic mice that overexpressed human α-synuclein with a PD-associated mutation, showed a significant increase of α-synuclein filament but not α-synuclein in the substantia nigra of wildtype mice (Ma et al., 2021). In contrast, Lewy Body injected mice in the parabiosis model showed decreased dopamine neurons and increased immunoreactivity of nigral phosphorylated α-α-synuclein immunoreactivity, while its partner mice did not show a lesion or change in S129 phosphorylated-α-α-synuclein levels, indicating that the disease was not 'transmitted' through the bloodstream (Yu et al., 2021). Clinical studies showed that young plasma infusions were safe, feasible, and well-tolerated, and they also decreased peripheral inflammation after four weeks of treatment in moderate-stage PD without serious adverse effects (Parker et al., 2020).
Stroke
The World Stroke Organization reported that stroke remains the third leading cause of death and disability in the world (Feigin et al., 2022), with 87% of strokes being ischemic (Benjamin et al., 2018). Aging is the most potent unadjustable risk factor for incident stroke, which increases dramatically with aging after the age of 55 (Yousufuddin and Young, 2019). As a part of the whole body system, disturbances of focal brain function caused by stroke could trigger the activation of the systemic immune response (Offner et al., 2006), stress response (Zi and Shuai, 2013), release of macrophages from the spleen (Anrather and Iadecola, 2016), release of bone marrow stem cells from bone marrow (Courties et al., 2015), and changes in intestinal permeability and microbiota (Crapser et al., 2016; Singh et al., 2016). These changes could lead to changes in peripheral blood and affect the ischemic brain in positive or negative ways. Clinical studies have shown that after 24 hours of ischemic stroke, the plasma level of BDNF and nitric oxide-derived metabolites in stroke patients reduced, which was associated with developing depression and a decreased cognition, emotion, and neurological status (Lasek-Bal et al., 2015; Casas et al., 2017). In vivo and in vitro studies showed that the miRNA Let-7i, miR-124, and miR-210 modulated BDNF expression after ischemic stroke (Eyileten et al., 2021), which was perhaps a promising therapeutic target for stroke therapy. Meanwhile, clinical and experimental studies found that brain-specific markers upregulated in blood after ischemic stroke and played a detrimental role in stroke prognosis. It has been proven that blood glutamate levels increase after acute ischemic stroke due to leakage of increased glutamate concentration in the ischemic brain. Peritoneal dialysis or bioconjugate glutamate scavenging ameliorate ischemic brain insult in rats by decreasing peripheral glutamate levels (Hawkins et al., 2006; Teichberg et al., 2009; Godino Mdel et al., 2013; Zaghmi et al., 2020). Taken together, there was an interaction between the ischemic brain and systemic blood circulation, which could play a decisive role in stroke outcomes. However, studies on the therapeutic effects of healthy blood/plasma therapy on ischemic stroke were limited. The main focus was on the anti-neuroinflammatory effects of healthy plasma in the acute phase and white matter repair in the subacute phase of ischemic stroke.
A clinical study found that no significant changes in peripheral inflammatory TNF-α, C-reactive protein, fibrinogen, and leukocyte during the first 24 hours of stroke onset (Masztalewicz et al., 2010). Systemic administration of young healthy plasma to elderly animals with ischemic stroke decreased infarct volume and neurobehavioral functional deficits, but elderly plasma injection to young ischemic stroke mice worsened stroke prognosis (Pan et al., 2017). A preclinical study showed that the administration of the young blood factor GDF11 promoted neurogenesis and angiogenesis, and contributed to functional recovery after stroke in mice (Lu et al., 2018). The level of insulin-growth factor-1 (IGF-1) in blood circulation decreased with age in humans and rodents (Yuan et al., 2019). Heterochronic parabiosis and young plasma injection ameliorated the results of intracerebral hemorrhage in aged mice (Yuan et al., 2019). They also found that IGF-1 administration to aged- intracranial hemorrhage (ICH) mice alleviated ICH outcomes (Yuan et al., 2019). Elevated peripheral CCL12 levels in elderly mice aggravate ICH-induced brain injury by recruiting macrophages and T cells (Huang et al., 2020). Replacing ischemic stroke mouse blood with healthy young mouse blood demonstrated that healthy blood substitution could improve ischemic stroke outcomes of mice by decreasing neuroinflammation and matrix metallopeptidase-9 activity (Ren et al., 2020). Significant changes in the expression level of IL-4, IL-6, and IL-10 in stroke patients were observed, but no significant changes over time were found in the IL-2, TNF-α, and interferon-γ expression levels (Deng et al., 2021). Our previous study also demonstrated that intravenous injection of healthy donor plasma to ischemic mice protected the integrity of the tight junction, decreased neuronal cell death, and improved neurobehavioral outcomes by increasing fibroblast growth factor 21 in blood (Mamtilahun et al., 2021).
From bench to bed: the limitations and future hopes
In preclinical studies, young blood plasma showed promising therapeutic effects on aging and age-related neurodegenerative diseases and stroke. However, clinical studies showed no significant improvement in the cognitive and neurological function of aged patients who received young plasma treatment. In addition to the small sample size of these clinical studies, there were several other reasons why young plasma treatment showed no significant improvement in elderly patients' prognosis. First, most of the preclinical studies used heterochronic parabiosis to study the effects of young or elderly systemic circulation on aging or aging-induced neuro disorders. Notably, in heterochronic parabiosis, the two mice that were joined together shared not only blood circulations, but also elderly or young organs that participate in modulating body functions. That means in parabiosis two animals shared the systemic environment, organs, and blood circulations, and it could not be repeated in clinical studies; rejuvenation of the aged brain may be the result of a combined effect of shared organs, environment, and blood circulations. Furthermore, blood exchanges in the parabiotic animal were relatively slow, with a total blood volume that exchanges approximately ten times a day (Huff et al., 1950; Harris, 2013). The half-life of proteins in the blood circulation is different, and blood or plasma infusion may not maintain a significant concentration of plasma proteins in the older blood circulation as heterochronic parabiosis does. The half-life of proteins could be another reason why plasma infusion did not show significant treatment effects in clinical trials. Young blood plasma infusion could alleviate aging-induced brain disfunction by diluting the circulating factors in aging blood. Blood or plasma is cocktail of proteins, microRNAs, exosomes, etc. The therapeutic effects of parabiosis or blood infusion could be the results of combined effect of more than one factor. Hence, the complexity of blood or plasma composition and its half-life is also needed to be taken into consideration in clinical studies.
Conflict of interest
None
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (82071284, YHT; 81974179, ZJZ; 81870921, YTW), the National Key Research and Development Program of China (2019YFA0112000, YHT), the Scientific Research and Innovation Program of Shanghai Education Commission 2019-01-07-00-02-E00064 (GYY), Science and Technological Innovation Act Program of Shanghai Science and Technology Commission, 20JC1411900 (GYY) and K. C. Wong Education Foundation (GYY).
References
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4409 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA