International bi-monthly journal of cell signaling, tissue protection, and translational research.
The current landscape of conditioning medicine: protecting the brain and heart and other organs
Maximillian C. Borlongan1, Jea-Young Lee1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair. Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida
Abstract
Organ tolerance to injury defines conditioning medicine. Laboratory research and clinical evidence have revealed the therapeutic potential of conditioning medicine for protecting the brain and the heart, among other organs, from insults. To highlight the recent progress in this field, we review recent reports from discovery-driven to mechanism-guiding studies, ranging from animal models to human trials. Accumulating data suggest the potential of conditioning medicine to confer therapeutic effects in stroke, cardiac arrest, and other organ failures.
Keywords: Ischemia, Conditioning, Cardiac, Neuroprotection, Cardioprotection
Abstract
Organ tolerance to injury defines conditioning medicine. Laboratory research and clinical evidence have revealed the therapeutic potential of conditioning medicine for protecting the brain and the heart, among other organs, from insults. To highlight the recent progress in this field, we review recent reports from discovery-driven to mechanism-guiding studies, ranging from animal models to human trials. Accumulating data suggest the potential of conditioning medicine to confer therapeutic effects in stroke, cardiac arrest, and other organ failures.
Keywords: Ischemia, Conditioning, Cardiac, Neuroprotection, Cardioprotection
What does conditioning medicine hold for protecting against organ injury?
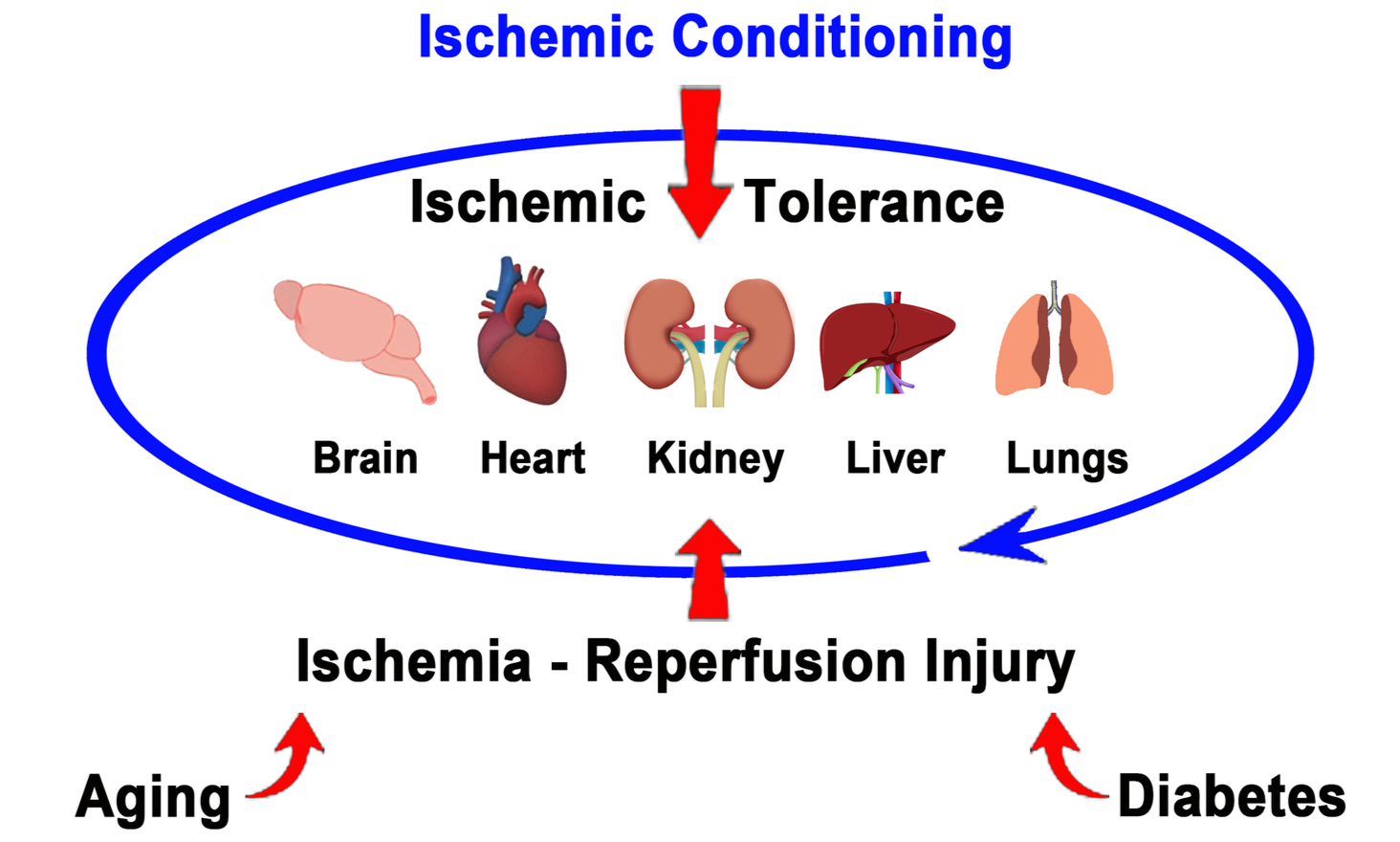
Conditioning medicine has emerged as a robust therapeutic regimen for protecting organs against injury (Kim et al., 2022). While originally explored in heart failure, ischemic conditioning has been extended to the brain and other organs, including the kidney, liver, and lungs (Figure 1). Ischemic conditioning consists of a sublethal ischemic injury that triggers multiple protective processes. Because ischemic injury is closely associated with vascular damage, ischemic conditioning has been indicated primarily for treating vascular diseases, such as stroke, coronary heart disease, and peripheral vascular disease (Kim et al., 2022). Ischemic conditioning has been applied remotely and intermittently, as well as via hypoxic, exercise, and pharmacological (chemical) triggers with generally safe and effective outcomes. Ischemic conditioning entails brief, reversible episodes of ischemia and reperfusion in one vascular bed to activate ischemia tolerance in remote tissues and organs, representing a new potential approach for organ protection (Hess et al., 2022). We discuss below some research and clinical evidence supporting the use of conditioning medicine for stroke, heart, and other organ failures.
In a new window | Download PPT
Figure 1. Ischemic conditioning for protecting the brain, heart, and other organs. Promoting ischemic tolerance via ischemic conditioning can protect the brain, heart, kidney, liver, and lungs from injury and its associated co-morbidity factors such as aging and diabetes via multiple degenerative pathways, including inflammation and oxidative stress.
Conditioning medicine for stroke brain
Effective treatment for stroke remains a significant unmet clinical need, as there are limited therapeutic options and a short therapeutic window. Ischemic conditioning, either via preconditioning, per-conditioning, or post-conditioning regimens, has been examined in stroke animal models and patients (Hess et al., 2022; Zhang et al., 2022). The procedure for ischemic conditioning in stroke has largely used short episodes of focal ischemic insult. In rodents, ischemic conditioning may involve a 10-minute occlusion of the middle cerebral artery (Zhang et al., 2022), while in humans, ischemic conditioning consists of an automated device applied to both arms twice per day with five cycles of inflation to 200 mmHg followed by deflation for 5 minutes for 14 days (Hess et al., 2022). In both laboratory and clinical settings, ischemic conditioning exerts functional benefits, reducing the behavioral symptoms of stroke (Wu et al., 2022, Jeon et al., 2022; Zhang et al., 2022; Hess et al., 2022). A mechanistic pathway implicated in ischemic conditioning is its potentiation of stem cell proliferation, especially vasculogenesis and angiogenesis. To this end, the combination of ischemic conditioning with stem cell transplantation appeals to stem cell-based regenerative medicine when contemplating enhancement of vasculogenic and angiogenic mechanisms to combat the vascular damage associated with stroke. Indeed, a bulk of laboratory and clinical trials of stem cell treatment for stroke suggests the use of this therapeutic regimen in conditioning medicine as an adjunct to ischemic conditioning (Wu et al., 2022). Investigations in such combination therapy of stem cell transplantation and ischemic conditioning may benefit from recognizing aging as a major risk factor for stroke. Accordingly, optimizing the timing of treatment intervention for both stem cell-based treatments should consider age-related chronic low perfusion, blood-brain barrier breakdown, immune alterations, neuronal loss, abnormal demyelination, and impairments in the stem cell proliferative capabilities during stroke progression (Zhang et al., 2022). An equally appealing therapeutic pathway implicated in ischemic conditioning is the repair of the energy-sensitive organelles mitochondria (Jeon et al., 2022). Targeting the mitochondria to confer ischemic conditioning-like protection of the stroke brain may be accomplished through pharmacological treatment. For example, a Korean red ginseng extract has been shown to protect astrocytic mitochondrial function against the in vitro ischemic insult produced by oxygen-glucose deprivation/recovery (Jeon et al., 2022). Similar to stem cell transplantation, pharmacologic treatment that enhances mitochondrial function may serve as an adjunct treatment to ischemic conditioning. An in-depth understanding of therapeutic pathways associated with stem cell proliferation and mitochondrial function, among other neuroprotective mechanisms, may reveal established as well as novel approaches to optimizing the beneficial outcomes of ischemic conditioning for stroke. Other brain disorders, such as traumatic brain injury, epilepsy, and cerebral palsy, which similarly manifest with ischemia-like pathology, may stand as additional disease targets for ischemic conditioning.
Conditioning medicine for heart failure
As noted above, ischemic conditioning was originally explored in heart failure. While the conventional method to induce ischemic conditioning in the heart is via cuffing (e.g., (3 cycles of inflation and deflation of a left lower limb cuff, for 5 min each) (Pires et al., 2023), recent studies have examined the use pharmacological treatments to mimic the ischemic-conditioning-like effects. Newly identified drugs with cardioprotective ischemic conditioning outcomes include the sodium glucose co-transporter 2 (SGLT2), which serves a key role in regulating glucose levels (Cong et al., 2022). SGLT2 is selectively expressed in human kidney and is overactive in patients with type 2 diabetes mellitus (T2DM), suggesting that its inhibition may counteract hyperglycemia (Cong et al., 2022). By reducing glucose levels, SGLT2 inhibitors exert cardioprotective effects not only in T2DM but also non-diabetic patients via multi-pronged therapeutic mechanisms, such as maintaining ketone body metabolism, ionic imbalances, and mitochondrial function, while dampening inflammation (Cong et al., 2022), altogether reminiscent of ischemic conditioning outcomes. Similarly, insulin has been shown to promote protection against myocardial injury based on the observation that impaired insulin signaling and depression of various voltage-dependent K+-channels propel prolonged cardiac action potentials and long-QT interval (i.e., the section on an electrocardiogram corresponding to the time between heart muscle contraction and recovery) (Turan, 2022). Accordingly, insulin treatment may foster proper cellular signaling during heart failure (Turan, 2022). Another major player in cardiac voltage-gated channels is Na+, which participates in the generation of cardiomyocyte action potential and the propagation of electrical impulses in the heart (Billur & Turan, 2022). New studies have documented that Na+ isoforms may contribute to mammalian heart remodeling (Billur & Turan, 2022), which should facilitate repair mechanisms following heart failure. A caveat to the success of these pharmacological treatments is the recognition of aging in exacerbating heart diseases (Turan, 2002; Billur & Turan, 2022), with hyperglycemia, impaired K+- and Na+-voltage-dependent channels, and abnormal insulin levels being more rampant in aged individuals. Thus, optimizing SGLT2 inhibitors, insulin, and drugs that target K+- and Na+-voltage-dependent channels should employ aging-incorporated animal models to fully capture cardiac failures and the safety and efficacy of the ischemic conditioning-based pharmacological treatments. Additional critical factors that need to be considered when contemplating ischemic conditioning treatments include the subtle and mostly undiagnosed coronary microvascular dysfunction (CMD) that accompanies ischemic heart disease (Lamprou, et al., 2022) and the timing of intervention after ischemia (Xiao et al., 2022). Interestingly, CMD triggers inflammation, among other myriad of secondary cell death pathways (Lamprou ewt al., 2022), which may be sensitive to ischemic conditioning. In terms of initiation of treatment, short duration ischemia of less than 30 minutes benefits primarily from lowering ischemic-induced oxidative stress and boosting endothelial nitric oxide synthase activity; intermediate duration ischemia between 30 and 60 minutes are mostly responsive to ischemic preconditioning, postconditioning, mitochondrial permeability transition pore (mPTP) inhibition, opioids, sevoflurane, and metoprolol. Long duration ischemia of more than 60 minutes is sensitive to postconditioning and mPTP inhibition (Xiao et al., 2022). Ischemic conditioning may be further optimized by recognizing CMD and timing of intervention, in addition to identifying specific patient populations who may or may not benefit from ischemic conditioning, including survivors of out-of-hospital cardiac arrest (Lane & Bulluck, 2022) and those diagnosed with coronary artery disease who receive percutaneous coronary intervention or coronary artery bypass grafting (Woodhead et al., 2022).
Conditioning medicine for other organ failures
In addition to the brain and heart, there are many studies documenting the therapeutic effects of ischemic conditioning in kidney, liver, and lungs. These other organs appear to be sensitive to ischemic conditioning with similar challenges faced by the brain and the heart, such as the age of the subjects and the timing of treatment initiation. Because Ischemia/reperfusion injury entails deficient oxygen supply and subsequent restoration of blood flow, the consequential pathogenesis manifests as complex, multifactorial and highly integrated cell, tissue, and organ damage (Lejay, et al., 2016). Designing ischemic conditioning to mount tolerance against ischemia-reperfusion injury requires an understanding of the disease manifestations, as well as the co-morbidities. To this end, among the many co-morbidities accompanying the brain, heart, and other organ failures, diabetes mellitus stands as a significant mitigating factor (Epps et al., 2022). Diabetes mellitus increases the brain (Dehondt et al., 2022), heart (Lejay et al., 2016), kidney (Baffour-Awuah et al., 2021), liver (McDonald et al., 2018), and lung (Feige et al., 2022; Viastos et al., 2022) susceptibility to ischemia-reperfusion injury, necessitating adjustments to ischemic conditioning strategies in order to enhance ischemic tolerance (Epps et al., 2022). Thus, focusing on both disease morbidities and co-morbidities, especially diabetes mellitus, may reveal valuable protective mechanisms associated with ischemia-reperfusion injury that will guide the translation of safe and effective ischemic conditioning strategies for brain, heart, and other organ failures.
Summary
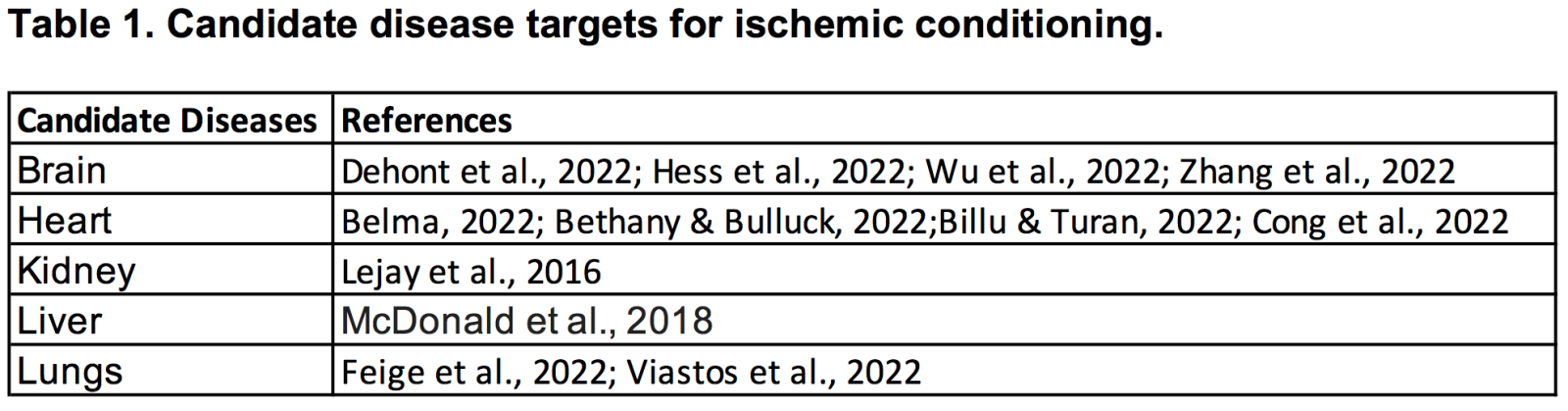
Ischemia-reperfusion injury in the brain, heart, and other organs manifests with devastating cell, tissue, and organ failures (Table 1). Limited therapeutic options exist to treat ischemia-reperfusion injury. Ischemic conditioning is a treatment strategy designed to promote ischemic tolerance. Safety and efficacy studies from animal models to human trials support the use of ischemic conditioning in stroke, cardiac arrest, and other organ failures. Future studies addressing mechanisms of action, co-morbidity factors such as aging and diabetes mellitus, and optimizing the timing of treatment intervention, will further advance the safe and effective application of ischemic conditioning in the clinic. Although ischemic conditioning is not associated with severe adverse effects, acute and long-term monitoring of patients will clarify the safety profile of the treatment.
Conflict of interest
The authors declare no conflict of interest.
References
Maximillian C. Borlongan1
1Department of Neurosurgery and Brain Repair. Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida
Jea-Young Lee1
1Department of Neurosurgery and Brain Repair. Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida
Corresponding author:
Jea-Young Lee
Email: jeayoung@usf.edu

In a new window | Download PPT
Figure 1. Ischemic conditioning for protecting the brain, heart, and other organs. Promoting ischemic tolerance via ischemic conditioning can protect the brain, heart, kidney, liver, and lungs from injury and its associated co-morbidity factors such as aging and diabetes via multiple degenerative pathways, including inflammation and oxidative stress.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6002 | 15 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA