Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The Regulatory Power of MicroRNAs in Mediating Cardioprotection
Time:2024-01-16
Number:5232
Aishwarya Prakash1,2, Sauri Hernandez-Resendiz1,2
Author Affiliations


- 1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
- 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
Conditioning Medicine 2023. 6(3): 70-77.
Abstract
The detrimental effects arising from acute myocardial infarction (AMI) due to ischemia/reperfusion injury (IRI) impair cardiac function through adverse cardiac remodeling. As such, new treatments are needed to protect the myocardium from IRI to prevent heart failure. In this regard, microRNAs are emerging as key regulators of several biological processes governing cardiac function and development, which can be leveraged to treat the adverse cellular processes arising due to acute myocardial IRI. Regulation of microRNA biogenesis and function are critical processes that determine normal functioning and development of the myocardium. Several new, promising microRNAs have been identified as key players of specific target genes in pathways of cell survival, apoptosis, metabolism, inflammation, fibrosis, and angiogenesis in the setting of IRI, which this review will aim to highlight, focusing on studies in the recent five years. This review will also elucidate the therapeutic capacity and potential challenges of microRNAs as a cardioprotective strategy to combat the adverse effects of IRI.
Keywords: Cardioprotection, MicroRNA, Acute myocardial infarction, Heart failure, Small non-coding RNA
Abstract
The detrimental effects arising from acute myocardial infarction (AMI) due to ischemia/reperfusion injury (IRI) impair cardiac function through adverse cardiac remodeling. As such, new treatments are needed to protect the myocardium from IRI to prevent heart failure. In this regard, microRNAs are emerging as key regulators of several biological processes governing cardiac function and development, which can be leveraged to treat the adverse cellular processes arising due to acute myocardial IRI. Regulation of microRNA biogenesis and function are critical processes that determine normal functioning and development of the myocardium. Several new, promising microRNAs have been identified as key players of specific target genes in pathways of cell survival, apoptosis, metabolism, inflammation, fibrosis, and angiogenesis in the setting of IRI, which this review will aim to highlight, focusing on studies in the recent five years. This review will also elucidate the therapeutic capacity and potential challenges of microRNAs as a cardioprotective strategy to combat the adverse effects of IRI.
Keywords: Cardioprotection, MicroRNA, Acute myocardial infarction, Heart failure, Small non-coding RNA
Highlights
MicroRNAs (miRNAs) have been identified as key regulators that can mitigate the damaging effects of acute myocardial ischemia/reperfusion injury. This review emphasizes the importance of miRNA biogenesis, and their functional pathways as integral to sustaining cardiac health and preventing the progression to heart failure. Moreover, it explores the promising therapeutic
applications of miRNAs addressing the complexities of advancing these molecular insights to clinically viable treatments for patients, altogether recapitulating the main tenet of conditioning medicine for cardiovascular diseases.
Introduction
Acute myocardial infarction (AMI) is one of the leading causes of morbidity and mortality in the world, the reason for which arises from the detrimental effects of acute ischemia/reperfusion injury (IRI) on the myocardium. Acute IRI is a highly complex injury arising from several dynamic changes in various cellular pathways across several cell types, starting with cardiomyocyte (CM) death, bioenergetic dysregulation, inflammatory response, angiogenesis, and myocardial fibrosis (Turer and Hill, 2010; Yang, 2018). This compensatory process, over time, induces adverse left ventricular (LV) remodeling, impairing cardiac function, leading to heart failure. Despite intensive investigations, there are currently no effective therapies to combat these adverse effects of IRI. As such, there is an urgent need to identify new strategies to treat acute myocardial IRI. With the advancement of precision medicine and next generation sequencing, small non-coding RNAs such as microRNAs have been discovered as critical regulators of most biological pathways regulating cardiovascular function (Barwari et al., 2016). This opens the door to studying the potential of these microRNAs to mediate various cardioprotective pathways, which can then be leveraged to develop therapies to mitigate the adverse effects of IRI. This review provides an overview of the role of microRNAs in cardioprotection, starting with the biology and mechanism of action, followed by its regulatory capacity in various cellular processes of IRI, and addressing the therapeutic potential and challenges in translating this therapy to the clinical setting.
MicroRNAs Biogenesis, Function, and Mechanism of Action
MicroRNAs (miRNAs) are a class of non-coding, single-stranded RNAs (~20-22 nucleotides long) that regulate gene expression post-transcriptionally of several genes involved in pathways of cell survival, stress response, proliferation, and apoptosis (Lu and Rothenberg, 2018; O'Brien et al., 2018; Ghafouri-Fard et al., 2020).
Biogenesis of miRNAs is mainly a four-step process: i) beginning with the transcription of primary miRNAs (pri-miRNAs) by the action of RNA polymerase II and III; ii) cleavage of pri-miRNAs by RNase II Drosha and DiGeorge syndrome critical region 8 (DGCR8) to form precursor miRNAs (pre-miRNAs); iii) shuttling of pre-miRNAs into the cytoplasm via the Exportin5/RanGTP complex, which then forms a mature miRNA duplex by the action of RNase Dicer III; iv) this mature miRNA duplex is then unwound to form a functional single strand miRNA by the action of an Argonaute (AGO) family protein (Figure 1). This single strand guide miRNA is then incorporated into the RNA-induced silencing complex (RISC) for targeted mRNA repression. This RISC facilitates post-transcriptional silencing by base pairing of the single strand guide miRNA to the miRNA response element (MRE) sequence on the mRNA. This RISC-MRE interaction causes either mRNA cleavage by AGO endonucleases or mRNA decay by deadenylation and decapping via specific effector proteins. The MRE sequence on most genes is found at the 3’ untranslated region (UTR) and more rarely at the 5’ UTR region, coding sequence, and gene promoters. Interestingly, in more recent studies, the RISC-MRE interactions have also been found to activate gene expression (O'Brien et al., 2018). It is also important to note that one guide miRNA may have binding affinity to MRE of several target mRNAs, and each mRNA may have MREs with binding affinities to several miRNAs. Therefore, the interactions and effect of miRNAs with their target genes are dynamic and depend on the abundance of miRNAs and target mRNAs, the affinity and efficacy of the RISC-MRE binding interaction, as well as the subcellular location of the miRNAs. Thus, miRNAs can influence gene expression by regulating the levels of available mRNA for translation.
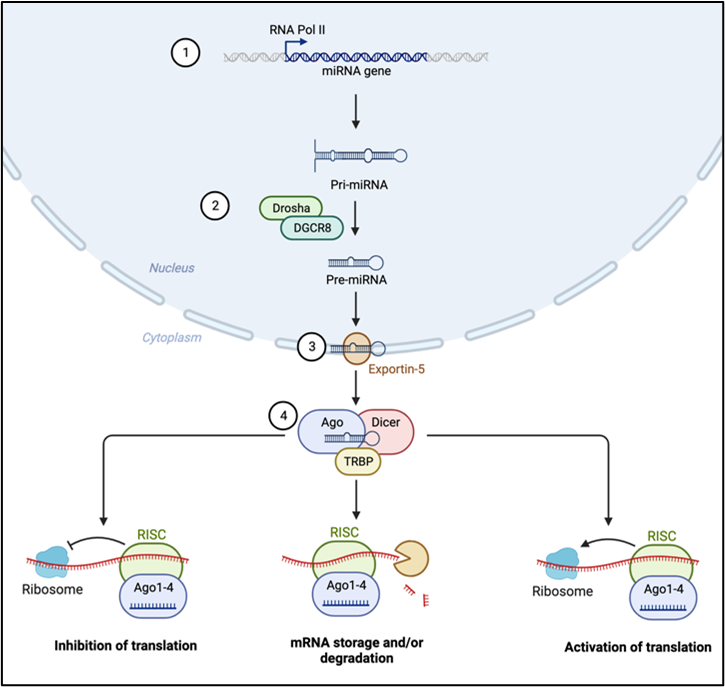
In a new window | Download PPT
Figure 1. Biogenesis and mechanism of action of miRNAs as potential regulators of cardioprotective processes. 1) Transcription of primary miRNA (pri-miRNA); 2) Clevage of pri-miRNA to precursor miRNA (pre-miRNAs); 3) Shuttling of pre-miRNA into the cytoplasm by Exportin; 4) Formation of RNA induced silencing complex (RISC) and the various RISC-miRNA response element (MRE) interactions.
While most miRNAs are found intracellularly, some miRNAs are also released into the blood circulation and are known as circulating miRNAs. Circulating miRNAs may be released in response to certain cell injuries or stimuli and enter the circulation in association with specific proteins (such as HDL, Ago2, nucleophosmin) or in cell-derived extracellular vesicles (such as exosomes, microvesicles, and apoptotic bodies). Once in the circulation, these miRNAs can travel to and target distal sites by fusing directly with the cell membrane or by receptor-mediated interaction, which implies their role in cell-cell communication (Zhou et al., 2018).
This function of miRNAs as cellular messengers represents their potential as novel therapeutic targets in treating AMI and preventing the resulting heart failure (Figure 2). More studies in recent years have also provided evidence that circulating miRNAs have an additional biological role as diagnostic biomarkers for several diseases, including AMI and heart failure (Xue et al., 2019; Guo et al., 2020b; Wang et al., 2021a; Mi et al., 2022), further highlighting the importance of the miRNAs in the pathogenesis of disease.

In a new window | Download PPT
Figure 2. MicroRNAs as potential regulators of cardioprotective processes. MicroRNAs induce translation, repression, or activation of target genes that regulate several biological processes involved in cardioprotective effects following acute myocardial ischemia/reperfusion injury, such as apoptosis, inflammation, metabolic regulation, fibrosis, and angiogenesis.
MicroRNAs and their potential to repair the heart after acute myocardial infarction
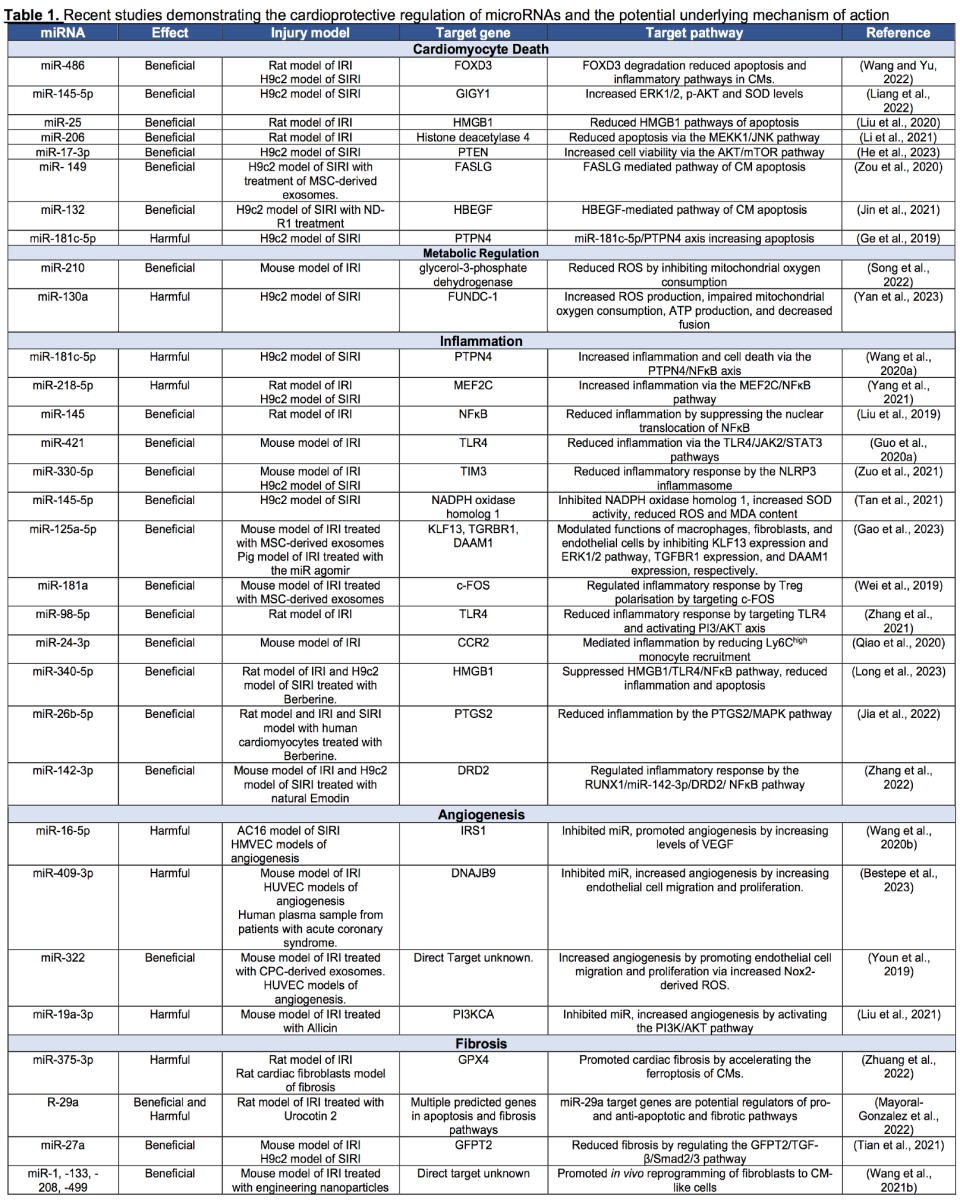
Acute myocardial IRI triggers a series of detrimental events, starting with cardiomyocyte death, leading to an inflammatory response that induces fibrosis to form scar tissue. The size of the progression of this scar tissue changes the architecture of the left ventricle (LV) in a detrimental manner, which ultimately impairs cardiac function and leads to heart failure. Continuous research over the years has aimed to understand the underlying molecular and cellular mechanisms of this adverse LV remodeling in which the participation of miRNAs has come to light in recent years. The first few studies in the early 2010s highlighted the importance of the biogenesis and regulation of miRNAs in normal cardiac functioning, elucidating the maladaptive effects in cardiac function upon dysregulation of the biogenesis pathway (da Costa Martins et al., 2008; Bauersachs and Thum, 2011). These studies discovered the posttranscriptional regulatory mechanisms of miRNA processing, but their therapeutic role in cardiovascular diseases had not yet been elucidated. The several studies that followed, especially in the last five years, have covered this knowledge gap and have demonstrated changes in the miRNA profile of the myocardium upon acute myocardial IRI and showed that genetic or pharmacological modulation of these identified miRNAs influenced the severity of the injury (Table 1). These studies demonstrated the critical role of miRNA-dependent regulation of cardiomyocyte death, metabolic regulation, inflammatory response, angiogenesis, and fibrosis upon acute myocardial IRI, all of which determine the adverse cardiac remodeling that leads to heart failure (Table 1). In the following section, we aim to highlight some of the new and novel miRNAs mediating cardioprotection through the above-mentioned cellular processes governing adverse LV remodeling and cardiac function.
MicroRNAs in Cardiomyocyte Death
Cardiomyocyte (CM) death is the key and central event occurring in acute myocardial IRI that initiates the cascade of events contributing to the pathogenesis of IRI that can eventually result in heart failure (Del Re et al., 2019). Recent studies have highlighted the regulation of specific miRNAs in alleviating cardiomyocyte death using cellular and animal IRI models. Wang and Yu (2022) elucidated the role of miRNA-486 in myocardial IRI using a rat model and a cellular model of IRI with H9c2 rat cardiomyoblasts, where they discovered the cardioprotective effect of miRNA-486 in reducing CM death by binding and inhibiting FOXO3 activity. Another study by Liang et al. (2022) demonstrated that overexpression of miR-145-5p in H9c2 cardiomyoblasts attenuated CM apoptosis by promoting cell proliferation by binding and inhibiting the expression of GIGY1, which in turn increased the expression of pro-survival protein ERK1/2 and p-AKT, and also improving the release of superoxide dismutase (SOD). Administration of a miR-25 agomir also reduced cell death by targeting HMGB1 in a rat model of myocardial IRI (Liu et al., 2020). Up-regulation of miR-206 by silencing histone deacetylase 4 reduced CM apoptosis via the MEKK1/JNK pathway in a rat model of IRI (Li et al., 2021). MiR-17-3p was found to induce cardioprotection in H9c2 cells exposed to in vitro simulated IRI (SIRI) by suppressing PTEN, thereby regulating the Akt/mTOR axis to promote cell viability (He et al., 2023). In addition to the targeted application of specific miRNAs for cellular regulation, it has been demonstrated that miRNA profiles can be influenced by treatments with various agents, including exosomes, as reported by Zou et al. in 2020 (Zou et al., 2020), and bioactive compounds such as notoginsenoside (NG)-R1 (Jin et al., 2021). Administration of mesenchymal stem cell (MSC)-derived exosomes in a study by Zou et al. (2020) demonstrated reduced CM apoptosis in H9c2 cardiomyoblasts exposed to SIRI. They found that treatment of MSC-derived exosomes increased the expression of miR-149 and Let-7c, which regulated Faslg to reduce CM apoptosis. Similarly, miRNA regulation was also observed upon treatment with notoginsenodie R1 (NG-R1) to H9c2 cells subjected to SIRI, where its cardioprotective effects were induced by the upregulation of miR-132 and downregulation of its target protein HBEGF (Jin et al., 2021). Apart from these studies showing the cardioprotective effects of miRNAs, an interesting study by Ge et al. (2019) showed the opposite. In their model of SIRI in H9c2 cells, they demonstrated that IRI increased the expression of miR-181c-5p, which aggravated apoptosis by targeting PTPN4. Therefore, they proposed that targeting the miR-181c-5p/PTPN4 axis may allow for the discovery of new cardioprotective strategies (Ge et al., 2019). Although these studies provide meaningful insights into the regulation of miRNAs for cardioprotection, a critical limitation they face is only considering one miRNA target when it is well known that miRNAs have several target MRE sequences. Therefore, considering only one miRNA target may not represent the actual biological effect of the miRNA of interest. Additionally, as a plethora of miRNAs are up- and down-regulated after IRI, the administration of just one miR may not yield beneficial effects in a clinical setting with patients with several co-morbidities. It is also noteworthy that most studies use an SIRI model in H9c2 cardiomyoblasts, which, though more widely accepted, have a genetic make-up different from that of humans and thus may impact the translational capacity of these identified miRNAs.
MicroRNAs in Metabolic Regulation
Acute myocardial IRI is fundamentally an injury of metabolic pathology as it is induced by the sudden halt of aerobic metabolic pathways during ischemia and exacerbated by the rapid restart of those pathways at the onset of reperfusion (Zuurbier et al., 2020). In recent years, microRNAs have been found to regulate specific metabolic pathways influencing the effect of IRI, which could be leveraged as therapeutic strategies. Song et al. (2022) elucidated the critical role of miR-210 in targeting mitochondrial glycerol-3-phosphate dehydrogenase to reduce mitochondrial reactive oxygen species (ROS) by inhibiting mitochondrial oxygen consumption during IRI using an in vivo mouse model and in vitro SIRI models (Song et al., 2022). On the other hand, a study by Yan et al. (2023) revealed that miR-130a is upregulated in IRI, impairing mitochondrial oxygen consumption and ATP production, in addition to increasing ROS production. Interestingly, it was also found to decrease mitochondrial fusion in a SIRI model with H9c2 cardiomyoblasts. This study showed that miR-130a influences FUNDC1-mediated mitophagy by down-regulating the mitochondria related gene GJA1, which is interesting as these authors, based on their results, suggest using antisense miRNA oligonucleotides or pharmacological inhibitors of miR-130a such as the 8-mer tiny locked nucleic acid (Monoe et al., 2021) to improve mitochondrial respiration and further increase mitochondrial fission and mitophagy (Yan et al., 2023). Pharmacological inhibitors of miR-130a have been explored, uncovering promising therapeutic possibilities. Monroig et al. (2015) and Nguyen and Chang (2017) discussed the potential of developing small molecule inhibitors for miRNAs like miR-130a, proposing them as a viable therapeutic approach. Stenvang et al.'s 2012 paper provided insights into using antisense oligonucleotides to inhibit miRNAs (Stenvang et al., 2012). Furthermore, Gumireddy (2008) described pioneering work on small molecule modifiers of miRNA function, including that of miR-21 8). These studies present a spectrum of potential pharmacological inhibitors for miR-130a, although further research is essential to confirm their effectiveness and safety. There are various controversies regarding whether increased or reduced mitochondrial metabolism at reperfusion is protective or detrimental. In this case, we believe the relevance of the model of IRI used in terms of the type of animal or cell model, including ischemia and reperfusion times, needs to be considered to interpret the meaning of these results fully.
MicroRNAs in Inflammation
The inflammatory response to IRI plays a key role in determining the extent of the adverse LV remodeling, as persistent inflammatory response affects the subsequent healing phase, making inflammation an important therapeutic target for improving cardiac function in IRI (Ong et al., 2018). MicroRNAs have been shown to regulate this inflammatory process post-IRI, thus they may have potential use in therapeutic strategies. A study by Wang et al. (2020a) found an increase in miR-181-c-5p levels in H9c2 cardiomyoblasts after SIRI, which upregulated PTPN4/ nuclear factor kappa-B (NFκB) signaling, promoting an inflammatory response. Therefore, they suggested targeting the miR-181c-5p/PTPN4/NFκB axis as a therapeutic strategy to reduce the inflammatory response. Yang et al. (2021) discovered another miRNA whose overexpression upon IRI was detrimental: miR-218-5p. They showed that by inhibiting this miRNA, the inflammatory response was perturbed by targeting the MEF2C/NFκB axis in a SIRI model with H9c2 cells and an in vivo rat model of IRI (Yang et al., 2021). Apart from the studies that found detrimental miRNAs overexpressed upon IRI, there were also studies that showed the opposite: beneficial miRNAs that were inhibited upon IRI whose pharmacological administration could elicit cardioprotective effects. Overexpression of miR-145 has been shown to alleviate the effect of IRI by suppressing the translocation of NFκB p65 in a rat model of IRI within two hours of reperfusion, thereby reducing the inflammatory response (Liu et al., 2019). Studies by Guo et al. (2020a) found that administration of miR-421 reduced the inflammatory response in a mouse model of IRI inactivating TLR4, JAK2, and STAT3. Zuo et al. (2021) discovered increased levels of miR-330-5p in both the in vivo mouse model of IRI and the SIRI model with H9c2 cells, which upon further overexpression, was cardioprotective by inhibiting the inflammatory response induced by the NLPP3 inflammasome. A study by Tan et al. (2021) also demonstrated that overexpression of miR-145-5p can be cardioprotective by inhibiting the inflammatory response by reducing the levels of NADPH oxidase homolog 1, which increased SOD activity and reduced ROS and malondialdehyde (MDA) content in a H9c2 model of SIRI. Apart from the direct regulation of miRNAs influencing inflammation, administration of MSC-derived exosomes and other chemical compounds such as berberine and emodin were also found to modulate the miRNA profile to be more cardioprotective. MSC-derived exosomes were found to deliver miR-125a-5p, miR-181a, miR-98-5p, and miR-24-3p to the cardiomyocytes, which were all found to ameliorate adverse inflammatory responses in various models of IRI (Wei et al., 2019; Qiao et al., 2020; Zhang et al., 2021; Gao et al., 2023). Certain chemical compounds, such as berberine, were found to regulate two different miRNAs in two different studies: miR-340-5p and miR-26b-5p, both of which inhibited the inflammatory response by suppressing HMGB1-mediated TLR4/NFκB and PTGS2/MAPK pathways, respectively in different models of IRI (Jia et al., 2022; Long et al., 2023). Another chemical compound, emodin, was found to upregulate miR-142-3p, which suppressed NFκB mediated inflammation via the dopamine receptor D2 in a mouse model of IRI (Zhang et al., 2022). It is vital to note that although most studies highlight the beneficial effects of a suppressed pro-inflammatory response, it is more of the balance and timely transitioning from the pro-inflammatory to anti-inflammatory response in IRI that would account for the cardioprotective effects. Complete suppression of the initial pro-inflammatory phase may also be detrimental as this response is critical to clear out the injured and dead cardiomyocytes. Therefore, when monitoring the regulation and effect of these miRNAs in modulating the inflammatory response, the type of IRI model and the reperfusion times studied would be critical to better understanding the results.
MicroRNAs in Angiogenesis
The blood supply of cells in the area at risk and the infarcted region in the myocardium gradually decreases post-IRI, thereby restricting oxygen, nutrient transfer and removal, and metabolic wastes, which results in CM death (Li et al., 2022). Therefore, angiogenesis is critical to restoring blood flow to the myocardium and improving clinical outcomes, which are regulated by miRNAs. MiR-16-5p knockdown was found to increase cell viability in a SIRI model with human adult ventricular cardiomyocytes (AC16) and promote angiogenesis in human microvascular endothelial cells (HMVEC) by silencing its target IRS1, which in turn elevated levels of the pro-angiogenic factor VEGF (Wang et al., 2020b). Overexpression of miR-409-3p was observed in the serum of patients with acute coronary syndrome and in a mouse model of IRI, which was associated with decreased endothelial cell proliferation and migration. Therefore, inhibition of miR-409-3p would allow increased angiogenic endothelial cell response to IRI (Bestepe et al., 2023). An interesting study by Youn et al. (2019) administered cardiac progenitor cells (CPC)-derived exosomes transfected with the pro-angiogenic miR-322 to mice after IRI and demonstrated enhanced angiogenesis in the border zones of the infarcted hearts. This pro-angiogenic effect was attributed to increased endothelial cell migration and capillary tube formation by increased Nox2-derived ROS (Youn et al., 2019). Another compound that increased angiogenesis was Allicin, which inhibited miR-19a-3p and activated the PI3/AKT pathway in a mouse model of IRI (Liu et al., 2021).
MicroRNAs in Fibrosis
During the final healing phase in myocardial IRI, the necrotic and apoptotic tissue is replaced with a fibrotic scar whose quality depends on the balance of the various pathways involved in fibrogenesis (( Scalise et al., 2021 )). These pathways are regulated by miRNAs, which have the potential to be leveraged as cardioprotective therapies. A study by Zhuang et al. (2022), elucidating the role of miR-375-3p in regulating secondary cardiac fibrosis, found that inhibiting this miR promoted the anti-oxidant capacity of the fibroblasts, reduced GPX1-mediated ferroptosis thereby reducing cardiac fibrosis in a rat model of IRI. Urocortin 2 was found to elicit cardioprotective effects by reducing fibrosis in a rat model of IRI by inhibiting the upregulation of six miRNAs in the myocardium: miR-29a, miR-103, miR-133, miR-339-5p, miR-423_1, and miR-451_1, of which miR-20a and miR-451_1 were predicted to modulate genes in fibrosis pathways (Mayoral-Gonzalez et al., 2022). Kruppel-like factor 5 (KLF5), a transcription factor, was found to induce cell death and fibrosis by downregulating miR-27a, which activated the GFPT2/TGF-β/Smad2/3 pro-fibrotic pathway. This study, therefore, described how a KLF5-specific inhibitor can ameliorate these effects by upregulating miR-27a and reducing cardiac fibrosis (Tian et al., 2021). Although most studies so far have engineered nanoparticles with one specific miRNA, a study by Wang et al. (2021b) engineered mesoporous silicon nanoparticles (MSNs) with a coating of FH peptide-modified neutrophil-mimicking membranes loaded with a combination of four miRNAs known to reprogram cardiac fibroblasts to cardiomyocyte-like cells, namely: microRNA-1, -133, -208, and -499. These MSNs delivered this cargo of miRNAs specifically to cardiac fibroblasts, allowing the in vivo reprogramming of the fibroblasts to cardiomyocyte-like cells, thereby reducing fibrosis and improving cardiac function.
The Promises and Challenges of miRNA targeted therapies
From the discussion in the previous section, there is sufficient evidence to suggest that miRNAs have the potential to be attractive therapeutic strategies to target the various cardioprotective processes in acute myocardial IRI to prevent adverse LV remodeling and heart failure. The attractive qualities of miRNAs include their pleiotropic ability to regulate multiple target genes, thereby influencing multiple processes simultaneously. This poses a significant advantage over the current drug-based approaches that target singular processes. Additionally, miRNAs are small and clearly defined sequences for which agomirs or antagomirs can be effectively mass produced for large clinical studies. Another advantage is that these miRNA-based therapies would bypass the limitations of cell-based approaches, including adverse immune response and rejection.
But however attractive this miRNA-based therapy sounds, it has not yet passed clinical trials and reached patients due to several challenges that should be considered. Firstly, nude miRNAs cannot be administered directly due to their quick degradation rates in the blood (Segal et al., 2020). Therefore, these small RNAs either need to be modified to prevent the degradation or administered via nanoparticles that can be loaded with miRNAs of interest or in extracellular vesicles (such as exosomes), which have both a natural abundance of miRNAs and can be loaded with specific miRNAs of interest (Ghasemiyeh and Mohammadi-Samani, 2018; Prakash et al., 2020). This presents challenges by itself as there are engineering difficulties loading several miRNAs in one nanoparticle. Even if we exploit exosomes, which have an abundance of miRNAs, the mechanism of action is still unknown, with batch-to-batch variations adding to this complexity. Secondly, it is interesting to note that most of these studies investigating cardioprotective miRNAs focus on a single microRNA influencing several processes through a single target gene in cellular models of IRI using rat H9c2 cardiomyoblasts or small animal models with a limited reperfusion time. Therefore, the exact time of action of this microRNA and the specific target cell cannot be clearly understood from these various studies, which questions whether this single administration of one single microRNA can confer long-term protection in humans. If ambiguity in the mechanism of action of these microRNAs and current limitations in delivery can be addressed in future studies, microRNA-based therapies have the potential to be a very potent cardioprotective therapy.
Conclusion
There is a need to discover novel cardioprotective strategies, as no current effective therapy has been found to improve clinical outcomes in patients with acute myocardial IRI. This review highlights and describes miRNAs as potent regulators of the various pathways inducing cardioprotection, including cardiomyocyte death, metabolism, inflammation, angiogenesis, and fibrosis. We have described studies in the past five years elucidating both protective and detrimental miRs that target several different cell types and can be leveraged for cardioprotective strategies. To further enhance these studies for clinical translation, a more detailed understanding of the mechanism of action and dynamic nature of miRNAs, along with a straightforward method of delivery, needs to be elucidated in appropriate cellular models, small animal models, and finally large animal models of IRI, before this may reach clinical trials. Additionally, apart from studying the effects in healthy animals, the miRNAs should also be tested in animals with co-morbidities and co-medications to recapitulate the patient population with IRI. This is key as the miRNA profile has been reported to be affected by the intake of common medications such as vinblastine, dexamethasone, fluoxetine, and enoxacin (He et al., 2015). With these critical points of clinical translation and mechanistic insights addressed in the upcoming years, this growing field of miRNA-based therapies for cardioprotection has the potential to one day reach the clinics to benefit patients and improve clinical outcomes.
Acknowledgments
This article is based upon work supported by the COST Action EU-CARDIOPROTECTION IG16225 supported by COST (European Cooperation in Science and Technology).
Conflict of interest
All authors have no relevant conflicts or disclosures.
References
Aishwarya Prakash1,2
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
Sauri Hernandez-Resendiz1,2
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
Corresponding author:
Sauri Hernandez-Resendiz
Email: sauri.hdz@duke-nus.edu.sg

In a new window | Download PPT
Figure 1. Biogenesis and mechanism of action of miRNAs as potential regulators of cardioprotective processes. 1) Transcription of primary miRNA (pri-miRNA); 2) Clevage of pri-miRNA to precursor miRNA (pre-miRNAs); 3) Shuttling of pre-miRNA into the cytoplasm by Exportin; 4) Formation of RNA induced silencing complex (RISC) and the various RISC-miRNA response element (MRE) interactions.

In a new window | Download PPT
Figure 2. MicroRNAs as potential regulators of cardioprotective processes. MicroRNAs induce translation, repression, or activation of target genes that regulate several biological processes involved in cardioprotective effects following acute myocardial ischemia/reperfusion injury, such as apoptosis, inflammation, metabolic regulation, fibrosis, and angiogenesis.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5232 | 13 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA