Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Ischemic conditioning, retinal neuroprotection, and non-coding RNAs
Time:2024-01-16
Number:6239
Cristina Franco1, Giovanna Lombardi1, Noemi Di Muraglia1, Lorella Maria Teresa Canzoniero1, Serenella Anzilotti1
Author Affiliations


- 1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Conditioning Medicine 2023. 6(3): 78-87.
Abstract
Retinal ischemia is a major cause of blindness worldwide. It is associated with various disorders such as diabetic retinopathy, glaucoma, optic neuropathies, stroke, and other retinopathies. Retinal ischemia is a clinical condition that occurs due to a lack of appropriate blood supply to the retina. In recent decades, the need to find new neuroprotective targets has led researchers to investigate the endogenous molecular mechanisms that the brain activates when exposed to a conditioning stimulus. Conditioning is an adaptive biological process triggered by those interventions capable of conferring resistance to a deleterious brain event through exposure to a sub-threshold insult. In particular, preconditioning and postconditioning occur when the conditioning stimulus is applied before or after the harmful ischemia, respectively. These neuroprotective strategies are widely used to identify new therapeutic strategies for retinal ischemia. The present review describes the most common methods for inducing retinal conditioning, including surgical, temperature-induced, and pharmacological approaches. Furthermore, the use of microRNAs and extracellular vesicles as conditioning agents in retinal diseases is investigated. The purpose of these new diagnostic and therapeutic approaches will be to manage this often-blinding retinal condition. The last few decades have provided fascinating insights into the mechanisms and potential application of strategies to induce retinal conditioning. Since identifying intrinsic cell survival pathways should provide more direct opportunities for translational neuroprotection studies, a thorough examination of different preconditioning and postconditioning patterns as possible pharmacological strategies for damage caused by retinal ischemia is mandatory.
Keywords: Ischemia, Animal models, Preconditioning, Extracellular vesicles
Abstract
Retinal ischemia is a major cause of blindness worldwide. It is associated with various disorders such as diabetic retinopathy, glaucoma, optic neuropathies, stroke, and other retinopathies. Retinal ischemia is a clinical condition that occurs due to a lack of appropriate blood supply to the retina. In recent decades, the need to find new neuroprotective targets has led researchers to investigate the endogenous molecular mechanisms that the brain activates when exposed to a conditioning stimulus. Conditioning is an adaptive biological process triggered by those interventions capable of conferring resistance to a deleterious brain event through exposure to a sub-threshold insult. In particular, preconditioning and postconditioning occur when the conditioning stimulus is applied before or after the harmful ischemia, respectively. These neuroprotective strategies are widely used to identify new therapeutic strategies for retinal ischemia. The present review describes the most common methods for inducing retinal conditioning, including surgical, temperature-induced, and pharmacological approaches. Furthermore, the use of microRNAs and extracellular vesicles as conditioning agents in retinal diseases is investigated. The purpose of these new diagnostic and therapeutic approaches will be to manage this often-blinding retinal condition. The last few decades have provided fascinating insights into the mechanisms and potential application of strategies to induce retinal conditioning. Since identifying intrinsic cell survival pathways should provide more direct opportunities for translational neuroprotection studies, a thorough examination of different preconditioning and postconditioning patterns as possible pharmacological strategies for damage caused by retinal ischemia is mandatory.
Keywords: Ischemia, Animal models, Preconditioning, Extracellular vesicles
Highlights
This study reviews the literature on retinal ischemia with a focus on pre- and post-conditioning methods to induce neuroprotection. Furthermore, the authors review the literature supporting the use of extracellular vesicles and microRNAs as preconditioning agents and as possible therapeutic targets for retinal diseases.
Introduction
Retinal ischemia, characterized by an insufficient blood supply to the retina, is a major cause of visual impairment and blindness. Inadequate blood supply results in a deficiency of essential oxygen and nutrients needed for the proper functioning of retinal cells. The retina shares many similarities with the brain but is relatively resistant to ischemic damage compared to the brain. While cerebral ischemia lasting a few minutes can result in widespread injury and death, the primate retina can withstand central retinal artery occlusion (CRAO) for up to 100 minutes without suffering permanent damage (Hayreh et al., 1980). The isolated retina can efficiently obtain ATP from glycolysis even without oxygen (Winkler, 1972). Thus, retinal neurons may be inherently more resistant to ischemia than cerebral neurons, although the underlying mechanisms are poorly understood. The most common cause of acute retinal ischemia is an embolus from a distant source, similar to cerebral infarcts of the anterior circulation, but other causes include occlusion of retinal blood vessels, systemic diseases that impair blood flow, or local factors that disrupt normal circulation in the eye (Yu et al., 2001). Retinal artery occlusion is an example of retinal ischemia, which occurs when a blood clot or embolus obstructs one of the retinal arteries and cuts off the blood supply to part of the retina (Dattilo et al., 2018). In addition, retinal ischemia can promote glaucoma by damaging retinal ganglion cells (RGC) and impairing optic nerve function (Rehak et al., 2022; Urbonavičiūtė et al., 2022). Another condition associated with retinal ischemia is diabetic retinopathy, which is caused by damage to the retinal blood vessels due to diabetes (Mohite et al., 2023). The consequences of retinal ischemia can vary depending on the severity, duration, and location of the ischemic event. In some cases, symptoms may be transient and disappear as blood flow is restored. In other cases, however, the damage may be irreversible and result in permanent vision loss. The severity of visual impairment can range from mild blurring to complete blindness (Hayreh, 2011).
The cellular mechanisms underlying ischemic retinal damage include a degenerative cascade initiated by energy deficiency and increased glutamatergic stimulation (Ju et al., 2008). When blood supply is inadequate, depolarization of retinal neurons occurs (Khalilpour et al., 2017), triggering a cascade of events leading to cell death. Oxidative stress, characterized by an imbalance between the production of reactive oxygen species and the ability of cells to neutralize them, further exacerbates retinal cell injury (Cho et al., 2015). In addition, the excessive release of the neurotransmitter glutamate, known as excitotoxicity, contributes to cell death and exacerbates the damage caused by retinal ischemia (Adachi et al., 1998). Several treatment approaches have been explored to mitigate the harmful effects of retinal ischemia and interrupt the destructive cascade. The goal is to restore blood flow, reduce neuronal damage, and preserve retinal function. Rapid intervention is critical to maximize the chances of successful treatment. Additionally, there is growing interest in developing neuroprotective agents to mitigate the cellular damage caused by retinal ischemia (Dilsiz et al., 2006). These agents aim to reduce oxidative stress, inhibit excitotoxicity, and promote cell survival. Several molecules such as growth factors, antioxidants, and anti-inflammatory agents have shown promise in preclinical studies and may have the potential for future therapeutic interventions (Yan et al., 2022; Chronopoulos et al., 2023; Yang et al., 2023; Zhao et al., 2023). The prevalence of retinal diseases is expected to increase in the next few decades due to the significant increase in obesity and diabetes, as well as the aging population (Lee et al., 2015). Therefore, it is critical that new and effective methods to treat or prevent these retinal diseases are developed. Over the past decade, the understanding of the molecular and cellular mechanisms underlying retinal ischemia has improved dramatically. Preconditioning and postconditioning strategies have been widely described as effective mechanisms for neuroprotection in retinal ischemia. The following sections discuss and summarize the most common methods for conferring neuroprotection through conditioning in retinal ischemia.
Retinal ischemia
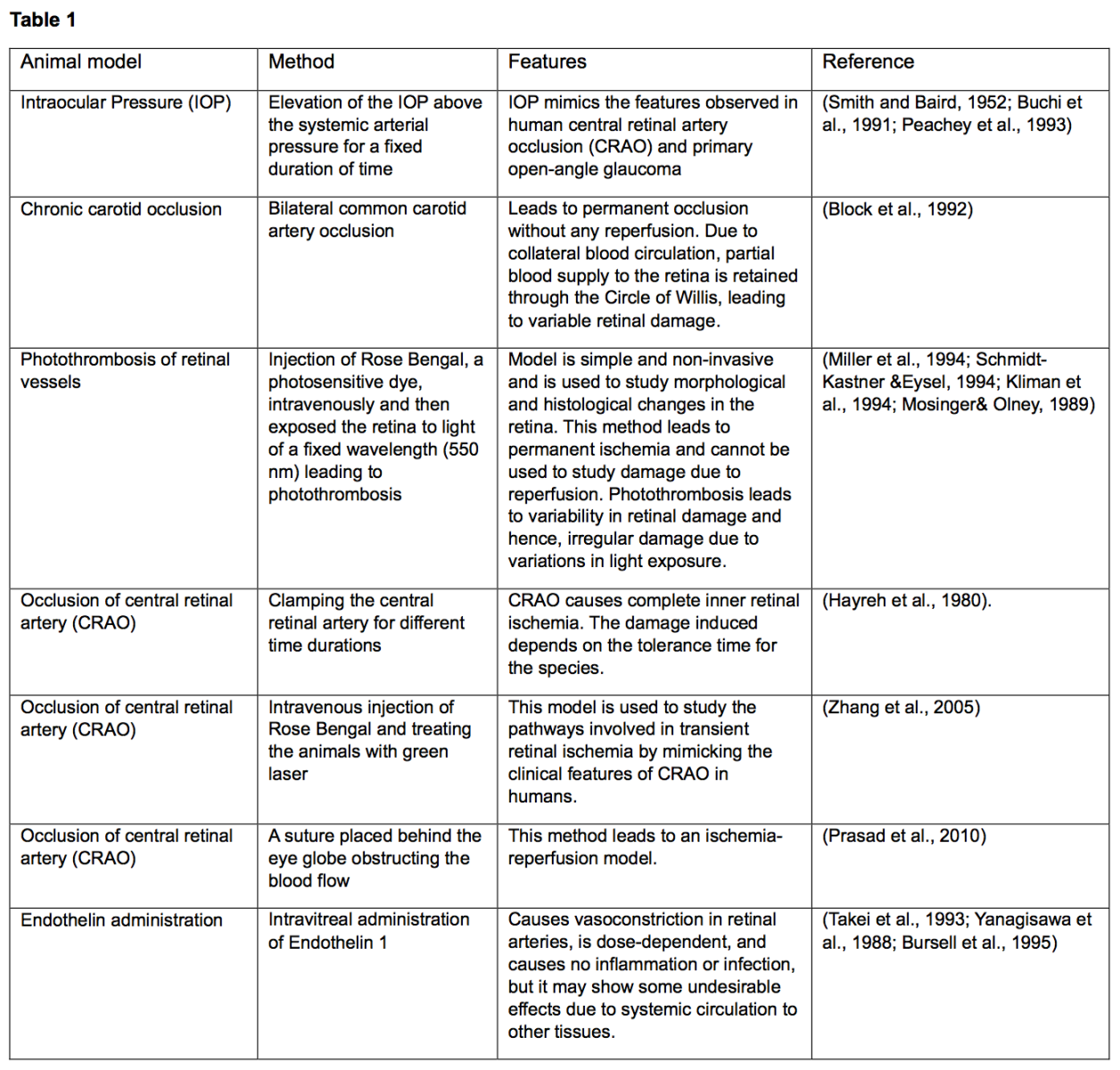
Ischemia is one of the main factors determining the pathophysiology of many retinal diseases, such as glaucoma, age-related macular degeneration, and diabetic retinopathy (Osborne et al., 2004). Ischemic retinopathy develops when blood flow in the retina is insufficient to meet the metabolic demands of the retina. Because the retina is one of the tissues that consumes the most oxygen, disruption of this phenomenon leads to morphologic and functional changes in retinal structure, which can extend to blindness (Böhm et al., 2022). Many mammalian models in vivo and ex vivo have been used to study retinal ischemia (Table 1). However, there are differences between the rat and human retina. In particular, the main blood supply to the rat retina is via a single posterior ciliary artery that runs along the ventromedial axis of the optic nerve and divides into three branches at the optic nerve head: a central retinal artery that supplies blood to the retina and a medial and lateral artery along the retinal arteries that supply blood to the choroid (Onda et al., 1995; Zhi et al., 2012). Complete transection of this vessel causes severe ocular trauma and inflammation and diffuse retinal infarction. Permanent occlusion of this vessel is not equivalent to central retinal artery (CRA) occlusion in humans, and even transient occlusion in rats likely causes more extensive lesions. Therefore, the degree of damage caused during a study must be considered because it may not accurately reflect the damage in the human eye. The elevation of intraocular pressure (IOP) is a commonly used model for studying retinal ischemia and has been described in several species (Li et al., 2000; Zhu et al., 2006). In 1952, Smith and Baird first described the induction of ocular ischemia in rats by introducing sterile fluid into the animal's eye under sufficient pressure to interrupt retinal circulation and thereby induce neuronal degeneration (Smith and Baird, 1952). The basic technique in rats was modified by Buchi et al. in 1991 (Büchi et al., 1991). These authors cannulated the anterior chamber of the rat eye with a needle connected to an elevated reservoir so that the intraocular pressure rose to 110 mmHg, making the intraocular pressure greater than the ocular perfusion pressure. This method leads to global ischemia that blocks both retinal and uveal blood flow, as evidenced by the flattening of the flash electroretinogram (ERG), leading to blanching, and pallor of the iris. This method results in pathological features almost identical to those seen after central retinal artery occlusion and may provide a model for acute angle-closure glaucoma. Lafuente et al. (2002), using an ocular traction technique that increases intraocular pressure over retinal perfusion pressure, demonstrated that an ischemia time of more than 45 minutes is required to produce quantifiable histopathological changes in the retina.
Preconditioning in retinal ischemia
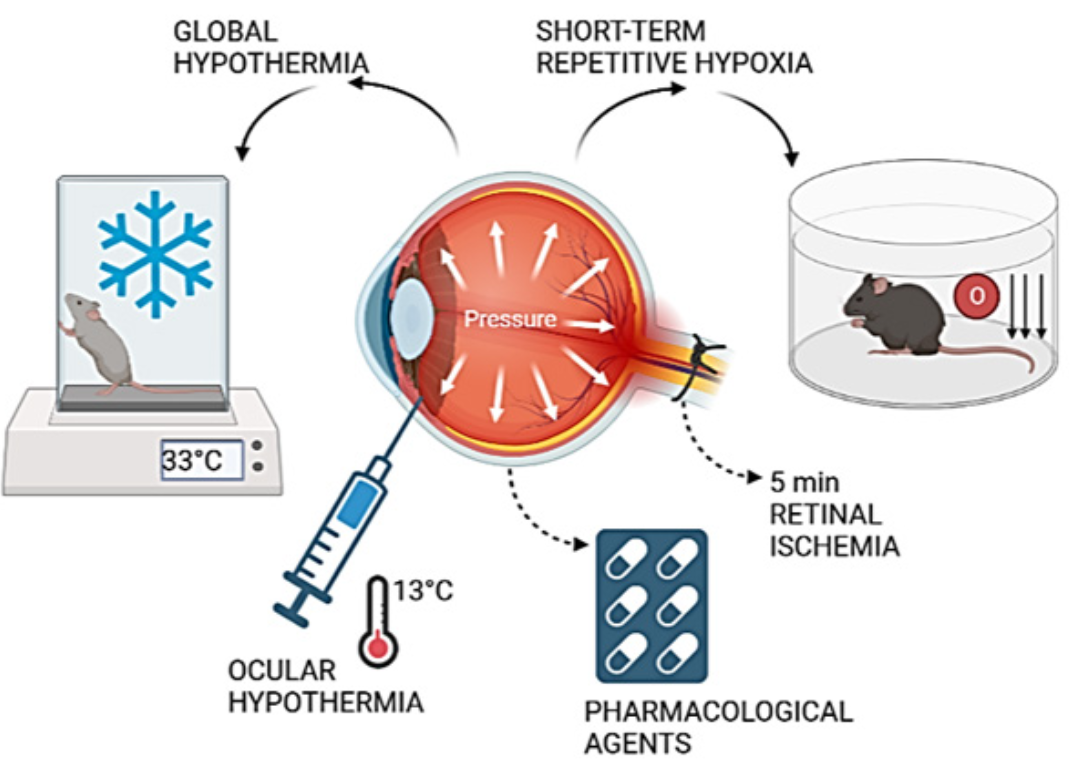
Ischemic conditioning is a neuroprotective approach that uses a sublethal stimulus to make an organ more resistant to an ischemic insult. This method provides neuroprotection when the conditioning stimulus is administered before or after a harmful ischemia, i.e., preconditioning or postconditioning (Pignataro et al., 2007; Pignataro, 2016). Indeed, ischemic preconditioning is an endogenous defense process triggered by a subclinical ischemic event that increases tissue resilience or, in other words, organ resistance to a subsequent, typically dangerous, ischemia (Vinciguerra et al., 2018; Cuomo et al., 2020). Since their original description in the brain by Kitagawa et al. (1990), nonischemic preconditioning stimuli have been classified as various stressors such as toxins, seizures, anoxia, hyperthermia, and spreading depression that, when applied at subthreshold levels, can induce tolerance to generally fatal episodes of the same type of insult. Importantly, nonischemic conditioning stimuli can have an additional neuroprotective effect on an ischemic insult, a phenomenon known as “cross-tolerance” (Plamondon et al., 1999; Gidday, 2006). When the subthreshold stimulus is administered after the ischemic insult, the neuroprotection achieved is comparable to that observed in models of ischemic preconditioning. The first in vivo brain preconditioning study demonstrated an acute increase in rat brain capacity for anaerobic glycolysis after brief anoxia, which increased animal survival after subsequent prolonged anoxia (Gidday, 2006). The first reference document on cardiac preconditioning in dogs with brief coronary ischemia was published in 1986 (Murry et al., 1986). Since then, many studies have shown that preconditioning stimuli induce ischemic tolerance in various tissues (Gidday, 2006; Pignataro et al., 2020). Since the treatments available for retinal diseases are not completely effective, more effective approaches for prevention and treatment must be developed. Based on highly effective protection induced by ischemic preconditioning against an acute ischemic episode, and since retinal ischemia (Stitt et al., 2011) is common in all retinal diseases, the effect of ischemic tolerance on retinal damage has long been studied. The results obtained are summarized below and in Fig. 1. Steven Roth et al. (1998) first described the phenomenon of preconditioning in retinal ischemia, which was induced by a silk suture placed around the optic nerve and blood vessels behind the globe of one eye, disrupting blood flow. A five-minute period of retinal ischemia was the preconditioning stimulus. To assess the time course of the preconditioning response, animals were first preconditioned, and the ischemic insult was then applied for 60 minutes, 1, 24, 72, or 168 hours later, or they were subjected to a five-minute sham treatment, and the ischemic insult was applied for 60 minutes 24 hours later. It was shown that a- and b-waves (spontaneous bursts of action potentials that propagate wave-like across the retina) fully recovered to preischemic amplitudes in preconditioned rats, in contrast to nonpreconditioned rats. The histological damage caused by the ischemic insult was completely prevented when preconditioning was performed 24 or 72 hours (but not 168 hours) before ischemia (Roth et al., 1998). Liu et al. (2017) identified a pattern of glaucoma tolerance by using short-term repetitive hypoxia as a preconditioning stimulus (RHP) in mice. RHP was able to prevent the degeneration of brn3-positive ganglion cells. In addition, axon density in the postlaminar optic nerve was strongly maintained in RHP-treated mice, and neurofilament immunostaining also showed preconditioning-induced improvement in axon integrity/survival in both the retina and optic nerves after ten weeks of experimental glaucoma (Liu et al., 2017). In another study, Salido et al. (2013) demonstrated that brief global or ocular hypothermia applied 24 hours before ischemia (i.e., hypothermic preconditioning, HPC) protected the retina from ischemia-reperfusion injury and that glutamate was involved in HPC-induced retinal protection. Retinal ischemia was induced by an increase in intraocular pressure to 120 mm Hg for 40 minutes. One day before ischemia, animals were subjected to global or ocular hypothermia. Fourteen days after ischemia, the animals underwent electroretinography and histological analyses. Global hypothermia was achieved by placing ice packs on the bodies of the animals to cool the body temperature to 33°C for approximately 30 minutes. To cool the eye, a flow of water-soluble standard ultrasound transmission gel cooled to 13°C was applied to one eye, and the temperature was monitored by a thermistor behind the ocular globe until it reached 32°C. The duration of ocular hypothermia was 20 minutes. Thus, the results of this study demonstrate that global or ocular hypothermia as a preconditioning stimulus significantly protects retinal function and histology from ischemia-reperfusion injury, possibly through a glutamate-dependent mechanism (Salido et al., 2013). There is a plethora of published studies that have examined the efficacy of preconditioning with pharmacological agents or with molecules that mimic a preconditioning stimulus against retinal ischemia. For example, preconditioning with intravitreal injection of leukemia inhibitory factor resulted in the preservation of photoreceptor function by preventing photoreceptor cell death from light-induced oxidative damage in a dose-dependent manner (Ueki et al., 2008). Other highly studied preconditioning agents include adenosine, a purine nucleoside product of high-energy phosphate metabolism, mediators such as protein kinase C, and potassium ATP channels (Roth et al., 2006). Potassium-ATP channels were also implicated in adenosine-mediated conditioning, as was protein kinase C (Dreixler et al., 2008). Preconditioning with prothymosin-alpha completely prevented ischemia-induced loss of ganglion cells with partial survival of bipolar and photoreceptor cells (Halder et al., 2015). Preconditioning with low concentrations of lipopolysaccharide-mediated toll-like receptor-4 activation was associated with increased retinal activity of nitric oxide synthase (Halder et al., 2015). Zinc preconditioning induced heat shock protein 72 expression in RGCs (Wong et al., 2017). Finally, cobalt chloride mimicking hypoxia increased glucose transporter- 1 and -3 expression in retinal tissues (Badr et al., 1999).
Postconditioning in retinal ischemia
The neuroprotective strategy of ischemic postconditioning, where a repeated series of brief reperfusions/occlusions are applied after ischemia, is a relatively new concept compared with ischemic preconditioning (Gidday, 2006; Zhao et al., 2006). After initially being shown to reduce infarct size after cardiac ischemia in both clinical (Staat et al., 2006) and preclinical (Gidday, 2010) conditions, ischemic postconditioning has recently been shown to be effective in attenuating neuronal damage in models of spinal cord injury in rodents (Jiang et al., 2006) in both focal models (Pignataro et al., 2008) and global models of cerebral ischemia (Wang et al., 2008). The postconditioning strategy was further refined when it was discovered that ischemic postconditioning could be performed on tissues (kidney or limb) other than the target tissue to be protected (heart or CNS) (Wang et al., 2008). This ischemic postconditioning is referred to as remote postconditioning and has the advantage that the target tissue does not need to be further manipulated or stressed by the postconditioning treatment. If postconditioning is performed on a limb (e.g., occlusion and release of the brachial artery or femoral artery), the procedure could also be performed noninvasively and used to treat many organs and tissues affected by ischemia (Lim and Hausenloy, 2012).
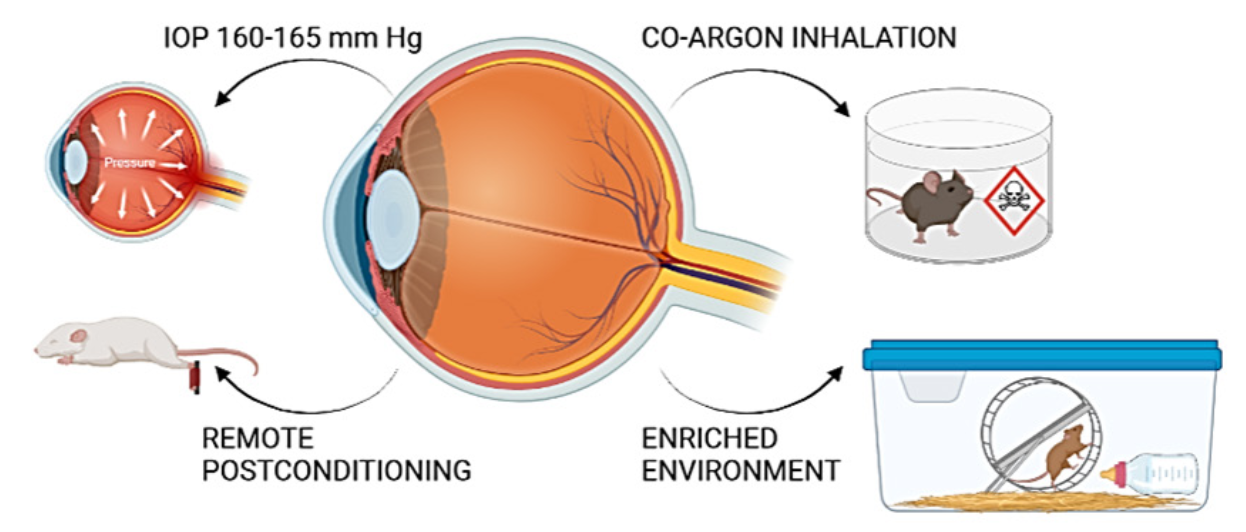
Interestingly, ischemic postconditioning of the retina in glaucoma or retinal ischemia improved retinal neuron survival and function (Belforte et al., 2011). In the literature, several authors have developed postconditioning protocols as a neuroprotective strategy against retinal pathologies (Fig. 2). For example, Fernández et al. (2009) described the postconditioning stimulus as a series of seven cycles of ischemia-reperfusion of one-minute duration (one minute of ischemia/one minute of reperfusion) performed five minutes after induced retinal ischemia, raising IOP to 120 mm Hg for 40 minutes. Another method to induce postconditioning was described by Dreixler et al. (2010), where the retinal ischemic insult was increasing the IOP of rats to 130-135 mm Hg for 45 or 55 minutes. Postconditioning was achieved by allowing five minutes of reperfusion after ischemia and increasing the IOP to 160-165 mm Hg for eight minutes (Dreixler et al., 2010). Other strategies have been developed to induce postconditioning, for example, chemical inhalation. Felix Ulbrich et al. (2015) found that argon inhalation immediately after reperfusion of retinal ischemia exerted neuroprotective effects in rat RGC through extracellular signal-regulated kinase-1/2-dependent regulation of heat shock proteins (Ulbrich et al., 2015). Another agent used as a postconditioning mediator is carbon monoxide. Carbon monoxide inhalation (250 ppm) by rats for one hour immediately after ischemia or with a latency period of 1.5 and 3 hours inhibited the proliferation of Mueller cells and microglia in the retina (Schallner et al., 2012). Remote limb ischemic conditioning, induced by a series of mild ischemia-reperfusion cycles at the hindlimb 10 minutes or six hours after optic nerve transection, increased ganglion cell survival seven days after injury compared with 10-minute postconditioning (Liu et al., 2013). Interestingly, housing in a post-ischemic enriched environment protected adult rat retinas from ischemic damage. In addition to functional protection, enriched environment housing resulted in a significantly increased retinal thickness, ganglion cell number, and decreased Müller cell number (Dorfman et al., 2013).
Extracellular vesicle-derived microRNAs as an alternative preconditioning strategy for the treatment of retinal ischemic disorders
The discovery of microRNAs (miRNAs) has revolutionized how we view post-transcriptional regulation of gene expression (Stefani and Slack, 2008). MiRNAs are categorized as small non-coding RNAs (sncRNAs) (Lee et al., 1993). They are evolutionarily highly conserved short, single-stranded, non-coding RNAs about 19 to 24 nucleotides in length that are endogenously transcribed by RNA polymerase II and III (Lee and Ambros, 2001). Functional miRNAs are generated by a two-step maturation process of primary-miRNA that leads to the formation of a hairpin-like precursor miRNA, which is further cleaved to form a miRNA:miRNA duplex of approximately 22 nucleotides in length (O’Brien et al., 2018). Upon complementary Watson-Crick base pairing, the miRNA directs the recognition of so-called miRNA response elements (MREs) on the target messenger RNA (mRNA) and interferes with its translation by either blocking protein synthesis, cleaving the mRNA, destabilizing its structure, or proteolytically degrading the nascent polypeptide chains (Filipowicz et al., 2008; Bartel, 2009; Jonas and Izaurralde, 2015). Interestingly, mRNAs have multiple MREs for different miRNAs, and the same miRNAs can bind MREs in different mRNAs. Thus, a single miRNA can simultaneously modulate the posttranscriptional availability of different protein-coding mRNA transcripts, control entire cellular processes, and thus affect any cellular function (Krek et al., 2005; Lim et al., 2005). Therefore, there is a growing interest in understanding altered patterns of miRNAs in terms of their deficiencies or excesses and how they are associated with pathological conditions. In this sense, many efforts are focused on understanding the putative role and involvement of miRNAs in the changes in the expression levels of target genes in various acute and chronic human diseases, including retinal ischemia (Condrat et al., 2020; Wang et al., 2020). MiRNAs are not only abundant within cells but are also highly represented in the extracellular milieu, including blood plasma, cerebrospinal fluid, tears, amniotic fluid, urine, breast milk, saliva, and sperm, where they act as paracrine and endocrine cell-cell messengers, ensuring crosstalk between different and distant tissues by transferring to recipient cells (Turchinovich et al., 2011).
In extracellular body fluids, miRNAs are associated with proteins, particularly Argonaute (AGO) proteins (Geekiyanage et al., 2020), but also in apoptotic bodies, high-density lipoproteins, and extracellular vesicles (EVs) (Mori et al., 2019). EVs are small particles enclosed in a lipid bilayer similar in composition to the cellular plasma membrane. They can contain bioactive molecules such as lipids, proteins, and, most importantly, miRNAs and are released extracellularly by all cell types (Colombo et al., 2014; Yáñez-Mó et al., 2015). Once released in the extracellular environment, EVs can reach neighboring cells, enter the blood, and reach distant tissues by overcoming biological barriers, allowing distant tissues and organs to communicate (Catitti et al., 2022). The specific cellular targeting of EVs is due to the expression of specific markers on the EV surface that reflect the status of the parent cell that released them (Richter et al., 2021). Originally, EVs were differentiated based on their size into exosomes (50- 100 nm in diameter), microvesicles (100-1000 nm in diameter), and apoptotic bodies (0.1- 5 µm in diameter) (ref). More recently, the International Society of Extracellular Vesicles has updated the guidelines on EV classification and has authorized the use of the term “extracellular vesicle” for all EV subtypes, with a subclassification based on size with “small EVs” (sEVs) being within 100 nm, and “medium/large EVs” (m/l EVs), 100–200 nm (Théry et al., 2018). EVs convey a broad range of messages by delivering specific miRNAs to target cells and modulating the expression of miRNA target genes in recipient cells (Kosaka et al., 2010). Therefore, the loading of EVs with miRNAs is a selective process that leads to the exosomal transfer of specific functional miRNAs into the extracellular environment, which are distinct from those that constitute the parental cellular repertoire and are the result of cellular sorting of miRNAs destined to be included in EVs (Squadrito et al., 2014) (Fig. 3A). For all these reasons, the components and cargo of EVs are considered an attractive therapeutic approach for the treatment of various retinal diseases in different clinical situations (Gowen et al., 2020). From this perspective, EVs released from adult bone marrow-derived mesenchymal stem cells (MSCs) are gaining attention as a cell-free strategy for treating retinal diseases (Mead and Tomarev, 2017; Mead et al., 2018; Mathew et al., 2019, 2023) and as an alternative to using MSCs themselves. Indeed, the ability of MSCs to self-renew and readily differentiate into neuronal cells initially paved the way for the use of stem cell therapy as a beneficial regenerative treatment of damaged retinal cells in retinal diseases (Mathew et al., 2017), as they can replace various cell types and overcome the limited regenerative capacity of all neuronal structures. Unfortunately, all MSC-based repair strategies tested in preclinical animal models of retinal diseases associated with neuronal cell loss and clinical trials have had poor results, mainly due to significant side effects and low regeneration rates (Zarbin, 2019). Nevertheless, MSCs remain the most commonly used cellular sources for the production of EVs because of their natural ability to produce them in large quantities (Yeo et al., 2013) (Fig. 3B, a). In contrast, MSC-derived EVs are ideal cell-free candidates for therapeutic purposes, firstly because it is now widely recognized that many of the benefits of MSCs are due to their derived EVs. In addition, MSC-derived EVs have significant bioengineering potential as drug delivery agents (Yeo et al., 2013).
In a new window | Download PPT
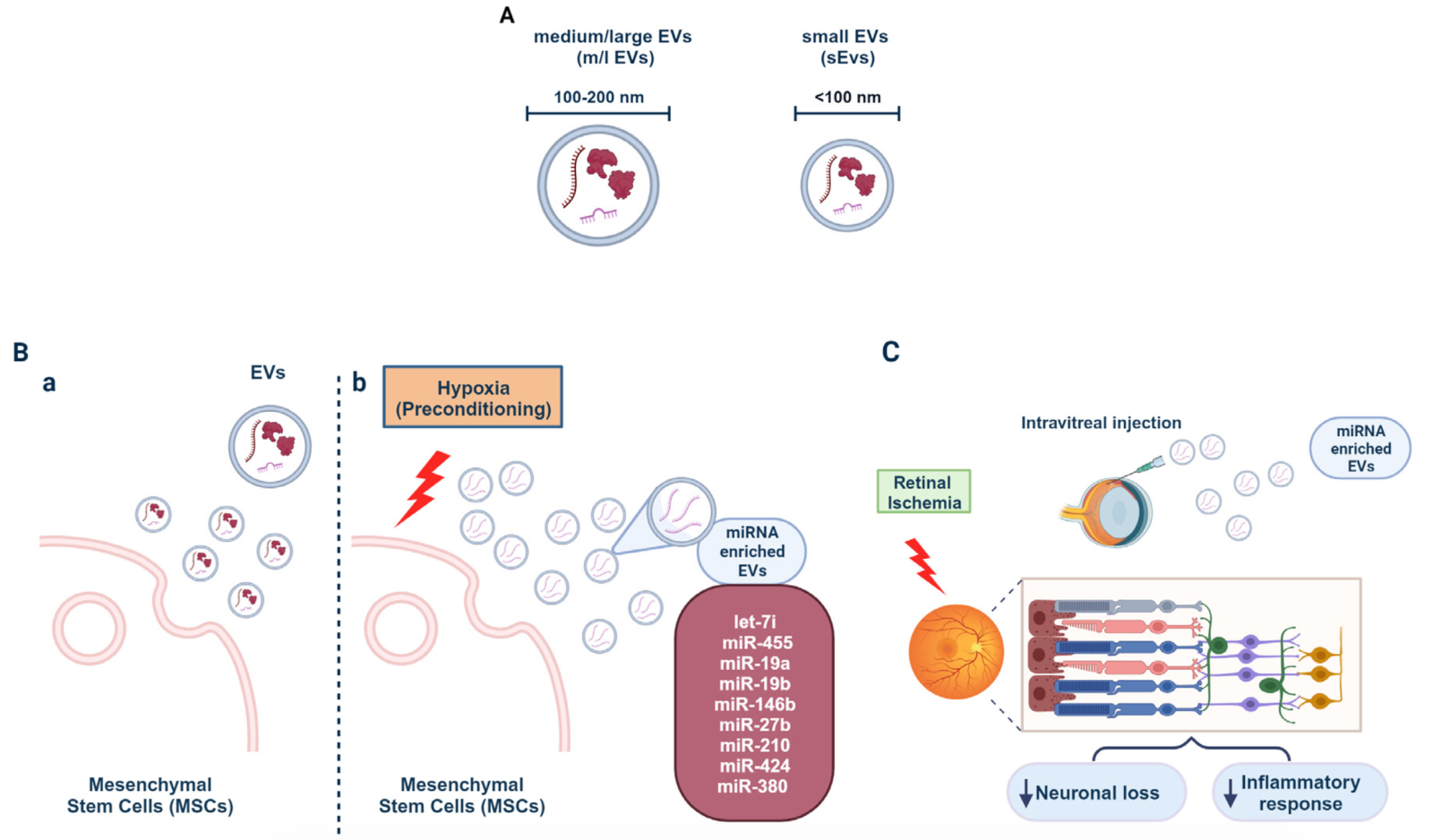
Figure 3. MSCs release miRNAs upon hypoxia and their potential for counteracting retinal neuronal loss and inflammation. (A) Schematic representation of extracellular vesicles (EVs) based on their size ranges. (B) MSCs release EVs thought to be responsible for their various beneficial effects (a). Hypoxic preconditioned MSCs release EVs enriched in miRNAs (b). (C) Intravitreal injection of EVs from hypoxically preconditioned MSCs reduces neuronal loss and inflammatory response in retinal ischemia.
The evidence, although limited so far, regarding the therapeutic significance of EVs in retinal ischemia is very encouraging. Indeed, Mathew and co-authors (Mathew et al., 2019) have obtained promising results with MSC-derived EVs in an in vitro and in vivo model of retinal ischemia. They demonstrated that MSC-EVs were readily internalized by RGC in culture via a caveolin-mediated process. Interestingly, preconditioning of retinal cells through prior exposure to MSC-EVs significantly reduced the cytotoxic effect of an ischemia-like insult. Accordingly, direct intravitreal injection of MSC-EVs supported their uptake by various retinal cell populations, such as RGC, retinal neurons, and microglia, thus reducing ischemic damage by protecting retinal cells from death and reducing insult-induced inflammatory processes. Remarkably, although EVs appeared to be rapidly taken up, as evidenced by their complete removal from the vitreous and migration into the retina, they had a longer retention time and were degraded many days after single-dose injection (Mathew et al., 2021). Such topical application of EVs is a promising and safe alternative to deliver therapeutic molecules directly to the site of damage by targeting the damaged cells. Another benefit that should not be overlooked is that MSCs release EVs that are particularly rich in precursor miRNAs. It is estimated that at least 150 different miRNAs are present in the EVs released by these cells (Chen et al., 2008). Moreover, conditioning of MSCs by biochemical signals such as cytokines, growth factors, and hypoxia exposure further enriches the miRNA content of EVs derived from these cells, increasing their therapeutic potential to modulate pathological targets (Lee and Kang, 2020) (Fig. 3B, b). In this context, the neuroprotective effects of EV-derived miRNAs released from MSCs have been observed after hypoxic preconditioning (Mathew et al., 2023). The latter leads to the enrichment of EVs with miRNA, which has a neuroprotective and regenerative profile that could help improve the functionality of MSC-EVs. Indeed, Mathew and colleagues (2023) found that EVs produced by MSCs after hypoxic preconditioning exhibited neuroprotective properties superior to those of normoxic MSCs. More detailed analysis of the miRNA content of EVs released during preconditioning of MSCs to hypoxia led to the identification of a subset of miRNA that were mainly enriched in EVs isolated under such conditions, including miRNAs -7i, -455, -19a, -19b, -146b, -27b, -210, -424, and -380. Both the neuroprotective and anti-inflammatory effects observed after treatment with EVs from hypoxic preconditioned MSCs appeared to require the presence of miRNAs in EVs (Fig 3C). Interestingly, Andreeva et al., 2015 studied the miRNA pattern associated with ischemia-reperfusion injury during the early (24 hours) and late (7 days) phases after ischemia-reperfusion injury in the retina of rats subjected to an ischemic insult. Specifically, they described that miR-495, miR-214, and miR-298 were involved in the early regulatory network of biological processes that occurred 24 hours after ischemic injury, whereas miR-873, miR-223, and miR-185 were important regulatory elements observed in the late phase after injury. Interestingly, the individual miRNAs were involved in all biological processes after ischemia-reperfusion injury, such as cell death, synaptic activity, apoptosis, and ion transport. Similarly, MSCs conditioning with pro-inflammatory cytokines can lead to the release of EVs enriched in miRNAs responsible for neuroprotection in models of retinal ischemia (Yu et al., 2022). In detail, MSCs exposure to tumor necrosis factor-α caused the upregulation of miR21-5p expression within MSCs as well as its enrichment within EVs released in these conditions. Most importantly, intravitreal injection of EVs containing miR21-5p negatively modulates the inflammatory response and alleviates apoptosis. Significantly, miRNAs derived from the EVs of MSCs have a completely different profile from that of injured retinal cells. This aspect represents an intriguing starting point for the development of new therapeutic strategies based on the use of EVs in retinal ischemia.
Conclusions
In summary, the last few decades have provided fascinating insights into the mechanisms and potential application of ischemic neuroprotection. While the data in the literature cannot compare with the explosion of experimental and clinical studies that define the field of cardiac and brain conditioning, the field of retinal resilience to acute and chronic damage is well advanced. However, choosing the right experimental model is advisable to obtain consistent results and valid indications of the direction needed to develop new effective treatments to manage retinal pathologies. Another aspect to consider in conditioning is the timing between the induction of preconditioning and postconditioning and the noxious stimulus. Correctly executing the experimental protocol is crucial to obtain better and repeatable results. In addition, there is more data in the scientific literature on the neuroprotective effects of preconditioning than on postconditioning models. Postconditioning is relatively new but more promising because it is more suited for the clinical setting (Vinciguerra et al., 2020). Although less studied, the influence of remote tissue on retinal responsiveness and protective conditioning is of paramount importance. To date, many patterns of retinal preconditioning and postconditioning appear to be neuroprotective. In particular, EVs from conditioning models are an excellent compromise for translational neuroprotection. Moreover, identifying miRNAs involved in retinal conditioning processes has opened new avenues for identifying potential therapeutic targets. In addition, innovative miRNA targeting strategies aimed at decreasing the levels of pathogenic or aberrantly expressed miRNAs or increasing the levels of miRNAs with beneficial functions have been developed and could be used to treat ischemic diseases. MiRNAs are also increasingly considered as potential diagnostic markers for disease stages. Indeed, miRNAs have been detected as nuclease-resistant moieties in various extracellular body fluids, including saliva, serum, plasma, milk, urine, and tears. These extracellularly circulating miRNAs enable cell-to-cell communication and provide information about physiological states or pathological disease progression within secreting cells (Redis et al., 2012). Further information on the formation and circulation of exosomal miRNAs could validate their prognostic potential in retinal diseases of the eye and help develop optimal miRNA delivery systems in vivo.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Support provided by FSE REACT-EU-Azione IV.4- Dottorandi e contratti di ricerca su tematiche dell’innovazione. Azione IV.6-Contratti di ricerca su tematiche green. DM1062 del 10/08/2021.
References
Cristina Franco1
1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Giovanna Lombardi1
1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Noemi Di Muraglia1
1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Lorella Maria Teresa Canzoniero1
1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Serenella Anzilotti1
1Department of Science and Technology, University of Sannio, 82100, Benevento, Italy
Corresponding author:
Dr. Serenella Anzilotti
Email: sanzilotti@unisannio.it

In a new window | Download PPT
Figure 3. MSCs release miRNAs upon hypoxia and their potential for counteracting retinal neuronal loss and inflammation. (A) Schematic representation of extracellular vesicles (EVs) based on their size ranges. (B) MSCs release EVs thought to be responsible for their various beneficial effects (a). Hypoxic preconditioned MSCs release EVs enriched in miRNAs (b). (C) Intravitreal injection of EVs from hypoxically preconditioned MSCs reduces neuronal loss and inflammatory response in retinal ischemia.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6239 | 15 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA