Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The potential of epigenetic modifications in stem cell therapy for stroke
Time:2024-01-15
Number:5543
Mia C. Borlongan1, Thomas Rodriguez1, Maximillian C. Borlongan1, Francesco D’Egidio1, Jea-Young Lee1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair; Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine; 12901 Bruce B. Downs Blvd., Tampa, Florida 33612
Conditioning Medicine 2023. 6(3): 65-69.
Abstract
Ischemic stroke is a condition characterized by reduced blood flow to the brain, often leading to severe brain damage, and it is typically caused by the blockage of blood vessels in the brain. Epigenetic therapy has emerged as a novel and promising approach for reducing the risk and damage associated with ischemic stroke. Epigenetic changes, which involve modifications to gene expression without altering the DNA sequence, may pose as therapeutic targets for ischemic stroke. These changes are attractive due to their specificity and potential reversibility, which makes them suitable for precision medicine. The potential targets for epigenetic modification in ischemic stroke primarily involve DNA methylation and demethylation. Alterations in DNA methylation patterns can influence gene expression and may play a role in stroke pathology. This review explores the efficacy of enhancing stem cell-based interventions for stroke via adjunctive epigenetic modifications. By modifying the epigenetic landscape of stem cells, eventually affecting their secretome, it may be possible to enhance their regenerative and neuroprotective properties.
Keywords: Cerebral ischemia, Genes, Cell transplantation, Regenerative medicine, Neuroprotection
Abstract
Ischemic stroke is a condition characterized by reduced blood flow to the brain, often leading to severe brain damage, and it is typically caused by the blockage of blood vessels in the brain. Epigenetic therapy has emerged as a novel and promising approach for reducing the risk and damage associated with ischemic stroke. Epigenetic changes, which involve modifications to gene expression without altering the DNA sequence, may pose as therapeutic targets for ischemic stroke. These changes are attractive due to their specificity and potential reversibility, which makes them suitable for precision medicine. The potential targets for epigenetic modification in ischemic stroke primarily involve DNA methylation and demethylation. Alterations in DNA methylation patterns can influence gene expression and may play a role in stroke pathology. This review explores the efficacy of enhancing stem cell-based interventions for stroke via adjunctive epigenetic modifications. By modifying the epigenetic landscape of stem cells, eventually affecting their secretome, it may be possible to enhance their regenerative and neuroprotective properties.
Keywords: Cerebral ischemia, Genes, Cell transplantation, Regenerative medicine, Neuroprotection
Highlights
Stroke is a significant health problem with very limited treatments. Stem cell transplantation may repair the stroke brain but with variable outcomes, in large part, due to low grafted stem cell viability. Epigenetic modifications of stem cells may increase graft persistence and optimize their secretome leading to improved functional benefits in stroke. Targeting stem cells via epigenetic strategies offers a conditioning medicine paradigm for optimizing the therapeutic response of exogenous stem cells to the hostile endogenous stroke microenvironment.
Introduction
Ischemic stroke is characterized by reduced blood flow to the brain due to the blockage of blood vessels, which can lead to severe brain damage. Limited therapeutic options for ischemic stroke warrants novel but safe and effective treatments (O’Donnell et al., 2010; Sarikaya et al., 2015; Guzik and Bushnell, 2017; Rinaldo et al., 2019; Cramer, 2020). Epigenetic therapy is emerging as a promising approach to reduce the risk and damage associated with ischemic stroke. This approach involves modifying gene expression without changing the DNA sequence, making it a specific and potentially reversible method suitable for precision medicine. The primary targets for epigenetic modification in ischemic stroke include DNA methylation and demethylation. In particular, changes in DNA methylation patterns can influence gene expression and may play a role in stroke pathology. In tandem, stem cell-based interventions have shown promise in stroke treatment, with the potential to promote tissue repair and neuroprotection. This review focuses on enhancing stem cell therapies with adjunctive epigenetic modifications to improve the regenerative and neuroprotective properties of stem cells, and eventually their secretome, potentially improving their efficacy in stroke treatment. We highlight the envisioned benefits of using epigenetic modifications in stem cell therapy for stroke. We focused on the literature published over the last three years to capture the recent discoveries in the field.
Epigenetics and Stroke
The field of epigenetics has examined treating multifactorial diseases such as stroke. Epigenetic changes are modifications to gene expression that do not alter the DNA sequence (Moore et al., 2013; Zhang et al., 2020). They have gained attention in research because they are reversible through pharmaceutical interventions. Epigenetic modifications can target various mechanisms, including DNA methylation and demethylation (Moore et al., 2013; Wu and Zhang, 2017; Zhang et al., 2020). DNA can be methylated (adding a methyl group) or demethylated (removing a methyl group). DNA methylation can be catalyzed by a family of DNA methyltransferases, with different DNA methyltransferases having distinct roles in establishing and maintaining methylation patterns. DNA methylation can be reversed through the action of ten-eleven translocation (TET) enzymes, which oxidize 5-methylcytosine (Wu and Zhang, 2017; Ross and Bogdanovic, 2019). Epigenetic changes can also involve histone modification, including histone methylation by histone methyltransferases and histone acetylation regulated by histone acetyltransferases and histone deacetylases (Zhang et al., 2021). Histone phosphorylation is another mechanism that can modify DNA activity (Zhang et al., 2021). Changes in histone proteins can affect chromatin structure and gene expression, contributing to stroke risk and damage (Zhang et al., 2021). Non-coding RNAs, specifically microRNAs (miRNAs), can regulate gene expression by inhibiting mRNA translation or promoting mRNA degradation (Correia de Sousa et al., 2019). Specific miRNAs are involved in post-transcriptional gene regulation and may be linked to stroke pathogenesis (Correia de Sousa et al., 2019).
Epigenetic modifications have been reported to influence the development and progression of atherosclerosis, a condition that contributes to stroke risk (Ng et al., 2018; Kumar et al., 2021). Epigenetic changes may also influence the inflammatory responses that occur following a stroke, affecting the extent of tissue damage and recovery. Accumulating evidence implicates the crucial role of epigenetics in neuroinflammatory responses to ischemic stroke. Whereas the primary cause of stroke cell death entails an ischemic injury, secondary causes, such as excitotoxicity, oxidative stress, free radical accumulation, mitochondrial dysfunction, impaired neurogenesis, angiogenesis, vasculogenesis, and aberrant inflammation, also participate in cell death and are equally detrimental (O’Donnell et al., 2010; Sarikaya et al., 2015; Guzik and Bushnell, 2017; Rinaldo et al., 2019; Cramer, 2020). Many of the epigenetic mechanisms noted above, including DNA methylation, histone acetylation, histone methylation, and miRNAs, have been shown to play a role in the neuroinflammatory responses to ischemic injury. Epigenetic changes can modulate the expression of genes involved in the inflammatory response following a stroke. However, the complex and unique epigenetic patterns seen in individual patients suggest that finding a universal epigenetic pattern that conforms to all ischemic strokes can vary significantly among patients, making it challenging to develop one-size-fits-all therapies. Nonetheless, epigenetics presents a fertile ground for developing therapies that can reduce neuroinflammation in stroke. By targeting specific epigenetic modifications, it may be possible to address the individualized nature of epigenetic patterns and develop more effective treatments for stroke-related inflammation.
The molecular mechanisms involved in epigenetic regulation underscore the diversity of targets and processes that can be modified for therapeutic purposes. Epigenetic research holds promise in developing treatments for complex diseases like stroke by manipulating these regulatory mechanisms.
Stem Cell Therapies for Stroke
Laboratory studies and clinical trials have demonstrated the safety of using stem cells in treating stroke by promoting tissue repair and recovery. Various types of stem cells have been shown to exert therapeutic effects in ischemic stroke (Shyu et al., 2004; Jin et al., 2006; Kranz et al., 2010; Borlongan, 2011; Ratajczak et al., 2012; Bhatt et al., 2015; Courties et al., 2015; Maya-Espinosa et al., 2015; Lee et al., 2016; Moisan et al., 2016; Uchida et al., 2016; Wang et al., 2016; Stonesifer et al., 2017; Yu et al., 2018; Huang and Zhang, 2019; Tuazon et al., 2019; Chen et al., 2020; Xie et al., 2020; Yang et al., 2020; Zhao et al., 2022). Stem cells can differentiate into various nervous system cells and can address both acute and chronic aspects of stroke damage (Kranz et al., 2010; Maya-Espinosa et al., 2015; Uchida et al., 2016; Wang et al., 2016; Stonesifer et al., 2017; Yang et al., 2020). Acute stem cell therapy aims to mitigate secondary injury processes, including proinflammatory responses, mitochondrial dysfunction, oxidative damage, and apoptosis, which exacerbate stroke-related damage. On the other hand, chronic stem cell therapy focuses on regeneration, stimulating processes like vasculogenesis, neurogenesis, angiogenesis, and synaptogenesis to aid recovery. Among the many types of stem cells tested in stroke, mesenchymal stem cells, induced pluripotent stem cells, embryonic stem cells, hematopoietic stem cells, and neural stem cells have been demonstrated to either replace ischemic cells or exert bystander effects (e.g., secrete paracrine signals, release anti-inflammatory factors, or transfer healthy mitochondria) to restore function (Shyu et al., 2004; Jin et al., 2006; Borlongan, 2011; Ratajczak et al., 2012; Bhatt et al., 2015; Courties et al., 2015; Lee et al., 2016; Moisan et al., 2016; Yu et al., 2018; Huang and Zhang, 2019; Tuazon et al., 2019; Chen et al., 2020; Xie et al., 2020; Zhao et al., 2022). Altogether, stem cells can aid in stroke recovery via neuroprotective and regenerative processes, making them a promising area of research and therapy in stroke treatment.
Enhancing Stem Cells with Epigenetics
The efficacy of stem cell-based interventions for stroke may be enhanced by incorporating adjunctive epigenetic modifications. Modifying the epigenetic landscape of stem cells may enhance their regenerative and neuroprotective properties (Figure 1). Here, we focused on the use of miRNAs in preclinical studies for targeting the stem cell epigenome, especially the bone marrow-derived stem cells (BMSCs) and their exosomes, which are shown to have a significant impact on neurological recovery in animal models of stroke (Table 1).

In a new window | Download PPT
Figure 1. Enhanced stem cell therapy with epigenetic modifications in stroke recovery. Modifications in the epigenome and exosomes of stem cells can enhance miRNA expression, increasing the efficacy of stem cell-related regenerative and neuroprotective properties, resulting in improved neurological recovery in models of ischemic stroke.
miR-133b-Overexpressing BMSC Exosomes: Exosomes harvested from BMSCs that were epigenetically modified to overproduce miR-133b and then administered to rats after middle cerebral artery occlusion (MCAO) improved neurological function and plasticity. These exosomes also increased neurite branching in cultured rat neurons (Xin et al., 2017b).
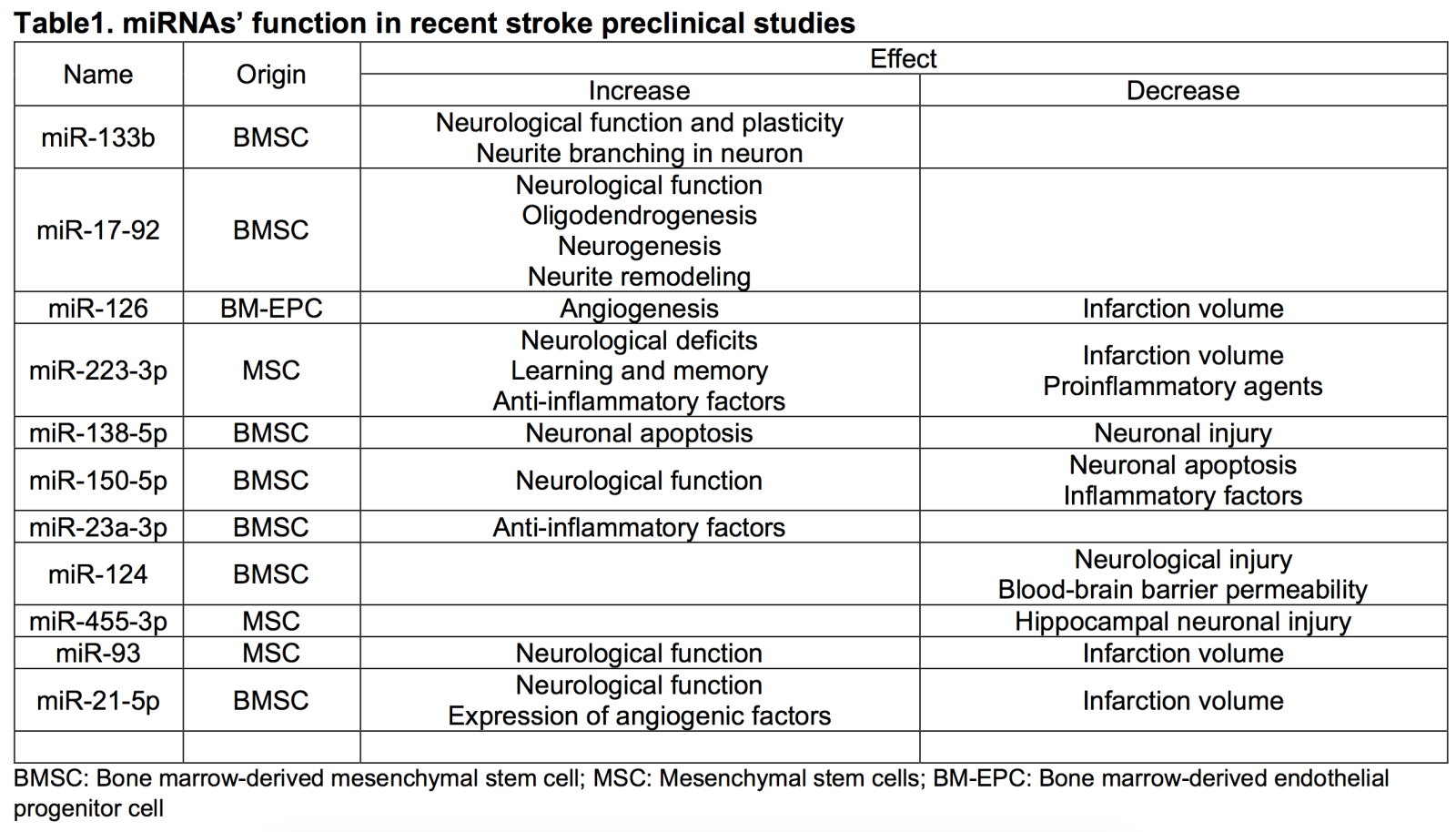
miR-17-92 Cluster-Enriched Exosomes: Rats that received miR-17-92 cluster-enriched exosomes from BMSCs showed improved neurological function, increased oligodendrogenesis, neurogenesis, and neurite remodeling within the ischemic site. These exosomes inhibit phosphatase and tensin homolog, activating the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin/glycogen synthase kinase 3β pathway (Xin et al., 2017a).
miR-126-Treated Bone Marrow-Derived Endothelial Progenitor Cells (EPCs): Mice infused with bone marrow-derived EPCs treated with miR-126 demonstrated decreased infarction volume and increased angiogenesis (Shan and Ma, 2018).
miR-223-3p-Overexpressing Mesenchymal Stem Cell Exosomes: Exosomes from mesenchymal stem cells overexpressing miR-223-3p reduced infarct volume and improved neurological deficits, learning, and memory in rat MCAO and microglia oxygen-glucose deprivation (OGD) models. They promoted M2 microglial transformation, decreasing proinflammatory agents and promoting anti-inflammatory factors (Zhao et al., 2020).
miR-138-5p-Overexpressing BMSC Exosomes: Exosomes from BMSCs overexpressing miR-138-5p reduced neuronal injury when administered to mice after MCAO. The mechanism involves miR-138-5p inhibiting lipocalin 2, an iron transport protein that promotes neuronal apoptosis (Bi et al., 2013; Dong et al., 2013; Deng et al., 2019).
miR-150-5p-Overexpressing BMSC Exosomes: Vesicles from BMSCs containing miR-150-5p improved neurological function and decreased neuronal apoptosis and inflammatory factors when administered to MCAO rat models. The mechanism involves the inhibition of Toll-like receptor 5 (Li et al., 2022).
miR-23a-3p-Transferring BMSC Exosomes: Exosomes from BMSCs transfer miR-23a-3p, which polarizes microglia to their M2 form (Dong et al., 2022).
miR-124 in Bone Marrow Stromal Cell-Derived Extracellular Vesicles: Extracellular vesicles from bone marrow stromal cells containing miR-124 ameliorated neurological injury and blood-brain barrier permeability in MCAO mice and microglia/astrocyte OGD models (Tian et al., 2022).
miR-455-3p-Containing Mesenchymal Stem Cell-Derived Exosomes: Exosomes from BMSCs containing miR-455-3p ameliorated hippocampal neuronal injury in MCAO mouse models and OGD cell models by targeting programmed cell death 7 (Gan and Ouyang, 2022).
miR-93 in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Exosomes from BMSCs upregulating miR-93 improved neurological function and reduced infarct volume in mouse stroke models. MiR-93 targeted histone deacetylase 4, deacetylating B-cell lymphoma 2, leading to increased infarct volume and neuron apoptosis (Shi et al., 2022).
miR-21-5p-Enriched Exosomes: Exosomes from BMSCs enriched with miR-21-5p improved neurological function and reduced infarct volume in mouse stroke models. These exosomes also increased the expression of angiogenic factors in vitro (Hu et al., 2022).
These findings highlight the potential of epigenetic modifications in stem cell-derived exosomes as a therapeutic strategy for ischemic stroke, demonstrating their ability to promote neuroprotection, neurogenesis, and anti-inflammatory responses in animal models. The specific miRNAs involved in these modifications mediate many of the observed beneficial effects in stroke models.
Conclusion
In summary, this review highlights the importance of epigenetics as a promising avenue for developing novel treatments for ischemic stroke. Indeed, epigenetic regulatory mechanisms can modulate stroke-related gene expression, injury response at cellular levels, and motor and cognitive functions (Peng et al., 2022). Evidence regarding the deep involvement of epigenomic writers, readers, and erasers in ischemic stroke has been collected during the last decades. Moreover, considering the clinical limits in stroke management, the epigenetic modifications offer a degree of specificity and reversibility that make them a valuable target for precision medicine approaches (Morris-Blanco et al., 2022), and the combination of these modifications with stem cell therapies holds promise for reducing the damage caused by stroke and enhancing recovery. As stem cells are historically known to exert neuroprotective and neurotrophic effects in ischemic stroke, their epigenetic enhancement could represent a reliable and effective way to manage this detrimental condition in a plethora of different modalities. However, more preclinical studies are needed to better comprehend how these epigenetic changes affect the injured environment to improve the focus of eventual targeted therapies (Monsour et al., 2022).
Conflict of interest
The authors declare no conflict of interest.
References
Mia C. Borlongan1
Department of Neurosurgery and Brain Repair; Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine; 12901 Bruce B. Downs Blvd., Tampa, Florida 33612.Thomas Rodriguez1
Maximillian C. Borlongan1
Department of Neurosurgery and Brain Repair; Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine; 12901 Bruce B. Downs Blvd., Tampa, Florida 33612.
Francesco D’Egidio1
Department of Neurosurgery and Brain Repair; Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine; 12901 Bruce B. Downs Blvd., Tampa, Florida 33612.
Jea-Young Lee1
Department of Neurosurgery and Brain Repair; Center of Excellence for Aging and Brain Repair, University of South Florida Morsani College of Medicine; 12901 Bruce B. Downs Blvd., Tampa, Florida 33612.
Corresponding author:
Jea-Young Lee
Email: jeayoung@usf.edu

In a new window | Download PPT
Figure 1. Enhanced stem cell therapy with epigenetic modifications in stroke recovery. Modifications in the epigenome and exosomes of stem cells can enhance miRNA expression, increasing the efficacy of stem cell-related regenerative and neuroprotective properties, resulting in improved neurological recovery in models of ischemic stroke.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5543 | 14 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA