Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Repositioning stem cell-based therapies for conditioning medicine in stroke
Time:2024-05-06
Number:5190
Felipe Esparza Salazar1, Germán Rivera Monroy1, Mauricio Muleiro Alvarez1, Joaquin Vega Gonzales-Portillo1, Jea-Young Lee1
Author Affiliations


- 1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, US.
- 2Universidad Peruana de Ciencias Aplicadas, Av. Alameda San Marcos 11, Chorrillos 15067, Lima, Perú.
Conditioning Medicine 2023. 6(5): 165-169.
Abstract
Stroke ranks as the second most common cause of sudden death and the leading cause of disability worldwide. It results from the interruption of cerebral blood flow, which consequently causes decreased cerebral oxygen and death of brain tissue. Clinically, damaged tissue appears in the brain region affected by blood obstruction. Its pathophysiological component depends on inflammation that damages the brain tissue and reduces its function, redefining stroke as a neurodegenerative disease. The efficacy and safety of current stroke treatments, i.e., drug thrombolysis and mechanical thrombectomy, highly depend on the short time elapsed since the blood occlusion began, thereby limiting the number of stroke patients benefitting from these treatments. Stem cell (SC) therapy has emerged as a potential treatment for stroke by extending the treatment window over current therapies. Several studies supporting the use of SC implicate the cells’ ability to promote direct cell replacement and the production of paracrine factors. The therapeutic effects of SC therapy in stroke have been demonstrated in vitro and in vivo models. In this article, we review the recent literature on the use of SC in stroke with an emphasis on repositioning its application for conditioning medicine.
Keywords: Cell therapy, Cerebral ischemia, Neuroprotection, Regeneration
Abstract
Stroke ranks as the second most common cause of sudden death and the leading cause of disability worldwide. It results from the interruption of cerebral blood flow, which consequently causes decreased cerebral oxygen and death of brain tissue. Clinically, damaged tissue appears in the brain region affected by blood obstruction. Its pathophysiological component depends on inflammation that damages the brain tissue and reduces its function, redefining stroke as a neurodegenerative disease. The efficacy and safety of current stroke treatments, i.e., drug thrombolysis and mechanical thrombectomy, highly depend on the short time elapsed since the blood occlusion began, thereby limiting the number of stroke patients benefitting from these treatments. Stem cell (SC) therapy has emerged as a potential treatment for stroke by extending the treatment window over current therapies. Several studies supporting the use of SC implicate the cells’ ability to promote direct cell replacement and the production of paracrine factors. The therapeutic effects of SC therapy in stroke have been demonstrated in vitro and in vivo models. In this article, we review the recent literature on the use of SC in stroke with an emphasis on repositioning its application for conditioning medicine.
Keywords: Cell therapy, Cerebral ischemia, Neuroprotection, Regeneration
Highlights
Stem cell therapy has been examined as a regenerative medicine approach that extends the treatment window for stroke intervention. Here, we argue that stem cell therapy can also be employed as a conditioning medicine modality, whereby its treatment can be initiated before stroke onset. Cognizant of stroke co-morbidities, such as obesity and hypertension, it may be possible to identify a cohort of patients who may be at risk of stroke. Intervening prophylactically with stem cells before these patients succumb to stroke may improve clinical outcomes in these patients. Accordingly, we advance the concept of stem cell therapy for conditioning medicine to protect the brain against an impending ischemic injury.
Stroke – an unmet clinical need
Ischemic stroke, or just stroke, is the most frequent cerebrovascular disease worldwide. Stroke results from brain blood flow interruption that consequently deprives affected cerebral tissue of oxygen and metabolites required for its functioning and survival (Putaala, 2022). Clinical manifestations depend on the specific brain region affected by the blood blockage. Stroke is ranked as the second most frequent cause of sudden death and the leading cause of disability around the world (Puutala, 2022; Mendelson et al., 2021). Despite stroke being considered an isolated event, it is now recognized that stroke has an important inflammatory component that continuously injures brain tissue and lowers brain function over the years. Thus, stroke is not just an acute event but also a neurodegenerative disease (Maida et al., 2020; Rabinstein, 2020).
Stroke pathophysiology comprises several events that lead to neuronal injury and, if not quickly repaired, neuronal death. Initially, adequate cerebral blood flow gets interrupted, restricting cerebral tissue from oxygen and nutrients (Puuatala, 2022). Blood interruption is frequently due to an embolus that blocks a cerebral artery, most commonly the middle cerebral artery. However, there are other causes of blood interruption, like native cerebral artery thrombus, vasospasm, or drug abuse. As a consequence of a disruption in blood flow, neurovascular cells suffer ischemia that triggers an inflammatory response and the beginning of a cytotoxic cascade (Jayaraj et al., 2019). M1 macrophages are recruited with consequent pro-inflammatory mediators such as interleukin 6 (IL-6), tumor necrosis alpha (TNF‐α), and interleukin-1 beta (IL-1 ß), as well as the release of metalloproteinases that together severely injure the endothelium and induce predisposition to blood-brain barrier leakage. Moreover, glutamate over-release from neurons can lead to hyperexcitation, electrical disbalance, mitochondrial dysfunction, and cellular death (Mendelson et al., 2021).
Current stroke treatments encompass different factors, such as the time since blood occlusion started, age, and patient comorbidities. However, pharmacologic thrombolysis and mechanical thrombectomy tend to lose efficiency over time (Rabinstein, 2020). Over the last few years, stem cell (SC) therapy has stood out as a novel therapy for treating stroke due to its multiple benefits over current treatments. Despite the exact mechanism in which SC aids brain repair, it is suggested that the benefits might come from direct cell replacement, neurotrophic factors production, anti-inflammatory cytokine release, and healing microenvironment conformation (Incontri et al., 2018). The success of SC therapy using in vitro and in vivo stroke models has prompted research with different SCs and implementation of complementary techniques like new routes of cell delivery, use of cell-free proteins, and extracellular vesicles secreted by SC, and lab-to-clinic translational optimization protocols (Borlongan, 2019).
Definition, epidemiology and risk factor for stroke
As noted above, stroke is a neurological condition in which clots form in the brain that interrupt cerebral circulation, causing hemorrhage that is secondary to the rupture of these vessels. Stroke accounts for 140,000 deaths in the US each year (Barthels et al., 2020; Kuriakose et al., 2020; Gandhi et al., 2020; Wafa et al., 2020) due to irreversible injury and death of brain cells from hypoxia (Barthels et al., 2020; Kuriakose et al., 2020; Toledo et al., 2022).
There are two types of stroke: 1) Ischemic cerebrovascular accidents, produced by blockage of an artery in the brain, represent 87% of all strokes. 2) Hemorrhagic strokes, accounting for approximately 13% of all strokes, are caused by the rupture of a blood vessel within the brain (Knight-Greenfield et al., 2019; Iadecola et al., 2020; Roberts et al., 2020; GBD, 2019; Spellicy et al., 2021; Hassani et al., 2021).
There are different risk factors that predispose up to 90% of the risk attributable to suffering a stroke worldwide. Indeed, pathophysiological factors that produce a negative impact on the cardiovascular system, such as arterial hypertension, obesity, smoking, high-fat diet, diabetes, apolipoprotein ratio, and alcohol consumption, have been shown to increase stroke occurrence with worsened morbidity and mortality (Caprio et al., 2019; GBD, 2019; Virani et al., 2020; Saft et al., 2020; George, 2020; Avellaneda et al., 2021). After a stroke, approximately 70% of survivors display motor neurological sequelae in the upper and lower extremities (Gandhi et al., 2020). Because of these societal burdens, therapies are urgently needed to improve the quality of life for stroke survivors.
Diagnosis for stroke
A brief patient history that includes symptoms, their onset, and any risk factors connected to the disease should be obtained early in stroke diagnosis (Hurford et al., 2020). It is critical to perform a focused neurological evaluation to determine the extent of the affected vascular area and to detect stroke-related physical impairment, especially when employing the National Institutes of Health Stroke Scale (NIHSS) for physical impairment quantification (Hurford et al., 2020).
Head imaging should be performed within the first 25 minutes of a patient's presentation with acute stroke suspicion (Mendelson et al., 2021). Non-contrast computed tomography (CT) is the gold standard for acute stroke imaging diagnosis, exhibiting 95% accuracy when distinguishing ischemic and hemorrhagic stroke (Musuka et al., 2015). Additionally, non-contrast CT can be utilized synergically with the Alberta Stroke Program Early CT Score (ASPECTS) to quantify extension and changes in the early stages of acute stroke (Zerna et al., 2018). However, non-contrast CT efficacy for stroke detection diminishes in cases of minor stroke due to lower sensitivity, whereas magnetic resonance imaging (MRI) exhibits greater sensitivity and utility for minor stroke diagnosis; however, its use is frequently limited to both non-critical scenarios (Musuka et al., 2015). Moreover, even though MRI is a highly effective imaging resource, CT is preferred due to its greater availability, higher time-efficient utility, and high sensitivity for stroke diagnosis (Maier et al., 2022). Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) of the head and neck is recommended for patients who exhibit significant impairments, possibly due to large vessel occlusion, to assess the occlusion location and eligibility for mechanical thrombectomy(Mendelson et al., 2021).
Actual treatment for stroke
Stroke treatment focuses on restoring brain tissue perfusion, mainly achieved by thrombolytic drug administration and/or mechanical thrombectomy (Zerna et al., 2018). Currently, alteplase is the only FDA-approved thrombolytic medication for stroke treatment (Bhalla et al, 2021). Nevertheless, intracerebral hemorrhage (ICH) must be excluded by non-contrast CT or MRI during the initial evaluation for IV alteplase administration to be considered suitable in patients with acute stroke (Powers et al., 2019). In addition, when provided within 4.5 hours of symptoms onset, alteplase has been linked to functional improvement three to six months later (Emberson et al., 2014; Lees et al., 2010). Moreover, alteplase’s favorable effects are associated with the time of administration within the therapeutic window, statistically exhibiting an outcome improvement rate of approximately one in three patients if treated between 1-3 hours from symptoms onset and one in six patients if treated between the 3-4.5-hour window. In contrast, a delay in treatment administration is associated with decreased beneficial effects and increased mortality (Lees et al., 2010). Nonetheless, actual treatment is only considered for a small proportion of stroke patients, with just 25% eligible for thrombolysis and 10-20% for thrombectomy (Zerna et al., 2018). Furthermore, patient eligibility for thrombolysis is predicated on a strict therapeutic window of 4.5 hours following symptoms onset and the existence of either disabling or major impairments. In contrast, patients outside the timeframe and/or display mild or no deficits are excluded from thrombolysis treatment (Mendelson et al., 2021). In short, current acute stroke treatments have therapeutic benefits with potentially significant clinical outcomes; however, limitations related to their application result in a small pool of suitable stroke patients. Therefore, new and more efficient therapeutic alternatives that can treat a broader population of stroke patients, particularly those who do not fit the criteria for traditional therapy, are needed.
Stem cells
Research on the use of SC therapy to treat brain injury secondary to stroke has been the main target of this treatment. SC therapy aims to repair, replace, and improve the biological function of injured cells and protect the integrity of the brain (Chang et al., 2018; Park et al., 2021; Berlet et al., 2021). Mesenchymal cells (MSC) have been one of the most widely studied SC in preclinical models of stroke, with consistent neurological recovery reported in transplanted animals (Kuang et al., 2020; Andrzejewska et al., 2021; Singh et al., 2020). MSC’s postulated mechanism of action implicates the reduction of inflammation both centrally and peripherally (Wang et al., 2018; Guo et al., 2021). Another type of cell is neural progenitor cells, which were among the first to be used to treat ischemic stroke. They have the advantage of being able to differentiate into neuronal and glial cell types but have the disadvantage of dividing a limited number of times (Toman et al., 2019; De Gioia et al., 2020). Growth factor-secreting cells are another cell type used for neuronal repair in stroke models. These cells secrete trophic factors that promote neurogenesis and angiogenesis, leading to the repair of the neurovascular unit (Chang et al., 2018; Song et al., 2020). Brain-derived neurotrophic factor (BDNF)-secreting neural SCs, vascular endothelial growth factor (VEGF), and glial cell line-derived neurotrophic factor (GDNF) secreting cells can promote the growth of neurovascular cells (Chang et al., 2018; Karantali et al., 2021). The secretion of paracrine factors by SCs has been viewed as a more robust mechanism of action than cell replacement regenerative processes (Chang et al., 2018).
During stroke, the immune response plays an important role in stroke progression. The immediate activation of innate immunity produces an inflammatory process. Inflammatory mediators, including cells and cytokines, are recruited from the periphery, eventually invading the central nervous system (Levard et al., 2021). Adaptive immunity is activated secondarily by the production of inflammatory mediators. Interestingly, SCs exert anti-inflammatory effects, thereby dampening inflammation-induced secondary cell death (Wang et al., 2018).
Stem cells transplanted in stroke animals reduce systemic and local inflammation in conjunction with remodeling of the neurovascular unit. Through secretion of paracrine factors and cell replacement (Kawabori et al., 2020; Suda et al., 2020 and Alessandrini et al., 2019), SCs extend the therapeutic window traditionally ascribed to thrombolytic drugs and mechanical thrombectomy from a few hours to days, weeks, months and even years, altogether establishing the notion of a regenerative medicine approach to treating stroke (Chrostek et al., 2019) (Figure 1).
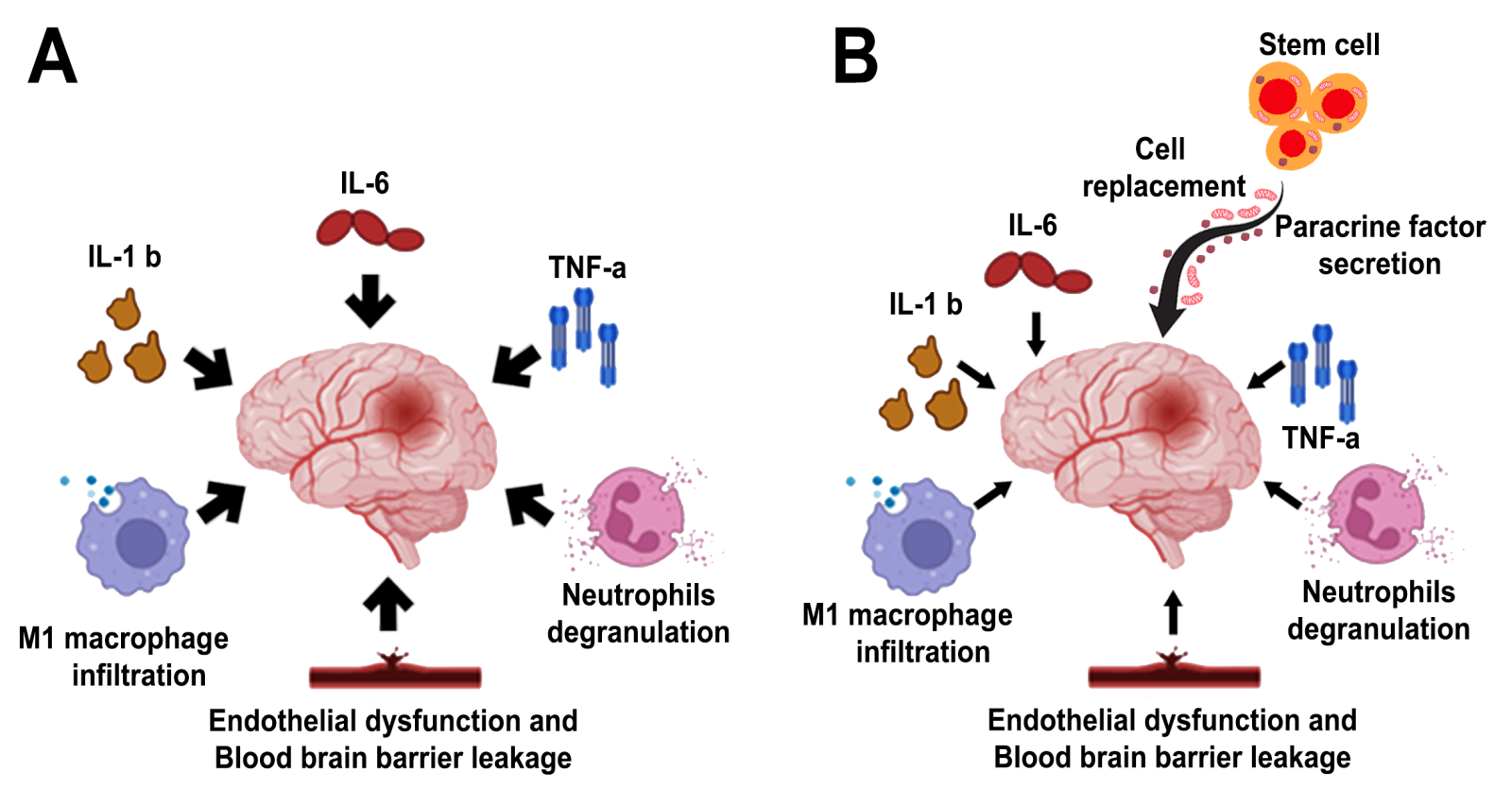
In a new window | Download PPT
Figure 1. Stem cell therapy in stroke. Multi-pronged mechanisms of action have been suggested to mediate the therapeutic effects of transplanted stem cells in stroke, generally by rescuing the neurovascular unit via cell replacement and paracrine factor secretion. Panel A: Among the many secondary cell death processes, inflammation has been widely implicated in stroke progression. Panel B: Stem cells mount multi-pronged neuroprotective and regenerative effects against stroke’s secondary cell death, including dampening inflammation.
Repositioning stem cell therapy for conditioning medicine
To date, most preclinical and clinical studies on SC therapy cater to its application as a regenerative medicine approach by extending the treatment window of intervention for stroke. In this line of investigation, SCs are transplanted after a prolonged period of time after stroke onset. However, we recognized that time is important to the brain after stroke as there is a higher likelihood of better functional recovery in stroke patients if the treatment can be initiated much closer to the time of stroke onset. Moreover, it may be more beneficial if SC therapy can be viewed as a preventive treatment. To this end, we posit that SC therapy can also be employed as a conditioning medicine modality, whereby its treatment can be initiated prior to stroke onset. By recognizing stroke co-morbidities, such as obesity and hypertension, it may be possible to identify a cohort of patients who may be at risk of stroke. With this patient history knowledge, intervening prophylactically with SCs before these patients succumb to stroke may improve clinical outcomes in these patients. Accordingly, we advance the conditioning medicine concept of SC therapy to protect the brain against an impending ischemic injury.
Conclusion
Stroke is a neurodegenerative disease that can be lethal and which considerably affects the quality of life for those who survive. Due to its increasing incidence, it is a main concern worldwide. Stem cell therapies have been vastly investigated during the 21st century, and most of the information obtained so far indicates that SCs exert beneficial effects against stroke, such as dampening inflammation, regulating immune cells, neurotrophic factors secretion, and at least in in vitro studies, cell substitution. The success of SCs has led to clinical trials for this therapy. Nevertheless, further studies are necessary to fully understand the mechanisms of action in tandem with optimizing the dose, timing, and route of cell delivery and identifying possible acute and long-term adverse effects. Whereas most studies on SC therapy focus on the regenerative approach, we speculate that there is room to test its application as a conditioning medicine. By identifying at-risk stroke patients, SC can be delivered prophylactically, and this new approach warrants additional studies.
Conflicts of interest
None
References
Felipe Esparza Salazar1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, US.
Germán Rivera Monroy1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, US.
Mauricio Muleiro Alvarez1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, US.
Joaquin Vega Gonzales-Portillo2
2Universidad Peruana de Ciencias Aplicadas, Av. Alameda San Marcos 11, Chorrillos 15067, Lima, Perú.
Jea-Young Lee1#
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, 12901 Bruce B Downs Blvd, Tampa, FL 33612, US.
Corresponding author:
Jea-Young Lee
Email: jeayoung@usf.edu

In a new window | Download PPT
Figure 1. Stem cell therapy in stroke. Multi-pronged mechanisms of action have been suggested to mediate the therapeutic effects of transplanted stem cells in stroke, generally by rescuing the neurovascular unit via cell replacement and paracrine factor secretion. Panel A: Among the many secondary cell death processes, inflammation has been widely implicated in stroke progression. Panel B: Stem cells mount multi-pronged neuroprotective and regenerative effects against stroke’s secondary cell death, including dampening inflammation.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5190 | 26 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA