Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Salvaging hearing loss in aging via NGF treatment
Time:2024-05-10
Number:6257
Hassan Choudhary1, Jea Young Lee1, Annamaria Cimini2, Michele d’Angelo2, Vanessa Castelli2
Author Affiliations


- 1Department of Neurosurgery and Brain Repair, University of South Florida, FL, USA.
- 2Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy.
Conditioning Medicine 2023. 6(5): 149-154.
Abstract
Hearing loss can significantly impact the quality of life. Significant societal and economic burden accompanies hearing loss. Unfortunately, a treatment for age-related hearing loss is lacking. Cochlear loss of mechanosensory hair cells commonly represents human hearing loss. Interestingly, cochlear hair cells can be repaired. Here, we discuss evidence of hair cell regeneration leading to salvaging of hearing loss in aging, highlighting the role of neurotrophins, specifically nerve growth factor (NGF), in hair cell regeneration. During development, NGF participates in the crosstalk between auditory receptors and efferent innervation in the cochlea. Accordingly, NGF may be a potential therapy for hearing loss. However, efficient delivery of NGF to the cochlea is a significant challenge for developing hearing impairment therapies. Our recent study revealed that intranasal administration of NGF may effectively reach the cochlea and afford therapeutic effects. In the first phase of our study, we performed the biodistribution analysis of NGF in mice at different time points (2h-48h) after intranasal administration. Tympanic bullas were sampled, and perilymph was collected to assess human NGF levels via enzyme-linked immunosorbent assay. The results of biodistribution in wild-type mice showed effective delivery of NGF to the cochlea starting two hours post-treatment, with a peak concentration at 12 hours. In aging-accelerated mice, NGF delivered intranasally counteracted hearing loss, coinciding with improved hair cell morphology. These findings suggest the therapeutic role of NGF in addressing age-related hearing loss and the importance of efficient drug delivery methods to the cochlea for successful treatment.
Keywords: Neurotrophic factor, Intranasal, Drug delivery, Auditory system, Therapy
Abstract
Hearing loss can significantly impact the quality of life. Significant societal and economic burden accompanies hearing loss. Unfortunately, a treatment for age-related hearing loss is lacking. Cochlear loss of mechanosensory hair cells commonly represents human hearing loss. Interestingly, cochlear hair cells can be repaired. Here, we discuss evidence of hair cell regeneration leading to salvaging of hearing loss in aging, highlighting the role of neurotrophins, specifically nerve growth factor (NGF), in hair cell regeneration. During development, NGF participates in the crosstalk between auditory receptors and efferent innervation in the cochlea. Accordingly, NGF may be a potential therapy for hearing loss. However, efficient delivery of NGF to the cochlea is a significant challenge for developing hearing impairment therapies. Our recent study revealed that intranasal administration of NGF may effectively reach the cochlea and afford therapeutic effects. In the first phase of our study, we performed the biodistribution analysis of NGF in mice at different time points (2h-48h) after intranasal administration. Tympanic bullas were sampled, and perilymph was collected to assess human NGF levels via enzyme-linked immunosorbent assay. The results of biodistribution in wild-type mice showed effective delivery of NGF to the cochlea starting two hours post-treatment, with a peak concentration at 12 hours. In aging-accelerated mice, NGF delivered intranasally counteracted hearing loss, coinciding with improved hair cell morphology. These findings suggest the therapeutic role of NGF in addressing age-related hearing loss and the importance of efficient drug delivery methods to the cochlea for successful treatment.
Keywords: Neurotrophic factor, Intranasal, Drug delivery, Auditory system, Therapy
Highlights
We discuss the potential of neurotrophic factor therapy for treating age-related hearing loss. In particular, we review the literature, focusing on our recent study that demonstrates the delivery of intranasal nerve growth factor to the cochlea and the efficacy of nerve growth factor in reducing hair loss and improving hearing function in a senescent-accelerated mouse model. Because aging is a predisposing factor in hearing loss, finding a treatment strategy that retards aging may likely prevent auditory dysfunction. Reminiscent of the conditioning medicine approach to neuroprotection, our study advances the concept of nerve growth factor treatment to protect against hearing loss during aging.
Current status of age-related hearing lost
Age-related hearing loss (ARHL) manifests as sensorineural hearing loss (Yutian et al., 2019; Bisogno et al., 2021). It typically starts in individuals over the age of 65. ARHL is a prevalent and increasingly recognized public health concern, highlighting the impact of hearing loss on various aspects of well-being (Huddle et al., 2017; Lohler et al., 2019; Ellis et al., 2021). Among the key components affected in the auditory system, the cochlea manifests as the primary site affected by aging in the auditory system. Aging leads to degeneration of both hair cells (HCs) and spiral ganglion neurons (SGNs) in the cochlea (Frisina et al., 2016). The aging process affects not only the peripheral but also the central auditory systems. Aging affects all aspects of the auditory system, indicating a widespread impact on auditory function.
ARHL is a socioeconomic burden, yet there are no treatments to prevent or counteract it. There is an urgent need to understand the challenges posed by age-related hearing loss. Recognizing the specific components of the auditory system affected by aging and exploring novel therapeutic interventions are paramount for improving the quality of life of individuals with ARHL.
Understanding the biological mechanisms contributing to hearing loss has attracted much research attention (Vlaikovic and Thorne, 2021). The primary factor mediating ARHL has traditionally been seen as an injury to HCs (especially outer hair cells or OHCs) (Tu and Friedman, 2018; Uchida et al., 2019). Rather than an acute injury, ARHL exhibits pathological hallmarks of neurodegeneration. Recent findings document damaged cochlea as the major pathology in ARHL (Jayakody et al., 2018; Paplou et al., 2021). This detection of progressive cell death in hearing loss highlights a transition from a primary focus on hair cell injury to recognizing the importance of neurodegeneration in the cochlea, particularly in the context of ARHL.
A careful examination of the pathological factors mediating ARHL points to an early pathology referred to as inner hair cell (IHC) synaptopathy (Kujawa et al., 2015; Mohrle et al., 2016). Although a complete understanding of the mechanistic factors underlying ARHL remains elusive, accumulating evidence suggests that cochlear synaptopathy is one of the initial pathological manifestations (Kujawa et al., 2015; Liberman and Kujawa, 2017). In the laboratory and the clinic, a crucial early pathology in ARHL manifests as the pruning of synapses between inner hair cells (IHCs) and spiral ganglion neurons (SGNs) (Makary et al., 2011; Sergeyenko et al., 2013; Viana et al., 2015; Wu et al., 2019). This condition is called cochlear or IHC synaptopathy, which seems to correlate temporally with IHC loss. Indeed, synaptopathy occurs before IHCs are lost in mice and humans (Makary et al., 2011; Sergeyenko et al., 2013; Viana et al., 2015; Wu et al., 2019). These observations support the notion that cochlear synaptopathy is an early event in ARHL, shedding light on the temporal sequence of events, particularly synaptopathy between IHCs and SGNs.
Finding treatment strategies for hair cell regeneration and protection of hearing loss
The loss of mechanosensory HCs in the cochlea is the pathological hallmark of hearing impairment (Wong and Ryan, 2015). Interestingly, experimental studies in birds reveal the potential for hair cell regeneration through cell proliferative and differentiation capacities of supporting cells (Cotanche, 1987; Cruz et al., 1987; Atkinson et al., 2015). To this end, probing the role of neurotrophic factors, particularly nerve growth factor (NGF), may reveal their utility in neuronal survival and differentiation, as well as their involvement in the repair of the cochlea (Despres et al., 1991; Terenghi, 1999; Anand, 2004). Neurotrophic factors play a crucial role in controlling neuronal developmental cues (Despres et al., 1991; Terenghi, 1999; Anand, 2004), including synapse formation and plasticity (Represa and Bernd, 1989; Lo, 1995; Datta et al., 1997). Four major neurotrophic factors have been identified, namely NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT4/5) (Represa and Bernd, 1989; Lo, 1995). Interestingly, NGF phosphorylates tropomyosin receptor kinase (TrkA) receptors and regulates downstream Akt, contributing to cell proliferation (Datta et al., 1997; Brain and David, 1999; Saito et al., 2005). Taken together, NGF may act as a therapeutic agent against hearing loss, possibly by its ability to promote hair cell regeneration and neuronal survival.
The therapeutic potential of NGF for preventing hearing loss can be further appreciated by probing its function in embryogenesis, characterized by the upregulation of NGF and its receptors in the inner ear (Staecker et al., 1996; Fritzsch et al., 2016). NGF plays a pivotal role in differentiating auditory ganglia and HCs (Represa and Bernd, 1989; Fritzsch et al., 2004). The administration of NGF decreases hair cell death and prevents hearing impairment (Gao, 1998; Han et al., 2017; Wang et al., 2017). Mouse NGF maintains auditory function in mice with advanced hearing dysfunction (Wang et al., 2017). Mouse NGF may act to protect auditory functions by decreasing apoptosis (Wang et al., 2017). Thus, NGF may exert its therapeutic effects against hearing loss by suppressing apoptosis, providing insights into the molecular pathways involved in its protective effects on auditory function.
The impact of NGF can be examined in the senescence-accelerated prone strain 8 (SAMP8) mice, characterized by their accelerated senescence phenotype. The mean lifespan of SAMP8 mice is about nine months, and they exhibit accelerated aging and progressive degeneration of various components of the auditory system (Takeda et al., 1991). The degeneration includes OHCs, SGNs, stria vascularis, and ultimately IHCs (Takeda et al., 1991). This degenerative pattern is noted to resemble human ARHL (Marie et al., 2017). The use of SAMP8 mice as an animal model of ARHL captures a clinically relevant aging pathology, particularly the accelerated senescence phenotype and the similarity of their degenerative patterns to human age-related hearing loss. Using SAMP8 mice to study age-related hearing loss offers advantages. Still, researchers must carefully weigh the limitations, including the limited translatability due to species differences, the ethical concerns, and the limited longevity compared to humans, which may impact long-term studies on age-related conditions. SAMP8 mice also have cognitive deficits and other pathologies beyond hearing loss (Peixoto et al., 2021). The use of this model allows researchers to investigate the potential therapeutic effects of NGF in the context of ARHL.
Treatment interventions for hearing loss face a significant obstacle in efficiently delivering drugs to the cochlea (Ma et al., 2019). Similar to the central nervous system’s blood-brain barrier, the blood–labyrinth barrier (BLB) defends the auditory system by limiting the entry of systemically administered compounds to the inner ear (Salt and Hirose, 2018; Nyberg et al., 2019). Systemic administration of drugs faces challenges due to the protective nature of the BLB, resulting in limited drug access to the inner ear. Thus, circumventing the BLB is a prerequisite for drug treatments for hearing loss. This delivery challenge applies to testing the therapeutic potential of neurotrophins in ARHL since their treatment regimens rely on exogenous delivery methods. Exogenous delivery involves infusion methods such as osmotic pumps or microcannulation, with various animal models showing their effectiveness in preserving auditory nerve function (Prieskorn and Miller, 2000; Guigou et al., 2021). These methods have demonstrated efficacy in protecting against drug-induced ototoxicity and noise exposure-induced hearing loss, suggesting repair of the cochlea (Hu et al., 2004; Hu et al., 2005; Sly et al., 2016; Cocchiaro et al., 2022; Castelli et al., 2023). In tandem, exogenously delivered NGF into the adult rat cochlea stimulates widespread neurite outgrowth of dorsal root ganglion (Sly et al., 2016; Cocchiaro et al., 2022; Castelli et al., 2023). Drug development for neurotrophins, including NGF, must consider the importance of overcoming delivery challenges to develop effective therapies for hearing impairment. Exogenous delivery of NGF and its positive impact on preserving auditory function in animal models provides insights into potential strategies for future therapeutic interventions.
Intranasal delivery of NGF for treating age-related hearing loss
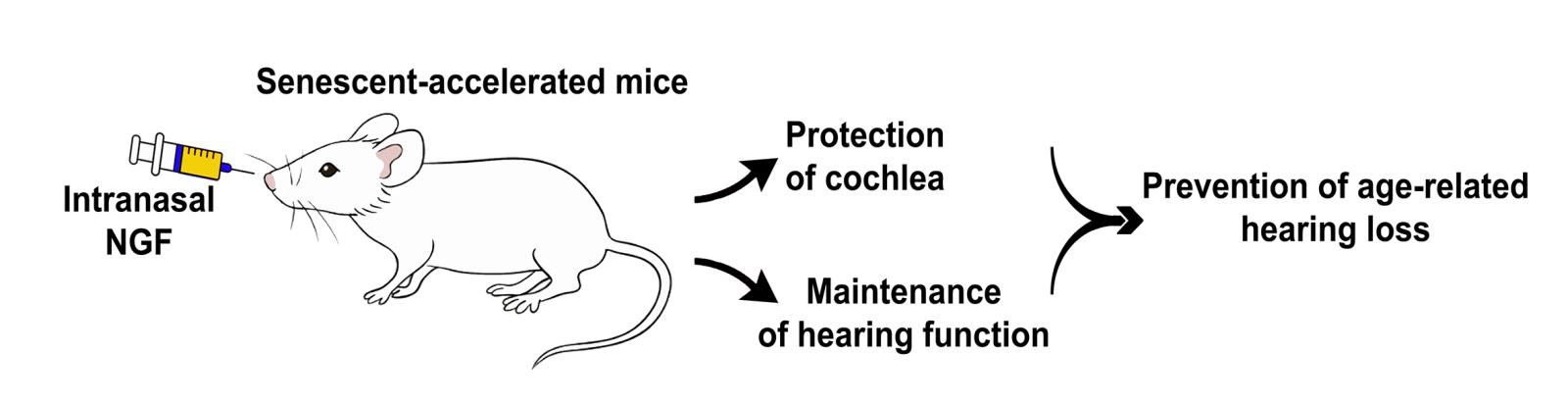
In contemplating NGF treatment for hearing loss, we recently tested the minimally invasive route of intranasal delivery (Castelli et al., 2023) (Figure 1). The intranasal route has numerous advantages compared to other routes. It is a convenient, easy, and painless route for self-administration. Due to its large surface area and rich vascularization, the nasal pathway is an intriguing portal for systemic delivery and delivery across the blood-brain barrier. Absorption is high, and there is no first-pass metabolism by the liver compared to other routes. Among the cons, we should mention the possibility of irritating or damaging the nasal mucosa, especially with long-term exposures, the limited volume and dose of drug that can be delivered, the requirement of high water solubility, and the variability of drug absorption (foster et al., 2022; Peixoto et al., 2022; Wong et al., 2024).
In a new window | Download PPT
Figure 1. NGF treatment for hearing loss. Intranasal delivery of NGF in senescent-accelerated mice protects the cochlea and maintains hearing function, suggesting its potential to treat age-related hearing loss.
In the first phase of our study, we performed a biodistribution analysis of NGF in mice after intranasal administration at different time points (2h-48h). Tympanic bullas were sampled, and perilymph was collected and assessed for NGF using the ELISA method. The results of the biodistribution analysis in wild-type mice showed effective delivery of NGF (single dose) to the cochlea two hours post-treatment, with a peak concentration at 12 hours that progressively decreased by 24 and 48 hours compared to the vehicle-treated group.
Second, we showed that intranasal NGF effectively reduced the pathological symptoms of ARHL in SAMP8 mice, improving both hearing performances and morphological changes (Castelli et al., 2023). The auditory brainstem response (ABR) threshold rose significantly in 37-day-old SAMP8 + vehicle mice, a manifestation of hearing loss. Interestingly, NGF-treated SAMP8 mice demonstrated significantly reduced ABR thresholds, suggesting the therapeutic effects of NGF against ARHL. Similarly, 37-day-old SAMP8 + vehicle animals displayed a significant reduction in distortion product otoacoustic emission (DPOAE) amplitude. In contrast, the NGF-treated SAMP8 mice exhibited a significantly elevated DPOAE amplitude, again implying a therapeutic effect of NGF against AHRL. Using the cochlear damage marker prestin (Dogan et al., 2018; Iliadou et al., 2021), SAMP8 + vehicle animals had increased plasma prestin levels, while the NGF-treated SAMP8 mice had significantly reduced plasma prestin levels. These data suggest that NGF prevented cochlear damage seen in the hearing loss animal model. A series of immunohistochemical markers for OHC and IHC loss corroborated the therapeutic efficacy of NGF against ARHL pathological conditions. SAMP8 + vehicle animals displayed cochlear injury, but the NGF-treated SAMP8 mice had a significantly higher survival of OHCs and IHCs. NGF must be delivered intranasally once a day for two months for the cell survival effects to remain stable. Additionally, healthy OHC and IHC stereocilia were maintained in NGF-treated SAMP8 mice compared to vehicle-treated SAMP8 mice. Finally, whereas cochlear ribbon synapses and neurons were reduced in aged mice treated with the vehicle, healthy postsynaptic morphologies were preserved in the cochlea of the NGF-treated mice. Taken together, these data provide solid evidence that NGF afforded robust and stable protective effects against ARHL pathological symptoms. These encouraging data highlight the potential benefits of intranasal delivery of NGF in treating ARHL, suggesting that this approach could be a promising therapy for hearing loss.
Advancing intranasal delivery of NGF for clinical application in age-related hearing loss
ARHL is a significant health problem (Huddle et al., 2017; Lohler et al., 2019; Bisogno et al., 2021). Various exogenous insults can lead to inflammation, oxidative stress, and cell injury, ultimately resulting in hearing loss (Yutian et al., 2019). Postulated underlying factors of ARHL include aging, radiation, sound-related trauma, and drug exposure, all of which can promote reactive oxygen species in the inner ear, leading to hearing loss (Gates and Mills, 2005; Jian et al., 2007; Caspary et al., 2008; Kovacic and Somanathan, 2008; Salt and Hirose, 2018; Shen et al., 2018). ARHL is the primary reason for hearing impairment in the population older than 70 years (Vlaikovic and Thorne, 2021).
Because of physiological barriers, specifically the BLB, which protects the cochlea and vestibular system from exogenous insults, these same barriers limit systemically administered therapeutics from reaching the inner ear (Ma et al., 2019; Peixoto et al., 2021). Minimally invasive intranasal delivery offers an effective approach to circumvent the BLB in treating ARHL. Our recent data showed that NGF reached the cochlea and improved ARHL-associated pathological symptoms after minimally invasive intranasal delivery.
The intranasal route likely achieves therapeutic effects on the cochlea by targeting the perilymph in the tympanic scale, which originates from cerebrospinal fluid (CSF), in particular from the scala vestibuli derived from blood plasma across a blood-perilymph barrier and the scala tympani originating from the CSF (Gates and Mills, 2005; Jian et al., 2007; Caspary et al., 2008; Kovacic and Somanathan, 2008; Engle et al., 2014; Cardin, 2016; Salt and Hirose, 2018; Shen et al., 2018). With the participation of both peripheral and central auditory systems in ARHL (Zhao et al., 2004; Martin de Campo et al., 2012), peripherally administered therapeutics that reach central targets like the cochlea are likely to afford clinical outcomes in ARHL. Our biodistribution data support the claim that intranasal NGF reaches the cochlea and exerts therapeutic effects in an animal model of ARHL.
In summary, we advance the notion of protective effects of NGF on ARHL, particularly in the context of an accelerated senescence model using SAMP8 mice. Our recent study (Castelli et al., 2023) employed NGF administered through the intranasal route, indicating that this method is less invasive and more efficient for delivering NGF to the cochlea. We provided evidence of NGF protection characterized by the preservation of OHCs and the maintenance of hearing function in senescent animals. NGF treatment decreased ABR threshold shifts in comparison to vehicle. The mechanisms of NGF protection and regeneration involve decreases in plasma prestin and cochlear hair cell loss. Histological examinations, including scanning electron microscopy and immunohistochemistry, confirmed the preservation of IHCs, OHCs, ribbon synapses, and cochlear neurons. These findings in SAMP8 mice concur with a similar report of the anti-apoptotic and pro-survival role of NGF in DBA/J2 mice.
Our recent study highlights that intranasal delivery of NGF reaches the cochlea and exerts protective effects on various cochlear components during aging (Castelli et al., 2023). These results suggest that NGF treatment may benefit ARHL. Altogether, we provide scientific evidence supporting the potential efficacy of NGF in protecting against age-related hearing impairment, emphasizing the importance of intranasal administration as a minimally invasive and effective route of administration and suggesting NGF as a potential therapeutic intervention for ARHL in humans. These observations warrant additional research to determine the optimal dose and long-term consequences of NGF treatment for hearing loss in humans.
Future perspectives
NGF exerts its protective effects on HCs through several pathways. Indeed, NGF supports the survival of auditory neurons (such as spiral ganglion neurons) by preventing their degeneration, preserving the health of these neurons probably by reestablishing the imbalance between proNGF and NGF, which is typical of neurosensorial disorders, by exerting anti-inflammatory activities and promoting the maintenance and functions of HCs, which are essential in the hearing process. In cases of damage or loss, we hypothesize that NGF may contribute to the regeneration of HCs by stimulating supporting cells to differentiate into new HCs, thus restoring hearing functionality. We hypothesize that NGF influences synaptic plasticity and enhances neurotransmitter release, facilitating efficient signal transmission from HCs to neurons. In light of this, NGF-based therapies could be beneficial not only for age-related hearing loss but also for hearing disorders caused by other factors such as drug therapies (e.g., antibiotics), environmental stress, and inflammatory conditions. Moreover, since it has been previously demonstrated that NGF exerted neuroprotective effects on acute cerebral ischemia (Li et al., 2017), it would be interesting to deepen our knowledge by testing this neurotrophin and others (such as brain-derived neurotrophic factors) in central nervous system disorders, such as neurodegenerative diseases.
Summary of results obtained from SAMP8 mice
Human NGF reaches the cochlea within two hours, with a peak at 12 hours in wild-type mice
NGF reduced age-related ABR threshold shift in SAMP8 mice
NGF increased DPOAE
NGF reduced plasma prestin levels
NGF reduced HC loss
Conflict of interest
The authors declare no conflict of interest.
References
Hassan Choudhary1
1Department of Neurosurgery and Brain Repair, University of South Florida, FL, USA.
Jea Young Lee1
1Department of Neurosurgery and Brain Repair, University of South Florida, FL, USA.
Annamaria Cimini2
2Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy.
Michele d’Angelo2
2Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy.
Vanessa Castelli2#
2Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy.
Corresponding author:
Vanessa Castelli
Email: vanessa.castelli@univaq.it

In a new window | Download PPT
Figure 1. NGF treatment for hearing loss. Intranasal delivery of NGF in senescent-accelerated mice protects the cochlea and maintains hearing function, suggesting its potential to treat age-related hearing loss.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6257 | 15 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA