Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Exercise conditioning in acute ischemic stroke
Time:2018-06-29
Number:7893
Eric Eugene Paul Cosky1, Yuchuan Ding1
Author Affiliations


- 1Department of Neurological Surgery, Wayne State University School of Medicine, Detroit, MI.
Conditioning Medicine, 2018. 1(4):204-211.
Abstract
In this article, we review the ways in which exercise promotes conditioning and neuroprotection in both clinical and scientific studies on acute ischemic stroke (AIS). The use of exercise as a preconditioning method for patients at risk for AIS is captivating to physicians because it is a cost-effective way to promote patient well-being. In fact, exercise is already an established rehabilitative method for AIS recovery. The effects of various exercise preconditioning intensities, intervals, and styles in animal and human studies are summarized in this review, including a brief summary of research trends in post-stroke exercise. The potential mechanisms involved in exercise conditioning and neuroprotection are described, including interactions at the blood-brain barrier (BBB) and regulation of neuronal apoptosis, inflammation, and metabolism. We also consider limitations and raise recommendations for prospective future research in exercise conditioning and neuroprotection.
Key words: acute ischemic stroke, conditioning, exercise, inflammation, neuroprotection
Abstract
In this article, we review the ways in which exercise promotes conditioning and neuroprotection in both clinical and scientific studies on acute ischemic stroke (AIS). The use of exercise as a preconditioning method for patients at risk for AIS is captivating to physicians because it is a cost-effective way to promote patient well-being. In fact, exercise is already an established rehabilitative method for AIS recovery. The effects of various exercise preconditioning intensities, intervals, and styles in animal and human studies are summarized in this review, including a brief summary of research trends in post-stroke exercise. The potential mechanisms involved in exercise conditioning and neuroprotection are described, including interactions at the blood-brain barrier (BBB) and regulation of neuronal apoptosis, inflammation, and metabolism. We also consider limitations and raise recommendations for prospective future research in exercise conditioning and neuroprotection.
Key words: acute ischemic stroke, conditioning, exercise, inflammation, neuroprotection
Introduction
Currently, acute ischemic stroke (AIS) is among the leading causes of mortality worldwide. In the USA, it is the fifth leading cause of death (Mozzafarian et al., 2016) and the leading cause of long-term mental disability and physical handicap. AIS risk factors include those that are modifiable (e.g., blood cholesterol, pressure, and sugar; obesity; and smoking) and non-modifiable (e.g., age, family history, gender, and race). It is imperative that physicians and scientists help prevent and treat AIS in patients through reduction of their modifiable risk factors.
The ability of physical exercise to condition or its ability to improve, maintain, and restore body function before disease, such as AIS, is an intense area of ongoing investigation. Several meta-analyses correlate physical activity with better neurological outcomes in hemorrhagic, ischemic, and total strokes (Lee et al., 2003; Wendel-vos et al., 2004). In clinical studies, previously active patients experienced decreased neurologic dysfunction after AIS, which is regarded to be the result of exercise-induced modifiable risk factors: favorable carbohydrate and lipid profiles, healthy body weight, and lower blood pressure and sugar levels maintained through exercise (Gillum et al., 1996; Macko et al., 1997; Evenson et al., 1999; Hu et al., 2004). In animal models of cerebrovascular disease, such as the middle cerebral artery occlusion (MCAO) model of AIS, exercise preconditioning confers endogenous neuroprotection as evidenced through the decreased infarct volumes and improved functional recoveries observed in several studies (Endres et al., 2003; Ding & Li, 2004; Ding, Luan et al., 2004; Ding et al., 2005; Ding, Ding et al., 2006; Ding, Li et al., 2006; Ding, Mrizek et al., 2006; Davis et al., 2007; Guo, Cox et al., 2008; Guo, Lin et al., 2008; Chaudhry et al., 2010; Curry et al., 2010; Liebelt et al., 2010; Zwagerman, Sprague et al., 2010).
AIS & Exercise Preconditioning:
In this section we will review the relevant animal studies that have contributed to our knowledge of exercise preconditioning in AIS with respect to exercise intensities, intervals, and styles. These elements of exercise are known to provide differential neuroprotection in animals experiencing cerebrovascular ischemia through MCAO. Afterwards, we will examine exercise intensities, intervals, and styles in human clinical studies. We will end this section by considering limitations of exercise preconditioning in clinical practice.
Neuroprotective effects are observed in rats preconditioned with both forced treadmill running (He, Wang et al., 2014) and voluntary wheel running (Kalogeraki, Pielecka-Fortuna et al., 2016). In forced treadmill running, the rodents run at a slow pace over long intervals. Unlike these rats, those exercising ad libitum run faster and longer, but in shorter spurts (Noble et al., 1999). Even though rodents preconditioned with forced treadmill exercise ran shorter total distances, researchers measured significantly smaller brain infarcts and less neurologic deficits in this cohort (Hayes et al., 2008). Similarly, rats forced to run on a treadmill five days a week for eight to nineteen weeks had reduced brain infarct volume after MCAO (Arrick, Yang et al., 2014). Rats that are exercise preconditioned for two to twelve weeks experienced a decreased lesion load and neuronal damage after MCAO (Stummer et al., 1994; Wang et al., 2001; Ang et al., 2003; Ding & Li, 2004; Davis et al., 2007; Jia, Hu et al., 2009; Curry et al., 2010; Liebelt, Papapetrou et al., 2010). This neuroprotective effect remains present for at least three weeks even after rodents stop exercising, which suggests that long-lasting protection is likely conferred through exercise training prior to cerebrovascular ischemia (Ding, Luan et al., 2004).
In a new window | Download PPT
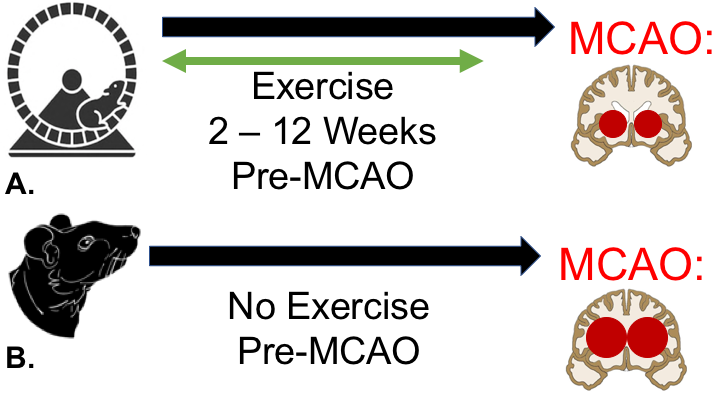
Figure 1: Exercise Preconditioning in the MCAO Model. A-B: In rats that are exercise preconditioned two to twelve weeks prior to MCAO (A), there are fewer neurological defects and reduced brain infarct volumes compared to sedentary rodents (B).
The two styles of exercise commonly employed in animal studies on exercise preconditioning are simple and complex. In simple exercise, subjects move on a running wheel or treadmill, which requires repetitive movements only. In complex exercise, subjects engage in enriched activities that demand equilibrium and proprioception (Ding et al., 2003). In rats conditioned with complex exercise, enhanced synaptogenesis and better functional outcomes were observed compared to rats conditioned with simple exercise consisting of treadmill running (Jones et al., 1999; Ding et al., 2003). Nevertheless, simple exercise still provides neuroprotection when compared to sedentary control animals (Ding et al., 2003).
Early clinical studies found that patients regularly exercising at moderate intensities and intervals had better functional outcomes after stroke (Larson et al., 2006). Similarly, a set of studies found that recovering stroke patients previously performing low to moderate intensity exercise prior to their AIS had less severe sequelae (Deplanque, Masse et al., 2006; Deplanque, Masse et al., 2012). However, these clinical studies were observational, which raises the possibility that confounding variables such as patient diet or smoking may have impacted their results. Moreover, the subjects were not randomized, making the results less generalizable among patients of diverse ages and races, which are important characteristics, as they are non-modifiable risk factors of AIS.
In a retrospective clinical investigation, patients reporting regular moderate physical activity had better functional outcomes than those reporting regular low or high intensity exercise at three months after AIS (Stroud, Mazwi et al., 2009). The authors state that their research results might have been skewed by both patient recall bias and sampling bias. A more recent clinical study found that patients engaging in high intensity physical activities had better neuroprotection against AIS: they experienced less severe strokes upon hospital admission, lower infarct volumes, and reduced length of in-hospital stays (Ricciardi, Lopez-Cancio et al., 2014). These findings are consistent with those obtained in murine models.
In a prospective cohort study, 27,348 patients were categorized as sedentary, moderately physically active (exercising one to three times a week), or highly physically active (exercising four or more times a week). The patients were assessed over six years for transient ischemic attacks and AIS, and those in the third cohort had the lowest prevalence of cerebrovascular disease (McDonnell et al., 2013). An important limitation of their research design is that patients might have changed their exercise habits over the six years of the study. A recent clinical investigation found that patients engaging in moderate to high intensity exercise even one to two times a week might be better protected against AIS (Jeong et al., 2017). In humans, there are four types of exercise: aerobic/endurance, balance, flexibility, and resistance/strength. However, data on exercise style in humans and functional outcomes after stroke are lacking; thus, it is essential to consider style of exercise in future research endeavors on exercise conditioning and stroke.
AIS & Exercise in the Recovery Phase / Rehabilitation:
In recovering animals engaged in exercise after MCAO, the effects of physical activity are either negative or positive depending on exercise intensity and interval or timing after cerebrovascular ischemia. In a recent study, rats underwent MCAO and were assigned to one of three exercise intensity regimens. Those performing high-intensity exercise recovered motor and physical functions earlier than control and treatment groups (Wang et al., 2016). As for temporal concerns, rodents exercising very early (6 hours) and early (24 hours) after MCAO had elevated stress response protein levels, such as HIF1-α (hypoxia-inducible factor 1-α) and Hsp70 (heat shock protein 70 kDa), and inflammatory cytokines like IL-1ß (interleukin 1ß) and TNF-α (tumor necrosis factor-α), factors exacerbating ischemic injury (Li et al., 2017). In contrast, rats engaged in physical activity one day after MCAO experience diminished BBB damage, cerebral edema, and inflammation (( Zhang et al., 2013 ); Zhang et al., 2016). Similarly, in exercise-trained rodents, depressed neuronal apoptosis occurs at one day after MCAO, which positively correlates with cognitive functions involving learning and memory (Li et al., 2014). Furthermore, rats exercising three days after MCAO had lower expression of inflammatory cytokines and stress proteins (Li et al., 2017). Moreover, rodents exposed to an enriched environment after MCAO have better neurological functioning and higher penumbral vascular density than time-matched controls (Xie et al., 2018). Thus, rats exercising after cerebrovascular ischemia injuries obtain neuroprotective effects associated with amplified angiogenesis (Pang et al., 2017; Xie et al., 2018), enhanced neurotrophin expression (Vaynman et al., 2005), and neurogenesis (Leasure et al., 2010; Zhang et al., 2013; Pang et al., 2017).
In patients surviving stroke, there are often profound physical deficits, such as apraxia, ataxia, and hemiparesis, which make exercising difficult especially in the early recovery phase. The most recent exercise guidelines for patients after AIS recommend aerobic exercise at twenty- to sixty-minute intervals three to five times a week, endurance and muscular strength training two to three days a week, and neuromuscular activities like tai chi or yoga two to three days a week (Billinger et al., 2014). In addition, it is recommended that physicians advise capable patients to perform low-intensity exercise and higher-intensity exercise for those who are physically fit enough, as the health benefits are greater (Boyne et al., 2014; Billinger et al., 2015). At the chronic recovery phase, patients engaged in exercises involving gait and weight-shift training have improved balance and coordination needed for walking (Van Duijnhoven et al., 2016). The Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) study found that both aerobic exercise and strength training significantly improved cognitive functions, including attention span and processing speed, in patients engaged in these physical activities in the first three months after stroke (Oberlin et al., 2017). These cognitive effects might be attributed to exercise increasing cerebral perfusion in patients after AIS (Robertson et al, 2017). A meta-analysis of controlled randomized clinical trials concluded that early exercise interventions in patients within the first six months after stroke coincided with significant reductions in blood cholesterol and pressure (Wang et al., 2018). In sum, post-stroke rehabilitative exercise has two important effects: it improves patients’ functional outcomes and is a cost-effective means for preventing future strokes by reducing modifiable risk factors in survivors.
Potential Mechanisms:
A better understanding of the cellular and molecular mechanisms involved in exercise conditioning and neuroprotection is needed to provide physicians and scientists with the theoretical foundation to develop and test future therapeutic interventions for AIS. In exercise conditioning, the increased release of growth factors such as neurotrophins upregulate neurogenesis and synaptic plasticity as explained briefly below. We will examine four potential mechanisms underlying exercise preconditioning that are currently believed to improve neuronal stability and viability after cerebrovascular ischemia injury, including its ability to: (1) diminish metabolic dysfunction, (2) mitigate apoptosis, (3) reduce cerebral inflammation, and (4) strengthen the blood-brain barrier (BBB). Taken together, these mechanisms enhance the brain’s resiliency against ischemia and reperfusion injuries.
Neurotrophins
In the central nervous system (CNS), exercise galvanizes growth factor cascades and neurogenesis. An earlier investigation found elevations in BDNF mRNA and protein expression after MCAO in recovering rats during the reperfusion phase, thus implying a function for this neurotrophin in maintaining neural network integrity (Schabitz et al., 1997). Another study determined that rodents preconditioned with endogenous nerve growth factor (NGF) and with exercise experience enhanced neuroprotection after MCAO (Ang et al., 2003). After exercise preconditioning, rats undergoing MCAO have elevated BDNF and NGF mRNA levels in their brain tissue, especially in astrocytes and neurons (Ding, Li et al., 2004). Now the neuroprotective effects of BDNF and nerve growth factor (NGF) after cerebrovascular ischemia are thought to include their functions of fostering neuronal development, promoting synaptic plasticity, and protecting CNS vasculature (Kim et al., 2004; Kuipers et al., 2006; Cohen-Cory et al., 2010). However, an outstanding issue is that in rodent hippocampi after MCAO, the nascent neurons are often dysmorphic or not properly integrated into the existing neuronal network (Niv et al., 2012; Woitke et al., 2017).
Metabolic Changes
There are metabolic changes that confer neuroprotection for subjects engaged in chronic exercise prior to cerebrovascular ischemia. In exercise-preconditioned rats, residual glucose metabolism is observed after MCAO (Bequet et al., 2001). This metabolic activity is associated with increased neuronal ATP synthesis after MCAO (McCloskey et al., 2001). A major protein regulator of these metabolic changes observed after exercise training is hypoxia-inducible factor-1α (HIF-1α). A known neuroprotective element in the post-ischemic setting (Bernaudin et al., 2002; Schubert, 2005), HIF-1α is upregulated after exercise preconditioning and induces both angiogenesis and glycolysis (Bergeron et al., 1999; Bernaudin et al., 2002; Schubert, 2005). These HIF-1α-induced metabolic changes promote neuronal survival (Kinni et al., 2011).
In response to exercise preconditioning, the cerebral microvasculature is remodeled to meet the metabolic demands of the brain observed in cerebrovascular ischemic injury. Chronic exercise preconditioning is associated with increases in blood vessel density (Swain et al., 2003), arteriogenesis (Lloyd et al., 2005), and angiogenesis (Ding, Luan et al., 2004), thus creating and maintaining extensive anastomoses that are important in ameliorating brain injury (Ding, Li et al., 2004). This underlying molecular mechanism is mediated through angiopoietin 1 and 2 along with vascular endothelial growth factor (VEGF) (Ding, Luan et al., 2004). In support of these earlier findings, it was recently reported that treadmill exercise upregulates VEGF receptor expression (Pang et al., 2017). A clinical study on stroke patients (n = 83) measured higher serum levels of VEGF on the seventh day after stroke in patients physically active prior to ischemic injury (López-Cancio et al., 2017). Perhaps their most meaningful finding was that patients’ VEGF levels were independently associated with functional outcomes at three months, and with smaller infarct volumes.
Neuronal Death & Survival
The signaling activity of neurons in tissues damaged by ischemia and reperfusion injuries is mediated by various regulatory genes and proteins that trigger cascades leading to either cell death or survival. According to data gathered in rat studies using MCAO, exercise preconditioning decreases the rate of neuronal apoptosis after cerebrovascular ischemia through Hsp70 and TNF-α (Chaudhry et al., 2010; Liebelt, Papapetrou et al., 2010). In addition, anti-apoptotic genes such as Bcl-2 and Bcl-xL, and pro-apoptotic genes such as AIF, Bad, Bak, and Bax modulate neurons’ responses to hypoxic conditions (Lazou et al., 2006). In particular, exercise-preconditioned rodents have increased expression of anti-apoptotic proteins and a corresponding decrease in pro-apoptotic proteins after MCAO (Chaudhry et al., 2010). As this ratio favors anti-apoptotic markers, neuronal survival is enhanced even in lethal ischemic injuries (Rybnikova et al., 2006). It is worth mentioning that treadmill training promotes functional memory recovery after MCAO through increased cell proliferation and decreased apoptosis (Seo et al., 2014). In exercise-preconditioned rats, the number of glial cells and neurons containing Hsp20 were higher than in sedentary rats after ischemic injury (Lin et al., 2015). Intriguingly, Lin et al. correlated increased Hsp20 levels with both decreased brain infarct volume and glial and neuronal apoptosis. Furthermore, these same authors concluded that chronic exercise training confers neuroprotection perhaps through increased expression of anti-apoptotic proteins.
The heat shock proteins are expressed in response to various stressors, including heat, hypoxia and ischemia, and have been observed to provide neuroprotection in the setting of AIS. In particular, Hsp70 has a neuroprotective effect through its downregulation of pro-apoptotic proteins, such as AIF (Matsumori, Hong et al., 2005) and its upregulation of anti-apoptotic proteins such as Bcl-2 (Liebelt et al., 2010). In addition, exercise preconditioning upregulates Hsp70 expression in neurons and nearby vasculature, and this effect is associated with enhanced neuroprotection (Masada et al., 2001). Another study demonstrated that Hsp70 and TNF-α function together in regulating the ratio of anti- to pro-apoptotic genes (Goel et al., 2010). It is therefore likely that exercise training generates elevated levels of Hsp70, in conjunction with TNF-α, thus promoting evasion of apoptosis and mediating neuronal survival.
Inflammatory Response
After cerebrovascular ischemia, astrocytes and microglia produce cytokines, such as IL-1ß and TNF-α, which stimulate the expression of cellular adhesion molecules (CAMs), including intracellular adhesion molecule-1 (ICAM-1), E-selectin, and P-selectin, on endothelial cells (Huang et al., 2006). These CAMs are well known for increasing leukocyte infiltration into the brain parenchyma, which worsens microvessel occlusion and neuronal damage in the salvageable penumbra. Exercise-preconditioned rats show decreased ICAM-1 levels, which correlates with depressed inflammation and leukocyte infiltration into the brain parenchyma during the reperfusion phase after MCAO (Ding et al., 2005; Barrientos, Frank et al., 2011). It was recently observed that aerobic exercise-preconditioned rodents have lower chemokine monocyte chemotactic protein-1 and IL-1ß levels after transient MCAO (Zhang et al., 2016). Therefore, it is likely that exercise preconditioning reduces secondary injuries in the reperfusion phase by impeding leukocyte migration and downregulating CAM and cytokine expression.
As mentioned above, TNF-α is a major inflammatory cytokine whose molecular actions have profound effects on the cerebral response to stroke. It is known to exert both toxic and trophic effects on neuronal tissues, depending on its concentration (Rothwell et al., 1995). It is thought that chronic low-level elevations of TNF-α, which are observed in exercise preconditioning, may result in the development of neuronal tolerance to this cytokine (Liu et al., 2000; Wang, Li et al., 2000). These gradual increases in TNF-α expression that are achieved through exercise preconditioning correlate with reduced brain injury in rats after MCAO (Ding et al., 2005). Conversely, TNF-α receptors (TNFRs) are downregulated through exercise preconditioning in rodents (Reyes et al., 2006), thus supporting a model in which chronic stimulation by low levels of TNF-α leads to desensitization of TNFRs.
These findings have yet to be fully confirmed in human patients. In a previous clinical investigation, researchers measured TNF-α plasma concentrations in patients at higher risk of stroke and did not detect significant changes between high-risk patients having high-grade carotid artery stenosis with unstable plaques and lower-risk asymptomatic patients with stable plaques (Jaroslav et al., 2012). A more recent clinical study on AIS patients (n = 619) and control patients (n = 612) determined that single nucleotide polymorphisms or SNPs in TNF-α are unlikely to contribute to AIS risk (Gu et al., 2016); thus, genetic heterogeneity at the TNF locus may not contribute to stroke risk. However, the relationships between cytokine expression, exercise preconditioning, and stroke risk in humans are not fully understood.
Another class of molecules, the toll-like receptors (TLRs), is involved in the inflammatory response to strokes by triggering cytokine cascades and influencing leukocyte infiltration. In TLR knockout mice, enhanced neuronal survival was observed as researchers found that ischemia and reperfusion injuries are significantly extenuated (Cao et al., 2007). In exercise-preconditioned rats, there is decreased expression of TLRs and diminished activation of its downstream targets, such as NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), which is a potent mediator of inflammation (McFarlin et al., 2006; Zwagerman, Plumlee et al., 2010; Ma et al., 2013; Zhu, Ye et al., 2016). Overall, exercise preconditioning helps to extinguish the inflammatory response observed after cerebrovascular ischemic injuries, thus protecting brain tissue against inflammatory damage after stroke.
Neurovascular Unit Integrity
The neurovascular unit is composed of capillary endothelial cells, glial cells and neurons. The stability of this structure requires extensive interactions between astrocytic end-feet, the basal lamina, endothelial cells, and neurons. Its permeability is regulated through the endothelial wall and basal lamina, including its extracellular matrix (ECM). This particular ECM consists of collagen type IV, fibronectin, heparin sulfate, and proteoglycans. The integrity of the neurovascular unit is threatened in AIS and is among the first structures to be damaged in cerebral ischemia (del Zoppo et al., 2000; del Zoppo et al., 2003). In AIS, the molecular interactions between collagen type IV, fibronectin, and laminin are altered, thus promoting inflammation and vasogenic edema (del Zoppo et al., 2003). Sedentary rats undergoing MCAO exhibit greater parenchymal edema, swollen astrocytic end-feet, and thinner basal lamina compared to exercise-preconditioned rats (Ding, Ding et al., 2006). In this manner, there is a positive correlation between blood-brain barrier (BBB) integrity and exercise preconditioning, in which exercise-preconditioned rodents experienced decreased brain injury and cerebral edema after MCAO (Masada et al., 2001; He, Wang et al., 2014; Shamsaei, Erfani et al., 2017).
In a new window | Download PPT
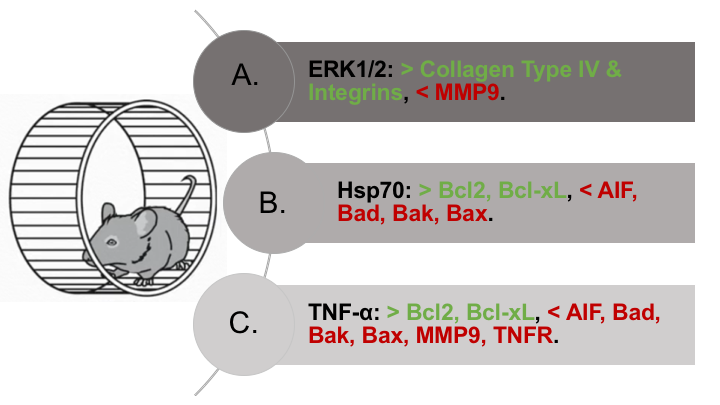
Figure 2: Three key regulatory proteins and their neuroprotective effects in exercise preconditioning. Foremost, exercise-preconditioned rats show increased activation, expression, and stimulation of ERK1/2, Hsp70, and TNF-α. (A) The proteins ERK1/2 stimulate collagen type four and integrin synthesis and inhibit MMP9 degradative activity, thus strengthening the blood-brain barrier under conditions of cerebrovascular ischemic injury. (B) The heat shock protein Hsp70 downregulates pro-apoptotic proteins (AIF, Bad, Bak, and Bax) and upregulates anti-apoptotic proteins (Bcl-2 and Bcl-xL), thus promoting neuron survival in exercise-preconditioned rodents undergoing MCAO. (C) The inflammatory cytokine TNF-α decreases pro-apoptotic protein (AIF, Bad, Bak, and Bax) and increases anti-apoptotic protein (Bcl-2 and Bcl-xL) expression together with Hsp70. It lowers MMP9 degradative activity, thus assisting the ERK1/2 proteins in maintaining blood-brain barrier integrity. In exercise-preconditioned rats, the gradual elevations in TNF-α concentration downregulate and sensitize responses at TNF-α receptors, thus alleviating inflammatory damage in these animals after ischemic insults.
Another class of CAMs, the integrins, enhances interactions between astrocytes and endothelial cells. The cerebral microvasculature uses integrin-mediated signal transduction pathways to regulate cell differentiation and proliferation (del Zoppo et al., 2003). After cerebral ischemia, the binding affinity of integrins for collagen and laminin decreases, inducing astrocytic swelling, BBB destruction, and cerebral edema (Hamann et al., 2002; Wang et al., 2003). However, in exercise-preconditioned rats, increased integrin expression is correlated with reduced ischemic brain injury after MCAO (Ding, Li et al., 2006). Additionally, there is elevated expression of the basal laminar protein, collagen type IV, in exercise preconditioned rodents (Davis et al., 2007). Altogether, these findings imply that exercise preconditioning strengthens the BBB through upregulation of collagen type IV and integrin expression, which alleviates cerebrovascular ischemic injury.
Another way that physical exercise bolsters the BBB is through downregulating matrix metalloproteinase (MMP) expression (Davis et al., 2007; Naderi, Alimohammadi et al. 2017). These proteins degrade the ECM and are produced and secreted by astrocytes, endothelial cells, and microglia. Increased MMP expression occurs after cerebrovascular ischemia in human and murine studies and is correlated with cerebral tissue inflammatory injury (Clark et al., 1997; Romanic et al., 1998; Asahi et al., 2001; Planas et al., 2001). The MMPs target caspases that trigger neuronal apoptosis (Gu et al., 2002). The death pathway is repressed through the actions of TNF-α, tissue inhibitors of metalloproteinases (TIMPs), and extracellular signal-regulated kinases (ERK1/2) (Brew et al., 2000; Arai et al., 2003; Zhang et al., 2010). The levels of these molecules rise after exercise and correspond with lower MMP expression and neuronal apoptosis after cerebrovascular ischemic injuries (Guo, Cox et al., 2008; Guo, Lin et al., 2008; Chaudhry et al., 2010).
Conclusions
The neuroprotective effects of exercise preconditioning are gathering strong support in both clinical and scientific studies. These effects include positive metabolic changes, reduced apoptosis and inflammation, a strengthened neurovascular unit, and upregulated neurotrophins. These cellular and molecular phenomena provide us with potential mechanisms and targets to investigate and manipulate for rational drug development. A pharmaceutical that mimics exercise-induced neuroprotection could potentially act through several signal transduction pathways to improve neurologic outcomes, which would be practical for patients with a history of transient ischemic attacks or AIS. The morbidity and mortality of AIS will continue to decline if physicians properly prescribe exercise-training programs for their patients with stroke risk factors. Moreover, the use of exercise preconditioning prior to neurologic surgery could take advantage of the known neuroprotective effects of exercise to achieve better surgical outcomes. Before physicians prescribe exercise preconditioning to patients, further research is needed, especially on evaluating the optimal exercise guidelines for ideal neuroprotection and examining the underlying cellular and molecular mechanisms mediating exercise-induced neuroprotection. The execution of prospective studies that are blinded, controlled, and randomized will be essential in resolving which exercise intensities, intervals, and styles confer the best patient outcomes.
References
Eric Eugene Paul Cosky 1
1Department of Neurological Surgery, Wayne State University School of Medicine, Detroit, MI.
Yuchuan Ding 1
1Department of Neurological Surgery, Wayne State University School of Medicine, Detroit, MI.
Corresponding author:
Eric Eugene Paul Cosky
Email: ecosky@med.wayne.edu
and
Yuchuan Ding
Email: yding@med.wayne.edu
In a new window | Download PPT
Figure 1: Exercise Preconditioning in the MCAO Model. A-B: In rats that are exercise preconditioned two to twelve weeks prior to MCAO (A), there are fewer neurological defects and reduced brain infarct volumes compared to sedentary rodents (B).
In a new window | Download PPT
Figure 2: Three key regulatory proteins and their neuroprotective effects in exercise preconditioning. Foremost, exercise-preconditioned rats show increased activation, expression, and stimulation of ERK1/2, Hsp70, and TNF-α. (A) The proteins ERK1/2 stimulate collagen type four and integrin synthesis and inhibit MMP9 degradative activity, thus strengthening the blood-brain barrier under conditions of cerebrovascular ischemic injury. (B) The heat shock protein Hsp70 downregulates pro-apoptotic proteins (AIF, Bad, Bak, and Bax) and upregulates anti-apoptotic proteins (Bcl-2 and Bcl-xL), thus promoting neuron survival in exercise-preconditioned rodents undergoing MCAO. (C) The inflammatory cytokine TNF-α decreases pro-apoptotic protein (AIF, Bad, Bak, and Bax) and increases anti-apoptotic protein (Bcl-2 and Bcl-xL) expression together with Hsp70. It lowers MMP9 degradative activity, thus assisting the ERK1/2 proteins in maintaining blood-brain barrier integrity. In exercise-preconditioned rats, the gradual elevations in TNF-α concentration downregulate and sensitize responses at TNF-α receptors, thus alleviating inflammatory damage in these animals after ischemic insults.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7893 | 16 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA