Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The effect of remote ischemic conditioning on clinical endpoints and treatment adherence in patients with acute ischemic stroke
Time:2024-09-17
Number:4223
Maria Kjølhede1, Grethe Andersen1,2, Claus Ziegler Simonsen1,2, Kim Ryun Drasbek3, Rebecca Best Jensen3, Jan Brink Valentin4, David C. Hess5, Rolf Ankerlund Blauenfeldt1,2
Author Affiliations


- 1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark.
- 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
- 3Center for Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark.
- 4Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
- 5Department of Neurology, Medical College of Georgia, Augusta University, Augusta, Georgia, USA.
Conditioning Medicine 2023. 6(6): 208-213.
Abstract
Remote Ischemic Conditioning (RIC) is a novel therapy that potentially activates protective pathways in the ischemic brain. Here, we present the clinical endpoints, compliance, and patient-reported outcome measures from a randomized sham-controlled trial investigating the effect of RIC on the rheo-erythrocrine function of red blood cells in patients with acute ischemic stroke (AIS). Patients were randomly assigned to RIC or sham twice daily for seven days. RIC or sham treatment consisted of five cycles, each cycle with five minutes of cuff inflation and five minutes of deflation on one upper extremity (total treatment time 50 minutes). The Montreal Cognitive Assessment (MoCA) and National Institute of Health Stroke Scale score (NIHSS) were obtained at baseline and after seven days. A total of 30 patients with AIS were included, and of these, 12 out of 15 patients assigned to RIC and 14 of 15 patients assigned to Sham completed the study. In the RIC group, the median (IQR) age was 67 years (52, 73) compared to 60 years (58, 72) in the sham group. We found no evidence of an association between RIC and improvement in the 7-day neurological outcome: NIHSS, -0.31 (-1.36-0.68), p = 0.51, and MoCA 1.03 (-1.26-3.32) = 0.38. All 12 patients (100%) in the RIC group and 7 (50%) in the sham group correctly identified which treatment they had received. The treatment compliance was high, and the majority of patients were willing to continue the treatment once daily for one year. ClinicalTrials.gov identifier: NCT04266639 (registered 2020-02-12).
Keywords: ischemic stroke, remote ischemic conditioning, stroke severity, compliance
Abstract
Remote Ischemic Conditioning (RIC) is a novel therapy that potentially activates protective pathways in the ischemic brain. Here, we present the clinical endpoints, compliance, and patient-reported outcome measures from a randomized sham-controlled trial investigating the effect of RIC on the rheo-erythrocrine function of red blood cells in patients with acute ischemic stroke (AIS). Patients were randomly assigned to RIC or sham twice daily for seven days. RIC or sham treatment consisted of five cycles, each cycle with five minutes of cuff inflation and five minutes of deflation on one upper extremity (total treatment time 50 minutes). The Montreal Cognitive Assessment (MoCA) and National Institute of Health Stroke Scale score (NIHSS) were obtained at baseline and after seven days. A total of 30 patients with AIS were included, and of these, 12 out of 15 patients assigned to RIC and 14 of 15 patients assigned to Sham completed the study. In the RIC group, the median (IQR) age was 67 years (52, 73) compared to 60 years (58, 72) in the sham group. We found no evidence of an association between RIC and improvement in the 7-day neurological outcome: NIHSS, -0.31 (-1.36-0.68), p = 0.51, and MoCA 1.03 (-1.26-3.32) = 0.38. All 12 patients (100%) in the RIC group and 7 (50%) in the sham group correctly identified which treatment they had received. The treatment compliance was high, and the majority of patients were willing to continue the treatment once daily for one year. ClinicalTrials.gov identifier: NCT04266639 (registered 2020-02-12).
Keywords: ischemic stroke, remote ischemic conditioning, stroke severity, compliance
Highlights
Remote Ischemic Conditioning (RIC) is a simple and low-cost intervention in which transient ischemia is induced in an extremity by repetitive inflation-deflation of a blood pressure cuff. Good adherence to the treatment protocol may be key in translating RIC into the clinic. RIC was well tolerated and only associated with mild to moderate pain compared to sham. The treatment compliance was high, and the majority of patients were willing to continue the treatment once daily for one year. Patients with higher treatment compliance were younger and had a better cognitive score at randomization, which may guide future trials on RIC in the subacute phases after stroke.
Introduction
Stroke remains a leading cause of death and disability worldwide (Johnson et al., 2019). Currently, revascularization is the only approved acute therapy for acute ischemic stroke (AIS) (Powers et al., 2019). Worldwide, these treatments are used in less than 7% of patients with AIS, and despite their beneficial effects, many patients remain disabled (Norrving et al., 2018). Protective therapies that can improve the brain’s tolerance to ischemia and reduce secondary ischemic injury are needed.
Remote Ischemic Conditioning (RIC) is a simple and low-cost intervention in which transient episodes of ischemia and reperfusion are induced in an extremity by repetitive inflation-deflation of a blood pressure cuff (Hess et al., 2015). The translatability of promising preclinical results into effective clinical therapies has proven difficult in pilot randomized controlled trials (Hougaard et al., 2014; England et al., 2017). However, recently, a large, randomized trial in AIS patients treated with RIC within 48 hours from symptom onset demonstrated improved functional outcomes at three months compared to controls. In contrast to previous studies, RIC was applied to both arms twice daily for 10-14 days (Chen et al., 2022; Hess et al., 2022). Dosing of RIC and compliance with the treatment protocol when it is continued for several days or weeks may be important in translating RIC to the clinic. Here, we present the clinical endpoints, compliance with treatment, and patient-reported outcome measures from a single-center randomized controlled trial.
Methods
We performed a pilot, single-center, randomized, patient-assessor blinded, sham-controlled study on patients admitted with AIS to the Department of Neurology, Aarhus University Hospital, Denmark. The primary objective of the Efficacy of Nitric Oxide in Stroke (ENOS) trial was to investigate whether RIC improved the rheo‐erythrocrine function of red blood cells in patients with acute ischemic stroke. Eligible study subjects were 18-80 years old and independent in activities of daily living (modified Rankin Score 0-2) with a magnetic resonance imaging (MRI) documented ischemic stroke, who could be included and randomized for study treatment within 48 hours of symptom onset. Patients with prior known neurological disease, upper extremity peripheral arterial stenosis, diabetes, and pregnancy were excluded.
The study was approved by the Central Denmark Region Committees on Health Research Ethics (ID no. 1-10-72-184-19) and the Danish Medicines Agency (jr. no. 2019081802, EUDAMED CIV-ID nr. 19-08-029484) and reported to the Region’s Internal List of research projects (ID no. 1-16-02-333-19). Clinicaltrial.gov identifier: NCT04266639. The study was monitored by the Good Clinical Practice unit in Aalborg/Aarhus, Denmark. All patients signed an informed consent form before enrolling in the study. The study adheres to the CONSORT guidelines.
Study design
Patients were randomly assigned to RIC/sham (1:1) using a simple, secure randomization procedure in REDCap without stratification. The RIC/sham treatment was initialized immediately after randomization and continued twice daily for one week. Patients discharged before day seven continued the treatment at home according to written instructions. Investigational automatic RIC/Sham devices were preprogrammed to five cycles of unilateral cuff inflation followed by five minutes of cuff deflation, a total treatment time of 50 minutes. The cuff pressure of the RIC device was dynamic to ensure complete arterial occlusion but with a minimum cuff pressure of 200 mmHg. If the systolic blood pressure exceeded 175 mmHg, 35 mmHg was automatically added. Maximum cuff pressure was 285mmHg. For the sham device, the inflation cuff pressure was fixed at 20mmHg, disregarding systolic blood pressure levels. The cuff was placed on the non-paretic side and ipsilateral to the side of the infarction. The investigational devices were designed and developed in collaboration with Aarhus University, the faculty of Biomedicine Technologies, the Department of Neurology at Aarhus University Hospital, and Seagull Healthcare, Slagelse, Denmark (Blauenfeldt et al. 2020). The manufacturer had no influence on study design, data collection, analysis/interpretation of results, or publication of results. The devices were returned to the stroke center after one week, and data on compliance was extracted from each RIC device. Received cycles out of planned cycles were calculated as the level of compliance to protocol.
Data on medical history, clinical characteristics, and treatment were obtained from medical records and the Danish Stroke Registry. MRI was performed at baseline as part of routine care. Further, Montreal Cognitive Assessment (MoCA) and National Institute of Health Stroke Scale (NIHSS) assessments were performed. The MoCA is a one-page, highly sensitive test administered by a healthcare professional for early detection of mild cognitive impairment (MCI) (Nasreddine et al., 2005). NIHSS is a 15-item neurologic examination scale for the assessment of acute stroke. MoCA and NIHSS were obtained at baseline and after seven days. A patient's perception of the treatment questionnaire was developed for this study. The original (Danish) and an English version (translated) are available in the supplemental material (see Supplemental Material). Patients and endpoint assessors were blinded. The investigators who performed the randomization were not blinded. No information regarding randomization was available in the patient record.
Sample size
The current study is an exploratory pilot study. The sample size was based on estimated improvements in the red blood cell deformability index, giving a sample size of 30 AIS patients (15 RIC and 15 Sham) (Kjølhede et al., 2023).
Statistics
Cohort characteristics and outcome at baseline were summarized using numbers and percentages, medians and interquartile range, and means and standard deviations (SD) as appropriate. Differences in NIHSS, MoCA, and physical activity scale for the elderly (PASE) and other continuous biomarkers over time (baseline, two hours, and seven days) between RIC (yes/no) were examined using mixed effects linear regression models with a random intercept on subjects. The model parameters were estimated using restricted maximum likelihood. Fixed effects in the model were RIC, time, and the two-factor interaction between RIC and time, the latter of which was considered the effect of interest. The adjusted model included reperfusion therapy (yes/no) and age (continuous). Data management and statistics were performed using Stata 18 software (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.), and effect estimates were presented with 95% confidence intervals (CI).
Results
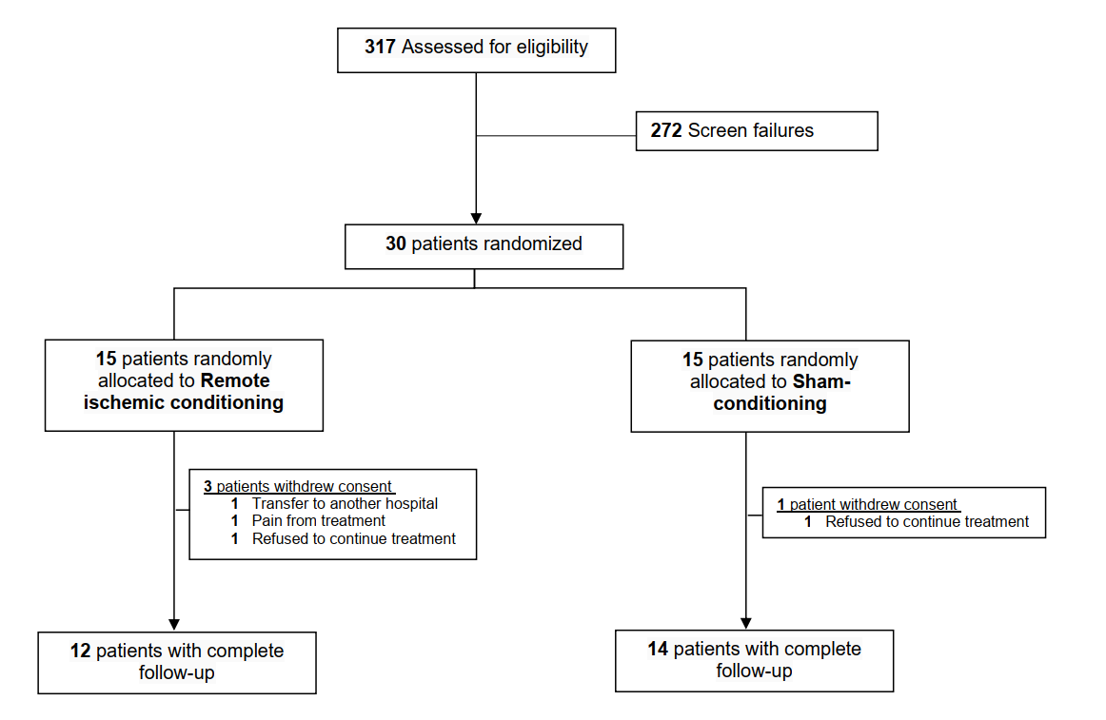
Between July 28, 2020, and July 5, 2021, 317 patients were screened for eligibility, and 30 patients with AIS were included in the study. Of these, 15 were randomized to RIC and 15 to sham treatment. Four patients withdrew consent: three RIC and one sham. Two patients refused to continue the treatment, one was transferred to another hospital, and one withdrew because the RIC cuff pressure was too painful (Figure 1).
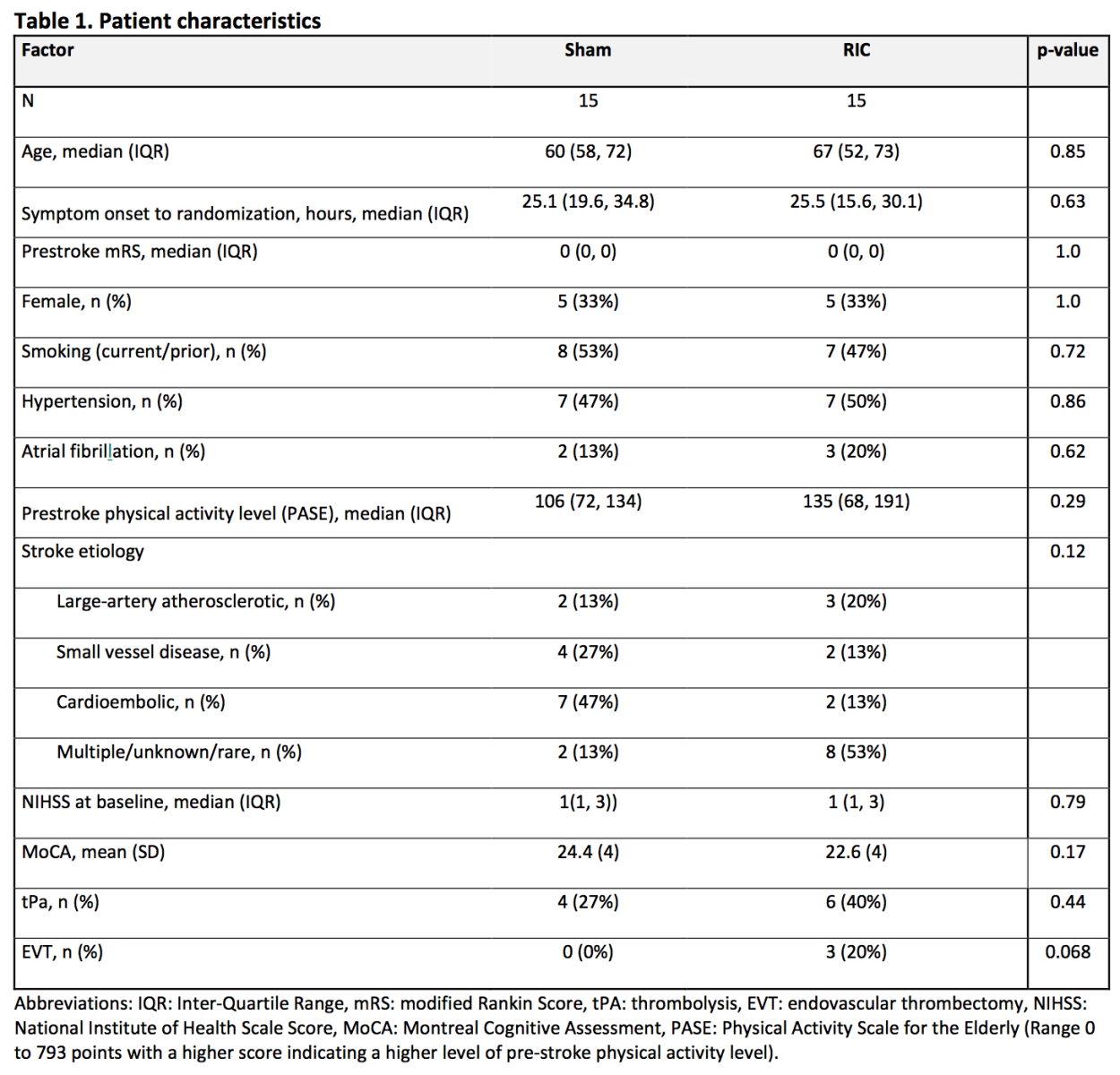
In the RIC group, the median (IQR) age was 67 years (52, 73) compared to 60 years (58, 72) in the sham group, and 33% were female in both groups. The mean (SD) NIHSS at baseline was 2 (1) in the RIC group and 2(1) in the sham group. Six patients (40%) in the RIC group and four in the sham group (27%) had received intravenous thrombolysis before enrollment, and three patients in the RIC group received endovascular treatment (EVT) compared to none in the sham group (Table 1).
Clinical endpoints
In both the RIC and sham groups, the mean stroke severity (NIHSS) was 2 points at randomization and 1 point at the seven-day follow-up visit (Table 2).
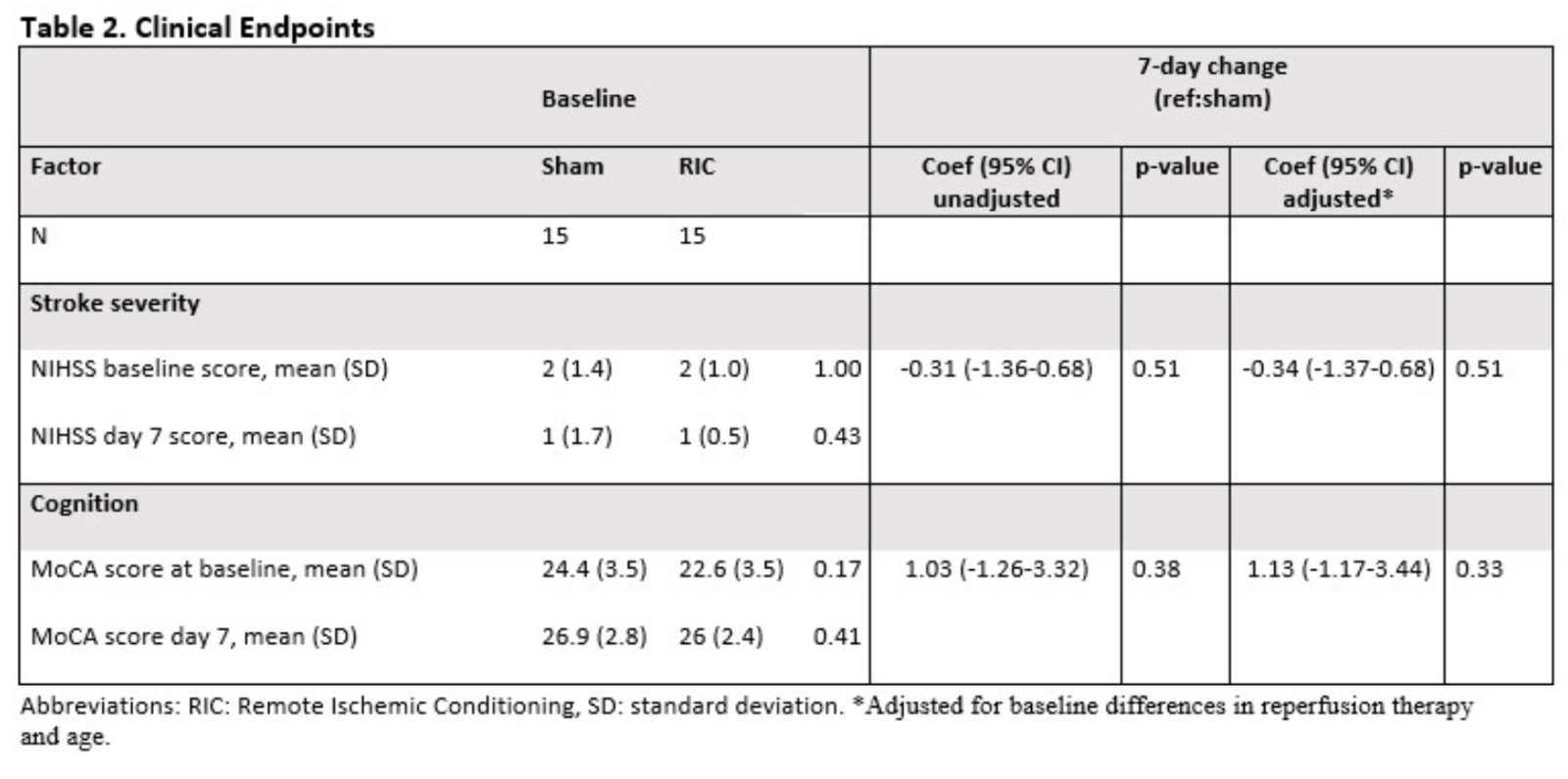
No statistically significant improvement was found between RIC and sham from randomization to day 7 in NIHSS, -0.31 (-1.36-0.68), p = 0.51, or MoCA 1.03 (-1.26-3.32) p = 0.38. Similar results were found in the models adjusted for reperfusion therapy and age (Table 3).
Treatment adherence and questionnaire responses
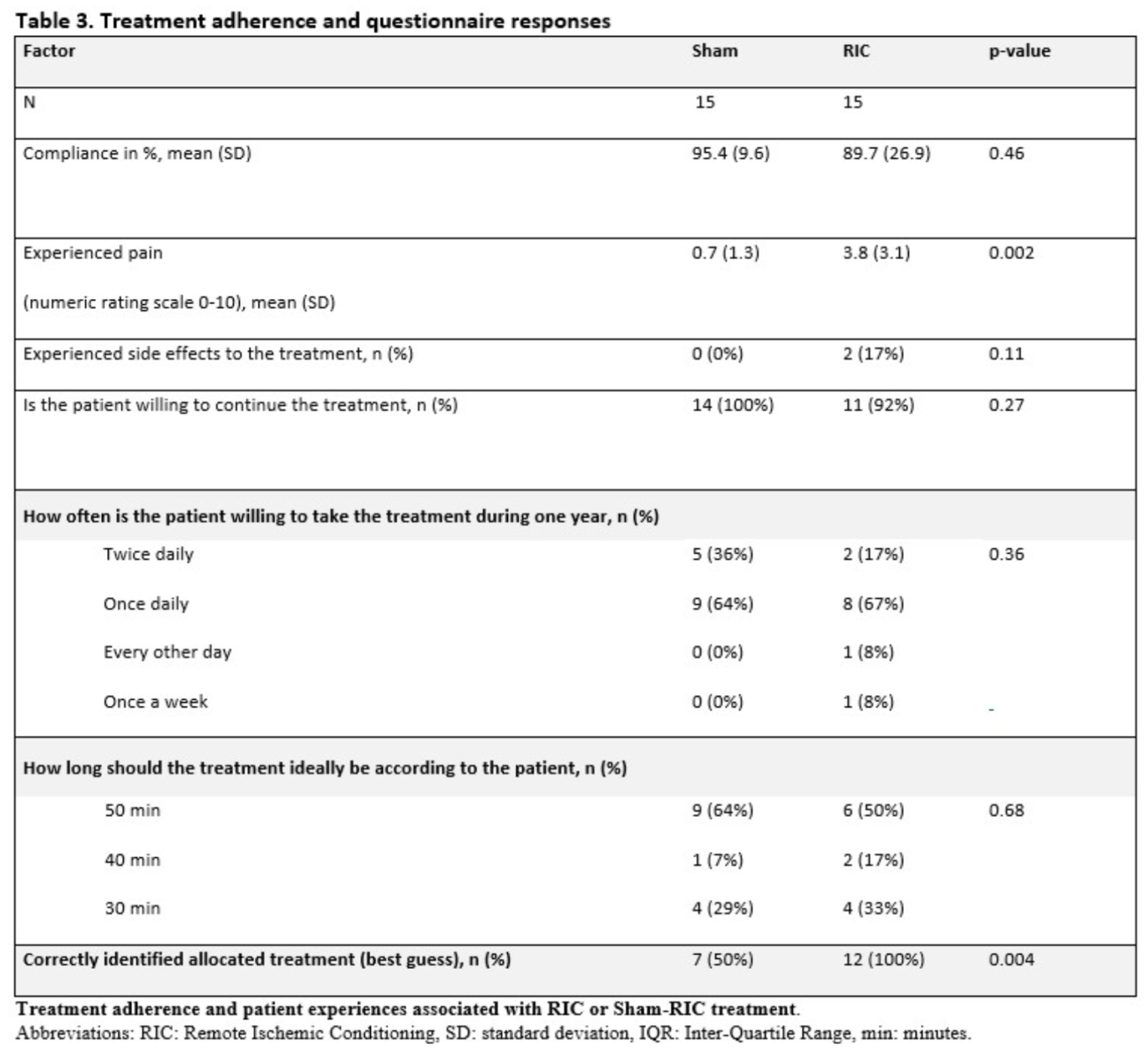
Mean (SD) treatment compliance was 89.7% (26.86) in the RIC group and 95.4% (9.54) for sham, p = 0.46 (Table 3). Two patients reported adverse effects, including mild bruising on the treated arm related to cuff pressure. No serious treatment-related adverse events appeared in the study. The mean (SD) self-reported pain/discomfort during treatment on a 0-10 numeric rating scale was 3.8 (3.1) for RIC and 0.7 (1.3) for sham, p = 0.002. Reasons for missing any RIC/sham cycles were reported as: pain from device/cuff (1), forgot to bring the device (1), technical issues with the device/battery (2), dropout from the study (2), or unknown (8).
All 12 patients (100%) in the RIC group and seven (50%) in the sham group could correctly identify which treatment they received. Ninety-two percent were willing to hypothetically continue the treatment beyond the seven days, and the majority of the patients (64% in the sham group and 67% in the RIC group) were willing to take the treatment once per day for 50 minutes for a year.
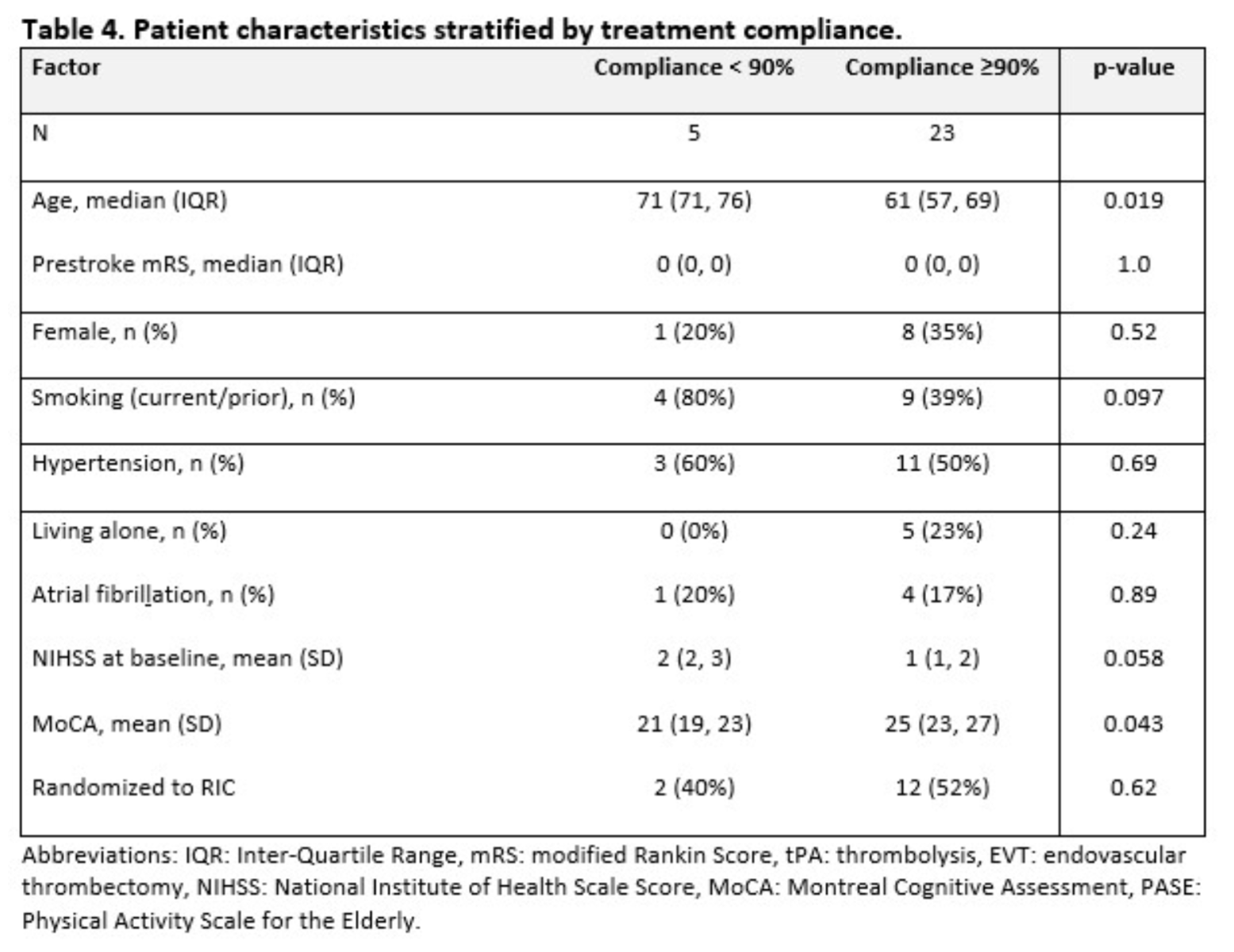
In a post-hoc analysis, we stratified the population according to treatment compliance of 90%. Five patients had treatment compliance below 90%, and in the remaining 23, it was 90% or above. The median age (71 vs. 61 years) was higher, and baseline MoCA (21 vs. 25) was lower in patients with low treatment compliance (Table 4).
Discussion
We found no evidence of an association between treatment with RIC twice daily for seven days and short-term improvement in neurological outcomes compared to sham-treated patients with AIS. RIC was well tolerated but associated with mild to moderate pain compared to sham. The treatment compliance was high, and the majority of patients were willing to continue the treatment once daily for one year. Patients with higher treatment compliance were younger and had better cognitive scores at randomization, which may guide future trials on RIC in the subacute phases after stroke.
The recent randomized sham-controlled RICA trial (Chronic remote ischemic conditioning in patients with symptomatic intracranial atherosclerotic stenosis) assessed whether daily RIC as a secondary preventive measure against new ischemic events in 3,033 patients with atherosclerotic intracranial arterial stenosis-related stroke or transient ischemic attack (TIA) (Hou et al., 2022). The trial was sham-controlled, and patients underwent RIC/sham for five cycles on both upper extremities once daily for one year. No significant difference was found in the primary endpoint of time to the first occurrence of ischemic stroke (hazard ratio (HR) 0.87, 95% confidence interval (CI) 0.74- 1.03; p = 0.12). Only 1,409 out of 3,033 (46%) patients had an acceptable treatment adherence (defined as 50% of the assigned treatments completed) and represented the per-protocol population. In the per-protocol analysis, RIC treatment significantly reduced the occurrence of AIS and the composite endpoints of AIS, TIA, and MI. Overall, the trial result was neutral but with a significant effect in patients with good treatment adherence (Blauenfeldt et al., 2023a). In the subacute phases after stroke, The RICAMIS trial (The Remote Ischemic Conditioning for Acute Moderate Ischemic Stroke) was the first large, multicenter, randomized clinical trial that was able to demonstrate improved functional outcomes in RIC-treated patients with AIS compared to controls (Chen et al., 2022). The recent RESIST (Remote Ischemic Conditioning in Patients With Acute Stroke) trial could not demonstrate an overall beneficial effect of RIC when applied within the first hours after a stroke (Blauenfeldt et 2023b; 2024). Based on the RICA, RICAMIS, and RESIST trials, the effect of RIC may be more pronounced and obtainable in the clinic in the subacute or chronic phases of stroke and will probably guide the direction of future RIC research. Several questions on treatment response related to comorbidities, physical activity level, medication, symptom duration, stroke severity, and the optimal conditioning regime remain unanswered (Hess et al., 2015; Blauenfeldt et 2023b).
The possible combinations are endless, and testing each factor in a clinical trial is not feasible. One solution would be to establish biomarkers to assess and predict responders of the RIC treatment. In the primary results of this trial, we could not demonstrate an effect of RIC on red blood cell rheology and endocrine function, but several studies are ongoing (Blauenfeldt et al. 2020). Compliance with treatment may be another key to translating RIC to the clinic. In the study, advanced age and cognitive impairment were significantly higher in the group with reduced short-term treatment compliance. Improving RIC compliance during home treatment is important, and future trials may consider implementing additional aids such as electronic reminders or phone calls (Hou et al., 2022; Blauenfeldt et al., 2023a).
The treatment paradigm in RICAMIS was comparable to the present trial. However, treatment was continued for 10-14 days, and bilateral upper extremity RIC was used. The neutral results in the present trial may be caused by an insufficient RIC dose and methodological limitations, including a small sample size and low stroke severity, limiting the ability to detect an improvement between groups. The current results should only be used in hypothesis generation, as they represent results from secondary endpoints and no correction for multiple comparisons has been made. Further, the current study has several limitations. It was a single-center study, and the sample size was very small (n = 30) and not powered to detect differences in clinical outcomes. The proportion of patients who received endovascular thrombectomy treatment was not balanced, possibly due to the limited sample size. Another major limitation was a high dropout rate of 4 out of 30 patients. Additionally, the study design required patients to continue the RIC treatment independently at home, and therefore, only minor strokes (median NIHSS of 1) were included, which may reduce the chances of detecting a clinical benefit.
Conclusion
In patients with minor AIS, we were unable to demonstrate an effect of treatment with RIC on improvement in short-term neurological outcomes compared to sham-treated patients. RIC was well tolerated but associated with mild to moderate pain compared to sham. Treatment compliance was high, and the majority of patients were willing to continue the treatment once daily for one year.
Conflict of interest
Dr. Blauenfeldt reported receiving grants 1R01NS112511-01A1 from the National Institutes of Health and lecture fees from Bayer, Pfizer and Novo Nordisk outside the submitted work.
Dr. Hess reported receiving grants from the National Institutes of Health biomarker substudy of the parent clinical trial during the conduct of the study; personal fees from Athersys Inc’s scientific advisory board outside the submitted work; holding a patent for multipotent stem cells in neurological disease issued to the Medical College of Georgia–Augusta University by Athersys Inc.
D. Hess serves as an associate editor of Conditioning Medicine, but did not participate at any level in the editorial review of this manuscript.
Dr. Simonsen reported receiving grants from Novo Nordisk Foundation and Health Research Foundation of the Central Denmark Region outside the submitted work. All other authors have no competing interests.
Acknowledgments
The study was funded by grants from the Lundbeck Foundation and the Novo Nordisk Foundation. There was no industry funding or involvement in any aspect of the trial.
References
Maria Kjølhede1
1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark.
Grethe Andersen1,2
1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark. 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Claus Ziegler Simonsen1,2
1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark. 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Kim Ryun Drasbek3
3Center for Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark.
Rebecca Best Jensen3
3Center for Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark.
Jan Brink Valentin4
4Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
David C. Hess5
5Department of Neurology, Medical College of Georgia, Augusta University, Augusta, Georgia, USA.
Rolf Ankerlund Blauenfeldt1,2
1Department of Neurology, Aarhus University Hospital, Aarhus, Denmark. 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Corresponding author:
Rolf Ankerlund Blauenfeld
Email: rolfblau@rm.dk
Supporting Information
Download Supporting Information (PDF)Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4223 | 23 | 4 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA