Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Time-restricted eating: A tool for hormesis in cardiovascular risk reduction
Time:2025-07-22
Number:12034
Elizabeth Epstein1, Irvin Xu2, Neeja Patel3, Pam R. Taub2
Author Affiliations


- 1Division of Cardiovascular Medicine, Department of Medicine, Scripps Green Hospital, 10666 N. Torrey Pines Road, La Jolla, CA, 92037.
- 2Division of Cardiovascular Medicine, Department of Medicine, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093.
- 3Department of Internal Medicine, University of California, Los Angeles, 757 Westwood Plaza Suite 7501, Los Angeles, CA 90095.
Conditioning Medicine 2024. 7(2): 48-61.
Abstract
The field of preventative cardiology is in the midst of a paradigm shift from treating established atherosclerotic cardiovascular disease (ASCVD) to identifying and treating early subclinical atherosclerosis to prevent downstream ASCVD events. Here, we discuss the foundational principle of hormesis, where exposure to sublethal stressors trains the body to develop resilience against subsequent stressors. Just as exposure to brief periods of ischemia may render the myocardium more resistant to subsequent ischemic insults, time-restricted eating (TRE) promotes metabolic switching and activation of adaptive cellular responses during finite periods of fasting. We discuss TRE as a lifestyle intervention that harnesses the benefits of hormesis for ASCVD risk reduction. Our review of animal models and human studies characterizes the current understanding of the promising cardiometabolic benefits of TRE and the mechanisms responsible for them. While human clinical trial data has yielded seemingly mixed results, we discuss the promising results from some of the largest and longest-duration trials and the challenges of studying TRE in human beings. In patients with multiple ASCVD risk factors and high downstream risk, TRE has the potential to be a low-risk, inexpensive, non-pharmacologic lifestyle intervention to prevent metabolic syndrome and ASCVD.
Keywords: Time-restricted eating, Ischemic preconditioning, Hormesis, ASCVD, Risk reduction
Abstract
The field of preventative cardiology is in the midst of a paradigm shift from treating established atherosclerotic cardiovascular disease (ASCVD) to identifying and treating early subclinical atherosclerosis to prevent downstream ASCVD events. Here, we discuss the foundational principle of hormesis, where exposure to sublethal stressors trains the body to develop resilience against subsequent stressors. Just as exposure to brief periods of ischemia may render the myocardium more resistant to subsequent ischemic insults, time-restricted eating (TRE) promotes metabolic switching and activation of adaptive cellular responses during finite periods of fasting. We discuss TRE as a lifestyle intervention that harnesses the benefits of hormesis for ASCVD risk reduction. Our review of animal models and human studies characterizes the current understanding of the promising cardiometabolic benefits of TRE and the mechanisms responsible for them. While human clinical trial data has yielded seemingly mixed results, we discuss the promising results from some of the largest and longest-duration trials and the challenges of studying TRE in human beings. In patients with multiple ASCVD risk factors and high downstream risk, TRE has the potential to be a low-risk, inexpensive, non-pharmacologic lifestyle intervention to prevent metabolic syndrome and ASCVD.
Keywords: Time-restricted eating, Ischemic preconditioning, Hormesis, ASCVD, Risk reduction
Highlights
Myocardial ischemic pre-conditioning and time-restricted eating (TRE) are both examples of hormesis, where exposure to sublethal stressors—transient ischemia or fasting—trains the body to develop greater resilience against subsequent stressors. TRE is a type of intermittent fasting that involves eating in a restricted time window every day. Metabolic switching, the process of switching from utilizing glucose stores to fatty acids for energy, and subsequent activation of adaptive cellular responses results in increased cardiometabolic resilience. Clinical study data has shown that TRE confers cardioprotective benefits, including improved body composition, insulin sensitivity, blood pressure, and blood lipids. Given its simplicity as a dietary intervention, TRE is sustainable and can be employed as a non-pharmacologic lifestyle intervention for ASCVD risk reduction.
Introduction
The field of preventive cardiology is in the midst of a paradigm shift from the treatment of established ASCVD to the identification of subclinical atherosclerosis and early risk reduction. With the goal of improving quality and quantity of life, risk assessment is growing more comprehensive, expanding focus to include early management of risk factors such as cholesterol, glucose, and body weight. Data continues to suggest that earlier intervention is better. Recent data from Mendelian randomization studies has shown that a 1 nmol/L reduction in low-density lipoprotein cholesterol (LDL-C) results in a 50% lower ASCVD risk in 52-year follow-up compared to a 23% lower 5-year risk (Ference et al., 2017). The feasibility of upstream risk reduction hinges upon identifying and implementing evidence-based, cost-effective, and low-risk treatment options. Lifestyle interventions are particularly well-suited to the task.
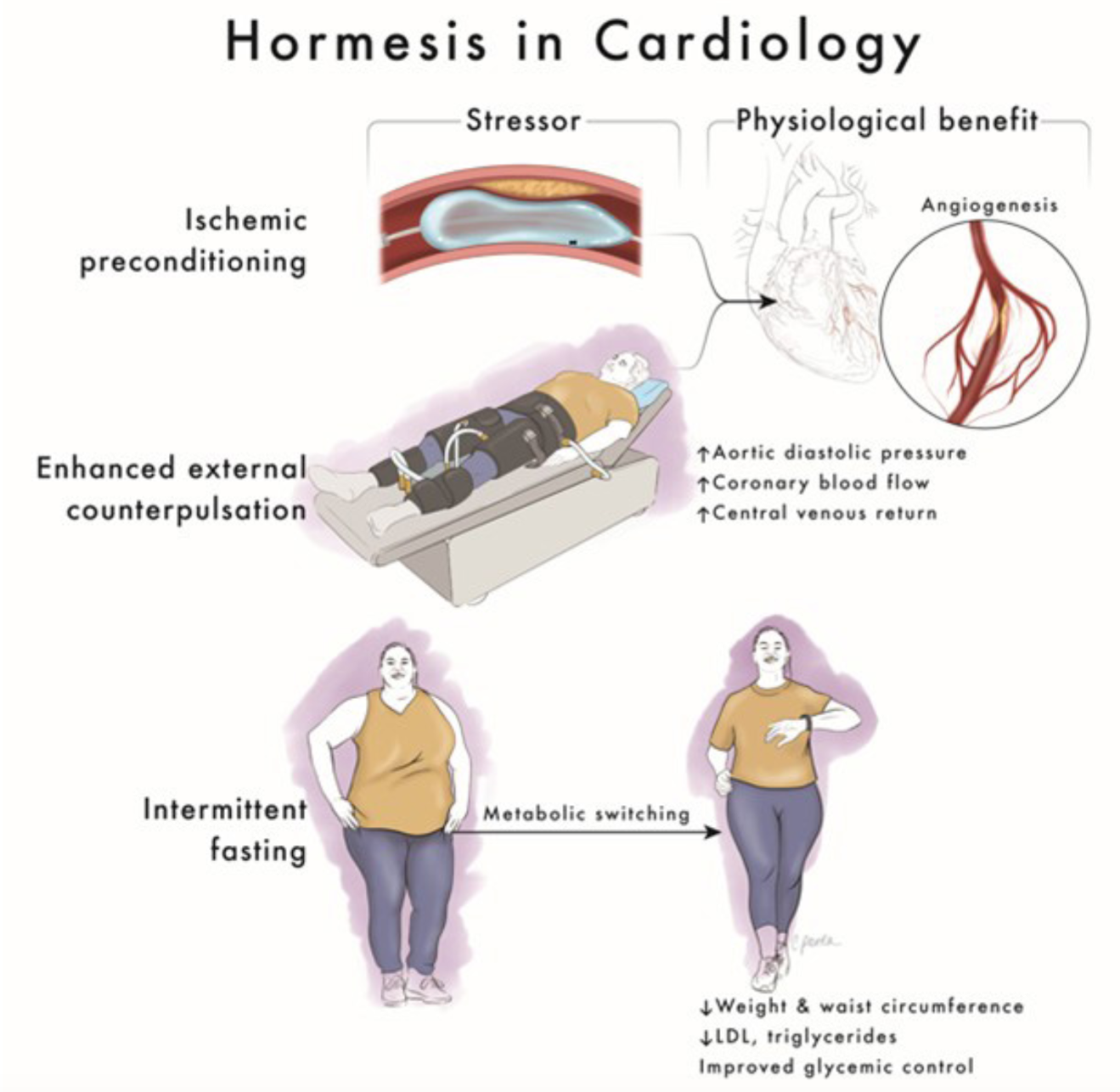
Determining the most potent lifestyle interventions that confer cardiovascular benefit is the key to defining the toolbox for lifetime risk reduction. One emerging mechanism responsible for the cardiovascular benefits of some lifestyle practices is hormesis: exposing the body to small doses of intermittent stress to activate biochemical and molecular pathways that evolved to sustain living things through hardship. Hormesis is the scientific term for the old adage, "What doesn't kill you makes you stronger." Research in animals is elucidating the molecular mechanisms that explain this phenomenon on a cellular level and the lifestyle practices that engage them. One such lifestyle practice is time-restricted eating (TRE). To better conceptualize the mechanism of TRE and its role in cardiology, it is helpful to review other well-established treatments whose mechanism is also rooted in hormesis (Figure 1).
Treatments that employ hormesis are not new to the field of cardiology, which has long recognized the benefits of intermittent stress. In the 1980s, Murry et al. (1986) demonstrated that short periods of ischemia render the animal myocardium more resistant to subsequent ischemic insult. In a study of 44 healthy adult dogs, 5-minute periods of ischemia prior to a sustained 40-minute occlusion of the left anterior descending (LAD) coronary artery decreased infarct size by 75% compared to the control group (Murry et al., 1986). In a study involving the hearts of anesthetized rats, short periods of ischemia significantly reduced ventricular arrhythmias during longer periods of subsequent ischemia (Shiki and Hearse, 1987). The mechanism of these benefits was hypothesized to involve increased recruitment of collateral vessels and preservation of intracellular ATP in cardiac myocytes (Speechly-Dick et al., 1995).
In humans, one retrospective study of 23 patients demonstrated that patients with unstable angina prior to acute myocardial infarction (MI) had faster reperfusion with thrombolytics and smaller infarcts (Andreotti et al., 1996). The effects and mechanisms of ischemic pre-conditioning were also studied prospectively with repeated balloon angioplasties, where subsequent balloon inflations resulted in decreased chest pain, decreased ST changes, and increased flow in collateral vessels (Kloner and Yellon, 1994). This effect was not seen when KATP channels were blocked, reinforcing intracellular ATP's role in ischemic pre-conditioning (Tomai et al., 1994). Next, studies investigated the effect of ischemic pre-conditioning on cardiovascular outcomes. In a prospective observational study of 350 patients presenting with acute MI, patients with prodromal angina in the 24 hours prior to infarction had lower in-hospital (6% vs. 14%, p = 0.02) and 5-year mortality (p = 0.009) (Ishihara et al., 1997). However, randomized controlled trials (RCTs) failed to demonstrate any benefits for patients who underwent ischemic pre-conditioning (four 5-minute inflations and deflations of a standard blood-pressure cuff on the upper arm) prior to cardiac surgery (Hausenloy et al., 2015; Meybohm et al., 2015).
Another classic clinical example of hormesis in cardiology is enhanced external counterpulsation (EECP), an FDA-approved, non-invasive treatment for angina that results in diastolic augmentation similar to the effect of an intra-aortic balloon pump. Three pairs of pneumatic cuffs are applied to the patient's calves, lower thighs, and upper thighs. The cuffs are inflated sequentially from distal to proximal during diastole and deflated during systole. Hemodynamically, this results in decreased afterload and increased systemic venous return, stroke volume, and cardiac output (Sinvhal et al., 2003). However, the clinical benefits of EECP are sustained far beyond the typical treatment course of seven weeks, raising the suspicion that the transient stress imposed during treatment may result in positive long-term adaptations. In the Multicenter Enhanced External Counterpulsation (MUST-EECP) trial, a multicenter RCT of 139 patients, EECP significantly increased time to exercise-induced ischemia (adjusted mean change in time to exercise-induced ischemia 37 ± 11 seconds in the EECP group vs. −4 ± 12 seconds in the control group, p = 0.01) and, in those who completed at least 34 sessions, EECP decreased angina (adjusted mean change in chest pain counts in the EECP group −0.033 ± 0.27 vs. 0.15 ± 0.27 in the control group, p < 0.035) (Arora et al., 1999). These changes were maintained for 12 months after completion of EECP treatment. While no RCT has evaluated the effect of EECP on major adverse cardiovascular events (MACE), a prospective, sequential registry of 3,000 EECP-treated heart failure patients demonstrated a significant decrease in heart failure exacerbations (14.9% vs. 27.9%, p = 0.001), Kaplan Meier mortality rate (9.5% vs. 17.6%, p = 0.015), MACE (15.8% vs. 25.4%, p = 0.018), and an improvement in Duke Activity Status Index score (13.8 ± 11 vs. 10.6 ± 10, p = 0.03) and Canadian Cardiovascular Society angina class (63% vs. 23%, p < 0.001) in the group who completed ≥30 hours of EECP compared with the group who did not (Lawson et al., 2008).
The mechanism behind EECP's persistent benefits is another example of hormesis. Diastolic augmentation increases coronary perfusion and shear stress, resulting in several positive downstream effects. One recent study in 12 healthy patients showed that shear stress (as measured by time-averaged shear stress over the entire femoral bifurcation) increased by 32.41%, 121.30%, 178.24%, and 214.81% during EECP with treatment pressures of 10 kPa, 20 kPa, 30 kPa, and 40 kPa, respectively (Du et al., 2023). The positive impacts of sheer stress occur in the endothelium, where there is an increase in nitric oxide, vasodilation, and improved endothelial function (Sharma et al., 2013). One prospective study in 11 patients showed an increase in nitric oxide levels (p < 0.02) after EECP as well as an increase in myocardial perfusion on exercise [13 N]-ammonia positron emission tomography (0.69 ± 0.27 to 0.85 ± 0.47 ml/min/g, p < 0.05). Another prospective study in 20 patients with refractory angina showed a significant improvement in endothelial function as assessed by flow-mediated dilation of the brachial artery (8.2 ± 2.1%, p = 0.01) compared with controls (3.1 ± 2.2%, p = 0.78) (Shechter et al., 2003). Lastly, a prospective study in 23 patients evaluated reactive hyperemia-peripheral arterial tonometry (RH-PAT), a non-invasive method to assess peripheral endothelial function by measuring reactive hyperemic response in the finger, after EECP. EECP was associated with a significant increase in average RH-PAT after each treatment (p < 0.05). In addition, the average RH-PAT at one-month follow-up was significantly higher than before EECP therapy (p < 0.05) (Bonetti et al., 2003).
Additional endothelial effects of sheer stress include decreased oxidative stress and increased angiogenesis. A randomized sham-controlled clinical trial in 42 patients with coronary artery disease (CAD) showed EECP-mediated reduction in pro-inflammatory cytokines (tumor necrosis-α (−16% vs. +12.1%), monocyte chemoattractant protein-1 (−13% vs. +0.2%), vascular cell adhesion molecule-1 (−6% vs. +1%), high sensitivity C-reactive protein (−32% vs. +5%), and lipid peroxidation marker 8-isoprostane platelet growth factor 2α (−21% vs. +1.3%) with treatment vs. sham (Braith et al., 2010). In a recent randomized sham-controlled trial in 50 patients with CAD, EECP resulted in the preservation of vascular endothelial growth factor (VEGF)-A and VEGFR-2, the types of VEGF that play the most crucial role in controlling angiogenesis, compared to sham (Ambari et al., 2021).
Just as ischemic pre-conditioning and EECP are examples of therapies that leverage hormesis to achieve lasting cardiovascular benefits, there is growing interest in lifestyle interventions that leverage hormesis to decrease cardiometabolic risk, decrease downstream cardiovascular risk, and promote longevity. One lifestyle intervention with a growing body of research suggesting cardiometabolic benefits is TRE.
Time-restricted eating in context
Poor dietary choices and excess calories from food have become widespread. They are linked to cardiovascular risk factors, including obesity, hypertension (HTN), diabetes, dyslipidemia, and metabolic dysfunction-associated steatotic liver disease (MASLD). Nutrition is an essential point of intervention to reduce downstream cardiovascular risk. However, diet and eating patterns are notoriously difficult to study, and dietary modification is equally difficult to implement. The best evidence for dietary content in cardiology comes from two large RCTs evaluating the Mediterranean diet in both primary and secondary prevention populations: the Prevention with Mediterranean Diet (PREDIMED) and the Coronary Diet Intervention with Olive Oil and Cardiovascular Prevention (CORDIOPREV) trials. The PREDIMED trial assigned 7,447 patients at high cardiovascular risk to one of three diets: a Mediterranean diet supplemented with extra-virgin olive oil, a Mediterranean diet supplemented with mixed nuts, or a control diet (advice to reduce dietary fat), and evaluated for MACE (Estruch et al., 2018). Results showed a hazard ratio (HR) of 0.69 (95% CI 0.53-0.91) for a Mediterranean diet with extra-virgin olive oil and 0.72 (95% CI 0.54-0.95) for a Mediterranean diet with nuts, as compared with the control diet. The CORDIOPREV trial randomly assigned 7,447 patients with established CAD to a Mediterranean diet or a low-fat diet and evaluated for the primary outcome of MACE with a follow-up of seven years (Delgado-Lista et al., 2022). The results showed multivariable-adjusted HRs for the different models ranged from 0.719 (95% CI 0.541–0.957) to 0.753 (95% CI 0.568–0.998) in favor of the Mediterranean diet.
Beyond dietary content, meal timing plays an important role in nurturing a healthy circadian clock, which is an integral part of human physiology essential for health. The daily patterns of feeding and fasting interplay with sleep, activity, exposure to light and darkness, and the cellular molecular clock within most cells of the body to influence the function of almost all organ systems (Manoogian and Panda, 2017). Epidemiologic studies in humans have shown that erratic eating patterns increase the risk of cancer, cardiovascular disease (CVD), obesity, immune disorders, infertility, and affective disorders (Hastings et al., 2003). Specifically, longitudinal studies in humans doing shift work demonstrate an association with weight gain and impaired glucose tolerance, both of which are linked to increased cardiovascular risk (Proper et al., 2016). To optimize health and metabolism, it is best to routinely eat during daytime hours of activity, with the bulk of calories consumed earlier in the day (Manoogian et al., 2019). While this was the natural eating pattern for human beings before the invention of the lightbulb, most modern humans now spread their daily food consumption throughout the day with a bias toward eating late in the day (Gill and Panda, 2015). In one study that used a smartphone-based monitoring method to collect the natural daily eating pattern of healthy adults, the daily food intake duration exceeded 14.75 hours for half of the cohort, with an estimated <25% of calories being consumed before noon and >35% after 6 pm (Gill and Panda, 2015). In the same study, when overweight individuals with >14-hour eating duration ate for only 10–11 hours daily for 16 weeks, they lost weight (average loss 3.27 kg, 95% CI, 0.9081–5.624 kg) and experienced improved energy levels and sleep. These effects were maintained at one-year follow-up (Gill and Panda, 2015). This practice of reducing the eating duration is a type of intermittent fasting (IF) called TRE.
IF is an umbrella term that encompasses various patterns of fasting and eating. The three most widely studied types of IF are alternate-day fasting (ADF), the 5:2 diet, and TRE. In ADF, people alternate between eating ad-lib (no restrictions on quantity or quality of food) for one day and drinking water for the other day (Varady et al., 2021). Individuals can opt to modify the fast day to ~25% of caloric needs (modified ADF). The 5:2 diet adopts a similar principle, where individuals eat ad-lib five days a week and limit themselves to ~25% of caloric needs for any two days out of the week (Varady et al., 2021). TRE differs from the other two fasting patterns in that individuals may eat ad-lib within a 6-10 hour time window and fast (zero calories) for the remainder of the day (Varady et al., 2021). Of the types of IF, TRE is of particular interest as a lifestyle intervention for patients with metabolic syndrome not just because of its simplicity and ease of implementation but also because of its potential to harness the health benefits of both hormesis—the fasting stressor in itself—and circadian rhythms.
Mechanisms of hormesis in time-restricted eating: The metabolic switch and circadian rhythms
In ischemic preconditioning and EECP, exposure to a stressor (balloon dilation or shear stress) activates a cascade of positive adaptations, resulting in lasting benefits beyond the treatment course. Similarly, dietary energy restriction as a stressor has long been recognized as the most potent intervention to extend lifespan and prevent disease in mammals (de Cabo and Mattson, 2019). In mice studies, there is an inverse linear relationship between caloric intake and lifespan (Weindruch et al., 1986). When ad-lib food intake of mice and rats is decreased by 30-60%, the average lifespan and maximal lifespan are increased by similar amounts (Weindruch et al., 1986). In addition, calorie-restricted animals develop fewer age-associated diseases. These observations dating back to the 1930s have led to decades of research into the mechanism behind the profound longevity benefits of caloric restriction (CR). Because the animals in these studies typically consumed their food within a few hours, incidentally, they were fasting for up to 20 hours per day. Subsequently, it has been demonstrated in mice that fasting drives the metabolic, molecular, and longevity effects of a CR diet (Pak et al., 2021). Therefore, the mechanisms of the benefits seen with CR most likely also apply to fasting, and the mechanisms of both will be discussed here.
In TRE, fasting between 12-36 hours results in a metabolic switch from glycogen-derived glucose metabolism to fatty acid-derived ketone metabolism (Figure 2). Ketone bodies can not only be utilized as an energy source but are also involved in signaling for multiple pathways that affect aging, most notably as histone deacetylation inhibitors, which results in increased gene transcription (Anon, 2020). Directly impacted proteins and molecules include the ubiquitous peroxisome proliferator-activated receptor, fibroblast growth factor 21, nicotinamide adenine dinucleotide, polyADP-ribose polymerase 1, and ADP ribosyl cyclase (Anon, 2020). The effect of ketone bodies on these major cellular pathways directly influences systemic metabolism. Because of the wide-reaching signaling capacity of ketones in the body, the metabolic switch has significance beyond just the provision of an alternative food source: it results in the activation of several downstream adaptations to increase antioxidant defenses, DNA repair, protein quality control, mitochondrial biogenesis, and decrease inflammation—all of which result in a robust resistance to subsequent damaging insults (Anon, 2020). In an animal model, ketone bodies have even been shown to be protective against ischemic injury. After acute proximal LAD coronary artery occlusion, animals pretreated with either empagliflozin or intravenous infusion of the ketone body beta-hydroxybutyrate had a significantly higher myocardial salvage, smaller MI size (both by cardiac magnetic resonance and histology), less microvascular obstruction, and improved cardiac function (left ventricle ejection fraction and strain)(Santos-Gallego et al., 2023). While evolutionarily this protective effect was meant to increase the odds of survival during periods of food scarcity, in modern times the positive cellular adaptations resultant from this powerful stressor can be harnessed to prevent chronic disease and potentially even promote longevity.

In a new window | Download PPT
Figure 2. the process of metabolic switching in time-restricted eating.
For many years, the primary mechanism of CR and fasting was thought to be a reduction in oxidative damage to cells. Indeed, the antioxidant effect of these interventions remains one of their main positive effects. Reactive oxygen species, which are generated during glucose metabolism, can cause oxidative damage to proteins, lipids, carbohydrates, and nucleic acids and impair their function—a process theorized to be a major determinant of lifespan (Bokov et al., 2004). In the yeast Saccharomyces cerevisiae and the fruit fly Drosophila melanogaster, CR extends lifespan by increasing the activity of sirtuin 2 (sir2), which encodes the silencing protein Sir2p, an NAD+-dependent histone deacetylase with a key role in regulating antioxidant and redox signaling (Guarente and Picard, 2005). When sir2 is deleted, CR no longer prolongs lifespan in yeast (Lin et al., 2000). In a subsequent study in mice, CR reduced oxidative stress, specifically through SIR3, the mammalian SIR2 homolog (Qiu et al., 2010). Daily CR for six months resulted in a decrease in the lipid peroxidation product 4-hydroxy-2-nonenal (4-HNE) and protein carbonyls (a marker of protein oxidation) while lowering the glutathione:glutathione disulfide ratio (a marker of oxidative stress), via SIR3-mediated superoxide dismutase-2 activation. An increase in oxidative damage is associated with aging and disease. One study, which localized oxidative damage in aging rhesus macaques in skeletal muscle, CR attenuated age-dependent increases in 4-HNE-modified proteins (Zainal et al., 2000). The effect of TRE on oxidative stress has specifically been assessed in humans. In a supervised controlled feeding trial, men with prediabetes were randomized to early TRE (eTRE, a 60-hour eating window with dinner before 3 pm) or a control schedule (12-hour eating window) (Sutton et al., 2018). eTRE decreased plasma levels of 8-isoprostane, a marker of oxidative stress to lipids, by 11 ± 5 pg/ml (p = 0.05) or about 14%.
Energy restriction regimens produce robust responses to a multitude of insults with fortified healing and repair (Anon, 2020). In mice, CR has been shown to strongly enhance DNA repair by non-homologous end joining, which is associated with elevated levels of DNA-dependent protein kinase and SIRT6 (Ke et al., 2020). In a study of 14 patients with metabolic syndrome who fasted from dawn to sunset, there was a significant increase in the levels of several tumor suppressor and DNA repair gene products at the end of the fourth week (Mindikoglu et al., 2020b). In another study of fasting from dawn until sunset for 30 days, the effect on the proteasome was evaluated (Mindikoglu et al., 2020a). IF was associated with an average 45-fold increase in centrosomal 164, a key DNA repair protein, at the end of the fourth week compared with baseline. In addition, IF stimulates autophagy and downregulates the mammalian target of rapamycin (mTOR) pathway to promote cell turnover and removal of damaged proteins. Throughout this process, unused molecules are also recycled for efficient utilization of resources and energy conservation (Anon, 2020).
Another mechanism through which IF exerts its effects is by reducing inflammation. CR has been shown to reduce inflammatory cytokines in overweight and obese adults (Imayama et al., 2012; Loria-Kohen et al., 2013; Ho et al., 2015). Intermittent and religious fasting have also been shown to reduce inflammatory cytokines, including tumor necrosis factor-alpha (TNFα), interleukin-6, and interleukin-1β (Aksungar et al., 2007; Faris et al., 2012; Moro et al., 2016). Another study showed that fasting reduces the numbers of circulating monocytes in healthy humans and mice and reduces monocyte metabolic and inflammatory activity (Jordan et al., 2019). In the same study, fasting improved chronic inflammatory diseases without compromising monocyte emergency mobilization during acute infectious inflammation and tissue repair.
While the cellular effects of CR and fasting are numerous, the key underlying mechanism is likely a profound impact on gene expression. One impactful study in mice examined gene expression changes in samples from 22 organs and brain regions collected every two hours over 24 hours (Deota et al., 2023). Nearly 80% of all genes showed differential gene expression during time-restricted feeding (TRF), the analog of TRE in animal studies. Sustained and consistent fasting duration consolidated gene expression into fasting and feeding phase peaks across all peripheral tissues, effectively harmonizing gene expression within the fasting vs. feeding phase and compartmentalizing catabolic and anabolic processes. The study also specifically demonstrated decreases in genes involved in inflammatory signaling and glycerolipid metabolism, increases in genes involved in RNA processing, protein folding, and autophagy, and multi-tissue rewiring of branched-chain amino acid, glucose, and lipid metabolism. The nearly ubiquitous impact of TRF on gene expression throughout the body appears to be the underlying mechanism for hormesis.
Lastly, TRE specifically harnesses the benefits of not just fasting but also synchronizing the light-driven suprachiasmatic nucleus’ circadian rhythm to the feeding-driven circadian rhythms in peripheral organs, including the liver, heart, kidney, and pancreas (Pickel and Sung, 2020). These peripheral rhythms control important cardiometabolic factors, including blood pressure (BP) rhythms, glucose levels, and lipid metabolism. By aligning feeding and fasting patterns with light, the dominant environmental cue for the master circadian clock of the suprachiasmatic nucleus, TRE, and specifically early TRE, can harness additional benefits. Several studies have suggested that skipping breakfast and eating dinner later in the day can have a negative effect (Yu et al., 2023). Recent data from the prospective NutriNet-Santé cohort of 103,389 adults in France demonstrated that having a later first meal (later than 9 am compared to earlier than 8 am) and last meal of the day (later than 9 pm compared to earlier than 8 pm) was associated with a higher risk of cardiovascular outcomes, especially among women (Palomar-Cros et al., 2023). Each additional hour delaying the time of the first meal of the day was associated with a higher risk of overall CVD (HR 1.06, 95% CI 1.01–1.12, p = 0.02). The HR linking time of the last meal with overall CVD risk was 1.13 (95% CI 0.99–1.29, p = 0.06) for having a last meal after 9 pm compared to before 8 pm. This is in keeping with robust data on the deleterious effects of circadian disruption. In circadian mutant mouse models, there is severe glucose and metabolic dysregulation (Rudic et al., 2004; Zarrinpar et al., 2016). In contrast, aligning food intake to the habitual wakeful or active period and maintaining a consistent eating window can reduce cardiometabolic disease in mice (Hatori et al., 2012). In one study, TRF of a high-fat diet in the active phase was compared to ad lib high-fat diet of equal calories (Chaix et al., 2014). The study demonstrated that TRF mice are protected against obesity, hyperinsulinemia, hepatic steatosis, and inflammation and have improved motor coordination. Mechanistically, the TRF regimen improved clock and nutrient sensor functions by improving cyclic-AMP response element-binding protein, mTOR, and 5’AMP-activated protein kinase pathway function and oscillations of the circadian clock and their target genes' expression. This study demonstrates not just the cardiometabolic benefit of TRF, but the resilience of that benefit despite poor nutrient content, and its interconnectedness with circadian rhythms.
In humans, insights into the negative impact of circadian disruption come primarily from data on shift workers, who engage in erratic light-dark, sleep-activity, and eating-fasting patterns (Boivin and Boudreau, 2014; Kosmadopoulos et al., 2020). Epidemiologic data on shift workers suggests they are at a significantly increased risk of CVD and cancer (Straif et al., 2007; Thomas and Power, 2010). In one retrospective study involving 706 patients in the Early Assessment of Myocardial Tissue Characteristics by Cardiac Magnetic Resonance (EARLY-MYO-CMR) registry, shift work was associated with increased infarct size on cardiac magnetic resonance imaging (β = 5.94%, 95% CI 2.94-8.94, p < 0.0001) and increased risk of MACE (HR 1.92, 95% CI 1.12-3.29, p = 0.017) (Zhao et al., 2022). The Healthy Heroes RCT evaluated the impact of TRE with a 10-hour eating window vs. standard of care eating window of ~14 hours in 137 firefighters working 24-hour shifts (Manoogian et al., 2022). Both groups were encouraged to eat a Mediterranean diet. Participants were able to decrease their eating window from 14 hours to 11.13 hours during the day, which resulted in a decrease in A1c and BP in patients with cardiometabolic risk. Through the mechanisms discussed in this section, these participants were able to harness the benefits of fasting and synchronizing circadian rhythms to achieve cardiometabolic benefits. Given the average American consumes food over a 14-hour period (Gill and Panda, 2015), many people could derive similar benefits from TRE.
Time-restricted eating for cardiometabolic benefit: Preclinical data
TRF has been shown in animal models to have beneficial effects on key cardiometabolic risk factors: body weight, lipids, glucose, and inflammation (Rothschild et al., 2014). Animal studies using an 8-9-hour feeding window demonstrate 12-28% decreases in body weight (Belkacemi et al., 2010; Hatori et al., 2012). One study compared 8-hour TRF with either a high-fat or regular chow diet to high-fat or regular chow ad lib feeding (Hatori et al., 2012). After 16 weeks, mice on 8-hour TRF with a high-fat diet consumed equivalent calories to mice on an ad lib high-fat diet but weighed 28% less. Weight loss has also been studied specifically in diabetic animals. In one study in which diabetic rats were transitioned from a purely vegetarian diet to a high-fat diet, the rats on TRF lost weight while the rats on ad lib feeding gained (Belkacemi et al., 2010). There is conflicting data on weight loss on a 12-hour TRF diet. Some studies demonstrated no change in weight or weight gain if rats or mice, which are nocturnal, are fed during the day in discordance with their natural circadian rhythm. When TRF of a high-fat diet in the day vs. night was compared directly, results demonstrated that rats fed in the night weighed 19% less than those fed in the day (Arble et al., 2009).
Animal studies have also demonstrated that TRF improves insulin sensitivity and lowers glucose in both diabetic and nondiabetic animals. One study compared a normal-chow 8-hour TRF diet to ad lib and demonstrated that four weeks of TRF prevented progressive deterioration of glucose tolerance seen in non-fasting animals who were switched from a purely vegetarian diet to a hypercaloric diet (Belkacemi et al., 2010). Another study also demonstrated that TRF improved glucose tolerance, increased plasma insulin, decreased insulin resistance as measured by homeostatic model assessment of insulin resistance (HOMA-IR), and increased β-cell mass, as well as individual β-cell and islet area in diabetic rats (Belkacemi et al., 2012). Twelve-hour TRF has also been shown to improve glucose handling. One study demonstrated 25-30% lower fasting glucose concentrations in rats fed during 12 hours either during the day or night (Farooq et al., 2006). Another study demonstrated that TRF of either a high-fat or low-fat diet at night, in alignment with rats’ circadian rhythm, resulted in decreased glucose and insulin levels (Tsai et al., 2013).
In terms of the effect on lipids, animal studies have shown significant reductions in total cholesterol (TC) and LDL in animals, even in the absence of weight loss. In one study, LDL was decreased by 29 and 36% for mice fasting during the day vs. the night, respectively (Farooq et al., 2006). While weight loss itself has been shown to lower LDL in humans and animals, in this study, the LDL reduction occurred in the absence of weight loss. In another study of mice fed a high-fat diet during an 8-hour window, there was a significant decrease in serum cholesterol levels (Hatori et al., 2012). The same study also demonstrated increased peak levels of Cyp7a1 mRNA, a rate-limiting enzyme in bile acid synthesis from cholesterol in the liver, suggesting this could be one mechanism for the effect of TRF on cholesterol. This study also evaluated the effect of TRF on inflammation and found that expression of proinflammatory genes, including TNFα, IL6, and C-X-C motif chemokine ligand 2 was significantly reduced in 8-hour TF mice on a high-fat diet compared to the ad lib group.
Hypothesizing that TRE could have the potential to prevent and treat obesity in humans, another mouse model study examined the effect of TRF when paired with obesogenic diets on weight and cardiometabolic risk factors (Chaix et al., 2014). Lean and obese mice were placed on either an ad lib diet or an 8-hour TRF diet whose content presented different nutritional challenges, including high-fat, high-fructose, and high-fat-plus-high-fructose diets, all of which have been shown to cause dysmetabolism. Calories were kept constant between the ad lib and TRF groups. The TRF groups on obesogenic diets gained significantly less weight than the ad lib groups. When the feeding window was increased to 9, 12, and 15 hours, weight gain increased: a 26% weight gain was seen for 9-hour TRF, whereas a 43% gain was seen for 15-hour TRF, in comparison to a 65% weight gain in the ad-lib group. To simulate TRF as a therapeutic intervention for mice eating an obesogenic diet ad lib, these mice were switched to a TRF regimen after 13 or 26 weeks on an ad lib diet. Transitioning to a TRF diet after 13 and 26 weeks ad lib resulted in a 5% and 12% weight loss, respectively, after crossover compared to a 24.8% and 10.6% weight gain in mice maintained on the ad lib diet. Differences in total body weight were explained by a decrease in fat mass: mice on TRF with either a fat-heavy diet or fructose-heavy diet exhibited reduced levels of fat mass (62% and 26% less, respectively). Along with the reduction in fat mass, the study demonstrated a reduction in mRNA levels of proinflammatory cytokines TNFα, IL1β, and the proinflammatory chemokine C-C motif chemokine ligand 8/monocyte chemotactic protein 2 in adipose tissue of mice maintained on TRF.
In addition, liver sections were stained with hemotoxylin and eosin to evaluate fat droplets and total triglyceride (TG) accumulation as observed in MASLD. Lipid droplets were reduced or absent, and TG was at least 70% reduced in mice on TRF. In serum, mice on TRF also had reduced TG and fasting insulin levels.
The researchers in this study went a step further to evaluate whether these changes in body composition and cardiometabolic factors translated into better exercise performance (Chaix et al., 2014). On a treadmill run-to-exhaustion protocol, mice in the 12-hour TRF high-fat diet group and 9-hour TRF high-fat diet group ran significantly longer than mice on the ad lib high-fat diet or the ad lib standard chow (73.3 ± 3.3 min and 141.3 ± 4.6 min vs. 50.2 ± 4.7 min and 77 ± 8 min, respectively). Because there was no difference in muscle fiber size nor glycogen content in sections of muscles between groups, the researchers postulated that better metabolic responses to mobilize energy stores in TRF mice might explain this difference. The results of this comprehensive study altogether suggest that TRF results in a significant improvement in cardiac risk factors, including obesity, hyperlipidemia, inflammation, and MASLD. Even when the nutrition content of the diet was high in fat and sugar, TRF resulted in significant health benefits and improved physical performance. The study clearly demonstrated the power of meal timing independent of meal content and, at the same time, the power of hormesis as a dietary intervention.
Time-restricted eating for cardiometabolic benefit: Clinical data
The human clinical trial data on TRE is growing significantly year by year, and most data suggest similar benefits to those observed in animal models (Table 1). While larger trials with longer follow-ups are still needed, several conclusions can be drawn from existing data. Firstly, TRE is safe among various patient populations and does not seem to negatively impact muscle mass or bone turnover (Moro et al., 2020, 2021; Lobene et al., 2021). TRE has been studied explicitly in elite athletes, patients with overweight/obesity, MASLD, prediabetes, diabetes, men and women, young and old—and all studies agree the lifestyle intervention is feasible and safe (Sutton et al., 2018; Lowe et al., 2020; Haganes et al., 2022; Liu et al., 2022; Correia et al., 2023; Domaszewski et al., 2023; Lin et al., 2023; Pavlou et al., 2023; Wei et al., 2023).
Next, TRE results in significant weight loss, equivalent to that of CR, and there is no additive benefit when TRE and CR are combined. One RCT evaluating 8-hour TRE (12 pm -8 pm) in 77 patients with obesity vs. CR vs. a 10+ hour eating window control group for 12 months found that both TRE and CR resulted in significant weight loss compared to the control group [-4.61 kg (95% CI -7.37 to -1.85 kg, p ≤ 0.01) and -5.42 kg (95% CI -9.13 to -1.71 kg, p ≤ 0.01), respectively]. However, there was no difference between the TRE and CR groups (0.81 kg [95% CI -3.07-4.69 kg, p = 0.68] (Lin et al., 2023). In the TREATY trial, 139 overweight/obese adults (body mass index [BMI] 28-45) were randomized to 12 months of 8-hour eTRE (eating window 8 am-4 pm) plus CR or CR alone to evaluate the primary outcome of weight loss (Liu et al., 2022). Mean weight loss from baseline was statistically significant in both groups [-8.0 kg (95% CI -9.6 to -6.4) in the TRE+CR group and -6.3 kg (95% CI -7.8 to -4.7) in the CR group], however there was no significant difference between groups [net difference -1.8 kg (95% CI, -4.0 to 0.4, p = 0.11)], suggesting that there was no incremental benefit to TRE if CR is already implemented. In the TREAT trial, which evaluated 8-hour TRE (eating window 12-8 pm) compared to consistent meal timing (3 meals per day) for three months in 116 patients who were overweight or obese, there was significant weight loss in the TRE group (−0.94 kg, 95% CI −1.68 to −0.20, p = 0.01) but not in the consistent meal timing group (−0.68 kg, 95% CI -1.41 to 0.05, p = 0.07). There was no difference between groups (−0.26 kg, 95% CI −1.30 to 0.78, p = 0.63) (Lowe et al., 2020). The effect of 8-hour TRE on weight in patients with type 2 diabetes mellitus (T2DM) and metabolic syndrome has specifically been evaluated, and TRE was shown to result in a greater decrease in body weight compared to a low-carbohydrate diet (He et al., 2022) and compared to CR (Pavlou et al., 2023). Similar results were also seen specifically in women with obesity (Schroder et al., 2021; Haganes et al., 2022) and in older adults (Domaszewski et al., 2023).
Within metabolic syndrome, TRE is equivalent to CR in reducing MASLD. In the TREATY-FLD trial, 88 patients with obesity and non-alcoholic MASLD were randomized to 12 months of 8-hour TRE (8 am-4 pm) vs. CR with consistent meal timing (Wei et al., 2023). Both groups demonstrated a significant reduction in intrahepatic TG content, 6.9% (95% CI -8.8% to -5.1%) in the TRE group and 7.9% (95% CI -9.7% to -6.2%) in the DCR group at the 12-month assessment, with no significant difference between groups (p = 0.45). In addition, liver stiffness, body weight, and metabolic risk factors were significantly and comparably reduced in both groups. When comparing three months of 8-hour TRE to the standard of care in 32 patients with MASLD, TRE resulted in a significant decrease in hepatic steatosis, weight, waist circumference, and BMI compared to the standard of care (Feehan et al., 2023). In patients with BMI in the normal range, TRE results in positive changes in body composition. In 16 elite cyclists, 8-hour TRE (10 am-6 pm) resulted in significant weight loss and reduction in fat mass percentage with no change in fat-free mass compared to control (3 meals per day between 7 am-9 pm) (Moro et al., 2020). In another study in 15 endurance-trained male runners, 8-hour TRE resulted in a significant decrease in whole-body fat mass, leg fat mass, and percent body fat compared to the 12-hour eating window group, with no change in fat-free mass (Richardson et al., 2023). Similarly, in 20 healthy patients, 8-hour TRE reduced body and fat mass significantly compared to three meals per day (8 am-8 pm) (Moro et al., 2021). RCTs thus far have demonstrated that normal-weight patients do benefit from TRE in terms of body composition, and importantly, do not experience loss of muscle mass.
Next, TRE can have beneficial effects on glucose and insulin sensitivity in patients spanning the spectrum from those at risk of diabetes to patients with a diagnosis of T2DM. In normal-weight patients, five weeks of 8-hour eTRE (6 am-3 pm), but not mid-day TRE (11 am-8 pm), improved HOMA-IR compared to control (Δ −1.08 ± 1.59 in eTRE vs. Δ −0.05 ± 0.75 in control, p < 0.001) (Xie et al., 2022), perhaps highlighting the role of circadian rhythms in glucose sensitivity. In the Diabetes Remission Clinical Trial (DIRECT), 209 patients at risk for T2DM were randomized to six months of: (1) 30% energy requirements between 8 am and 12 pm followed by a 20-hour fasting period on three nonconsecutive days per week, and ad lib eating on other days), (2) CR (70% of energy requirements daily, without time prescription), or (3) standard care (weight loss booklet) (Teong et al., 2023). The intermittent TRE group had a greater improvement in the primary outcome, glucose area under the curve (AUC) in response to a mixed-meal tolerance test, at month six compared with CR [-10.10 (95% CI -14.08 - -6.11) vs. -3.57 (95% CI -7.72-0.57), p = 0.03]. Another RCT evaluated the effect of seven weeks of TRE (≤ 10-hour daily eating window) vs. high-intensity interval training (HIIT) vs. combination TRE plus HIIT compared to a non-intervention control group on glucose AUC in 131 women who were overweight/obese (Haganes et al., 2022). While all three intervention groups decreased glucose AUC, neither the change from baseline nor the difference between groups was statistically significant. However, the combination of TRE plus HIIT did result in a significant reduction in A1c (-1.1 mmol/mol) compared to the other groups.
In overweight patients, TRE has also been shown to reduce mean glucose and glucose excursions (Jamshed et al., 2019). In overweight patients with prediabetes, TRE improved postprandial insulin and insulin sensitivity (Sutton et al., 2018). One large RCT in patients with T2DM demonstrated that six months of 8-hour TRE resulted in a statistically significant improvement in the secondary outcome A1c from baseline (−0.91% [95% CI −1.61% to −0.20%]), equivalent to that of CR (−0.94% [95% CI −1.59% to −0.30%]) (Pavlou et al., 2023). The TREATY-FLD trial in 88 adults with obesity and MASLD also saw a significant reduction in fasting plasma glucose (FPG) level, A1c, and HOMA-IR at six months in both the 8-hour TRE and CR groups, and TRE significantly reduced HOMA-IR compared with CR at 12 months (Wei et al., 2023). While some studies showed improvement in insulin resistance measures, others question the mechanism through which TRE improves glucose homeostasis. In one small study in 14 patients with T2DM, 10-hour TRE increased the time spent in the normoglycemic range (15.1 ± 0.8 vs. 12.2 ± 1.1 h per day, p = 0.01); however, neither hepatic glycogen content nor hepatic and peripheral insulin sensitivity (as assessed by mitochondrial oxidative capacity in a muscle biopsy) differed between the TRE and control group (Andriessen et al., 2022).
RCTs have demonstrated that TRE has a BP-lowering effect. BP was specifically evaluated as a primary outcome in one trial of 74 patients with stage 1 primary HTN. Six weeks of the dietary approaches to stop hypertension (DASH) diet combined with TRE resulted in a greater decrease in systolic BP (SBP) and diastolic BP (DBP) compared to the DASH diet alone (Zhou et al., 2024). The reduction of SBP and DBP were 5.595 ± 4.072 and 5.351 ± 5.643 mmHg in the DASH group and 8.459 ± 4.260 and 9.459 ± 4.375 mmHg in the DASH + TRE group (p < 0.01). TRE also resulted in a greater reduction in 10 pm BP (p < 0.01). The study went a step further to investigate the mechanism behind TRE’s impact on BP. They first noted that the reduction in BP in the DASH + TRE group preceded the reduction in body weight. They also demonstrated that extracellular body water decreased significantly, and night urinary sodium excretion increased significantly in the DASH + TRE group.
BP was a secondary outcome in all large RCTs evaluating TRE as an intervention; however, whether or not a significant reduction in BP was observed in these patients with normal BP at baseline seemed to depend on the duration of the TRE intervention. In the TREAT trial, which evaluated three months of TRE, there was no significant difference in BP between the TRE group and the control group (Lowe et al., 2020), nor ¬was there a significant change in BP in another large trial that evaluated TRE for six months (He et al., 2022). However, in the DIRECT trial, which assessed 18 months of intermittent TRE, there was a significant decrease in SBP and DBP in both the TRE and CR groups after six months (Teong et al., 2023). The TREATY trial also saw significant decreases in BP in both the TRE plus CR and the CR groups after 12 months (Liu et al., 2022), and the TREATY-FLD trial saw significant decreases in SBP and DBP in both the 8-hour TRE and CR groups after 12 months (Wei et al., 2023).
Lastly, TRE can have a positive effect on blood lipids. Some small RCTs have included lipid measures as primary outcomes. However, larger RCTs have assessed lipids as a secondary outcome. Small trials with short interventions did not show significant changes to lipids. One non-randomized trial in 20 obese women showed no significant changes to the lipid panel after three months of 8-hour TRE (Schroder et al., 2021). Another trial of 72 adults with impaired fasting glucose also failed to show any significant changes to the lipid panel with one month of TRE (Suthutvoravut et al., 2023). Similarly, in the TREAT trial, which investigated the effect of TRE for six months in 116 overweight/obese adults, there was no significant effect on the lipid profile (Lowe et al., 2020). However, in one RCT of 169 patients with metabolic syndrome, three months of TRE with or without a low-carbohydrate diet significantly reduced TG and improved TG-HDL ratio compared to the low-carbohydrate diet and control groups (He et al., 2022). In the TREATY-FLD trial, 12 months of 8-hour TRE resulted in a significant reduction in TC (-10.2, 95% CI -18.5 to -1.9), LDL (-12.5, 95% CI -20 to -5), and TG (-39, 95% CI -56 to -21) from baseline, equal to that of CR (Wei et al., 2023). In addition, HDL was significantly increased from baseline in the TRE group (6.5, 95% CI 4.2-8.9), with no significant difference from the CR group.
The small pilot trials and larger RCTs that have been completed to date suggest several positive effects that the temporary stress of fasting can induce in humans through hormesis, similar to the effects seen in animal models. However, the reality of studying this dietary intervention in human beings brings unique challenges, and whether or not significant benefits are seen in any given trial seems to depend on (1) whether or not cardiometabolic abnormalities are present at baseline, (2) participants’ compliance with TRE, (3) duration of the intervention, and (4) the alignment of the eating window with natural circadian rhythms. The Healthy Heroes trial illustrates the first challenge: while there was no significant difference between the 10-hour TRE group and standard of care group in changes in A1c when the entire group was analyzed, in a subgroup analysis of participants with elevated fasting glucose or A1c at baseline, there was a significant improvement in A1c in the TRE group compared to control (Manoogian et al., 2022). There was also a significant reduction in DBP in participants with elevated DBP at baseline in the TRE group compared to control.
Compliance is also an issue when studying any dietary intervention in humans. Overall, self-reported compliance with TRE tends to be high in RCTs, even as high as 98.2% in one large RCT involving five weeks of TRE, suggesting that this tends to be a feasible lifestyle recommendation (Xie et al., 2022). However, when the trial length increases, patients may drop out, become lost to follow-up, or decrease adherence to the fasting pattern—all of which decrease the efficacy of the intervention in an intention-to-treat analysis. In the TREAT trial, there were no statistically significant differences in weight loss, fat mass, fasting insulin, fasting glucose, A1c, or energy expenditure between the TRE and control group (consistent meal timing) after three months (Lowe et al., 2020). However, there was a discrepancy between groups in adherence to the treatment regimen (92% in the control group and 83.5% in the TRE group). With an intention to treat analysis, there may have been insufficient power to detect a difference between the treatment arm and the control group. In another trial in 72 adults with impaired fasting glucose, there was no significant difference between the TRE and the control group in mean body weight, FPG, A1c, fasting insulin, and lipid profiles after one month; however, when considering only patients who were able to comply with the TRE protocol, the TRE group showed significantly lower mean FPG, A1c, and fasting insulin levels compared to the usual care group (Suthutvoravut et al., 2023). In addition, the RCTs with longer interventions, such as 12-18 months, showed much more significant reductions in weight, BP, glucose parameters, and lipids (Liu et al., 2022; Lin et al., 2023; Wei et al., 2023) compared to shorter trials (Sutton et al., 2018).
Compliance and confounding variables are a concern when evaluating retrospective studies. A recent controversial abstract published as part of AHA Scientific Sessions 2024 titled “Association of 8-Hour Time-Restricted Eating with All-Cause and Cause-Specific Mortality” presented a retrospective analysis of over 20,000 adults in which those following an 8-hour TRE schedule had a 91% higher risk of death from CVD (Ezpeleta et al., 2024). They also noted that 8-hour TRE was not associated with a mortality benefit compared to a 12-16 hour eating window. The study utilized only self-reported data and based the categorization of TRE groups on only two days of dietary intake. Without a reliable assessment of these participants’ long-term dietary patterns, it is difficult to draw conclusions about any association of TRE with the participants’ outcomes. There were 31 cardiovascular events in 414 individuals in the 8-hour TRE group over a median eight-year follow-up period. This TRE group represented only 2% of the total sample (414 out of 20,078 individuals). In addition, there were 60% more smokers in the TRE group (23.2% in the TRE group versus 6.6% in the control group) and 300% more Black Americans in the TRE group. Both smoking and the Black race are associated with worse cardiovascular outcomes, confounding this analysis.
Some studies also demonstrated the importance of not just fasting but also aligning feeding and fasting patterns with light, the dominant environmental cue for the master circadian clock of the suprachiasmatic nucleus. In one study that specifically compared eTRE to mid-day TRE in 90 normal-weight adults, although similar changes in energy intake occurred in both groups, only the eTRE group showed a reduction in body mass compared to the control group (Xie et al., 2022). Similar findings were seen with reductions in body fat, fat mass, and A1c percentage. Timing the fasting window earlier in the day may be a way to maximize the benefits of TRE.
Conclusion
What doesn’t kill you makes you stronger. Temporary stress is an impetus for positive adaptations in the body. Just as ischemic preconditioning and EECP can result in protection from future ischemic insults, TRE is emerging as a potent stressor that results in widespread changes in gene expression and subsequent adaptations on a cellular level, which can result in cardiometabolic resilience. Clinical trial data suggest that TRE is a feasible and safe lifestyle intervention that can be employed to target obesity, hypertension, insulin resistance, and hyperlipidemia.
Conflicts of interest
Drs. Xu, Patel and Epstein have no disclosures. Dr. Taub is a consultant to Amgen, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, Medtronic, Edwards and Esperion.
Acknowledgements
Dr. Taub has research funding from Argenx, Novartis, and NIH.
References
Elizabeth Epstein1
1Division of Cardiovascular Medicine, Department of Medicine, Scripps Green Hospital, 10666 N. Torrey Pines Road, La Jolla, CA, 92037.
Irvin Xu2
2Division of Cardiovascular Medicine, Department of Medicine, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093.
Neeja Patel3
3Department of Internal Medicine, University of California, Los Angeles, 757 Westwood Plaza Suite 7501, Los Angeles, CA 90095.
Pam R. Taub2
2Division of Cardiovascular Medicine, Department of Medicine, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093.
Corresponding author:
Pam R. Taub
Email: ptaub@health.ucsd.edu

In a new window | Download PPT
Figure 2. the process of metabolic switching in time-restricted eating.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 12034 | 20 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA