International bi-monthly journal of cell signaling, tissue protection, and translational research.
Recent clinical trial advances in remote ischemic conditioning for stroke
Lu yang1, Wenbo Zhao1, Changhong Ren2, Xunming Ji3
Author Affiliations


- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
- 2Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University.
- 3Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Abstract
Remote ischemic conditioning (RIC) refers to the body's adaptive response to brief, nonfatal ischemic and reperfusion processes that can improve tissue tolerance to subsequent more serious ischemic events, and it is generally induced by several brief cycles of limb ischemia followed by reperfusion in clinical practice. In recent years, RIC has been widely investigated in cardio-cerebrovascular diseases, especially in stroke. In both animal and clinical studies, RIC has been shown to have neuroprotective effects against brain ischemic injuries, and lots of studies have been published recently. This review summarized the recent advances in clinical studies on RIC in cerebrovascular diseases and discussed the opportunities and challenges for future research in this research field.
Keywords: Ischemic conditioning, Ischemic stroke, Intracranial hemorrhage, Moyamoya disease
Abstract
Remote ischemic conditioning (RIC) refers to the body's adaptive response to brief, nonfatal ischemic and reperfusion processes that can improve tissue tolerance to subsequent more serious ischemic events, and it is generally induced by several brief cycles of limb ischemia followed by reperfusion in clinical practice. In recent years, RIC has been widely investigated in cardio-cerebrovascular diseases, especially in stroke. In both animal and clinical studies, RIC has been shown to have neuroprotective effects against brain ischemic injuries, and lots of studies have been published recently. This review summarized the recent advances in clinical studies on RIC in cerebrovascular diseases and discussed the opportunities and challenges for future research in this research field.
Keywords: Ischemic conditioning, Ischemic stroke, Intracranial hemorrhage, Moyamoya disease
Highlights
Remote ischemic conditioning (RIC) has shown promise in enhancing tissue tolerance to ischemic events, particularly in stroke management. Recent clinical trials have shown that RIC improves neurological prognosis in patients with acute moderate ischemic stroke. However, its efficacy varies across stroke subtypes and patient populations. In cerebral hemorrhage, the impact of RIC remains uncertain. In moyamoya disease, RIC has potential benefits in improving cerebral blood flow and slowing disease progression. Despite these advances, challenges remain in standardizing RIC protocols and ensuring patient compliance. Future research must address these issues to fully utilize the therapeutic potential of RIC.
Introduction
Remote ischemic conditioning (RIC) is a strategy that enhances the tolerance of tissues or remote organs to subsequent more serious ischemic events by performing short, nonfatal ischemic and reperfusion treatment of tissues in advance or at specific times (Heusch et al., 2015). In clinical practice, RIC is typically induced by several brief cycles of limb ischemia followed by reperfusion, usually applied to the upper arms or legs using a blood pressure cuff to intermittently occlude blood flow for a few minutes, then release it (Zhao et al., 2018). This process is repeated several times to trigger the body's adaptive response. A wealth of preclinical evidence has demonstrated the therapeutic potential of RIC in stroke (Baranova et al., 2023; May et al., 2021).
Ischemic stroke is mainly caused by localized cerebral tissue ischemia and hypoxia due to cerebral vascular obstruction, which in turn triggers cell death (Walter et al., 2022). RIC can promote the establishment of collateral circulation, increase cerebral blood flow, and improve oxygen and nutrient supply to brain tissue, thereby reducing ischemic injury (Saito et al., 2024). In addition, RIC modulates the inflammatory response, reduces oxidative stress, and decreases apoptosis, all of which help to attenuate brain injury after ischemic stroke (Sun et al., 2023). Atherosclerosis is one of the major causes of ischemic stroke, which is characterized by the formation of atherosclerotic plaques due to lipid deposition and fibrous tissue proliferation in the blood vessel wall, leading to the narrowing of the blood vessels (Libby et al., 2021). RIC can improve the function of the vascular endothelium, reduce the infiltration of inflammatory cells in the blood vessel wall, and reduce the instability of plaques, thus reducing the risk of stroke caused by atherosclerosis (An et al., 2024). In contrast, moyamoya disease (MMD) is a chronic cerebrovascular disease characterized by progressive stenosis or occlusion of the end of the internal carotid artery and its branches, as well as the formation of an abnormal vascular network at the base of the brain (Ihara et al., 2022). The potential benefits of RIC in MMD may be related to its ability to promote neovascularization and establish collateral circulation. By enhancing the tolerance of brain tissue to ischemia, RIC helps alleviate the symptoms of cerebral ischemia due to vascular stenosis or occlusion and slows disease progression (Heusch et al., 2015). At the same time, RIC may also improve cerebrovascular pathology by modulating immune and inflammatory responses, providing an adjunctive therapy for patients with MMD (Pearce et al., 2021) (Figure 1).
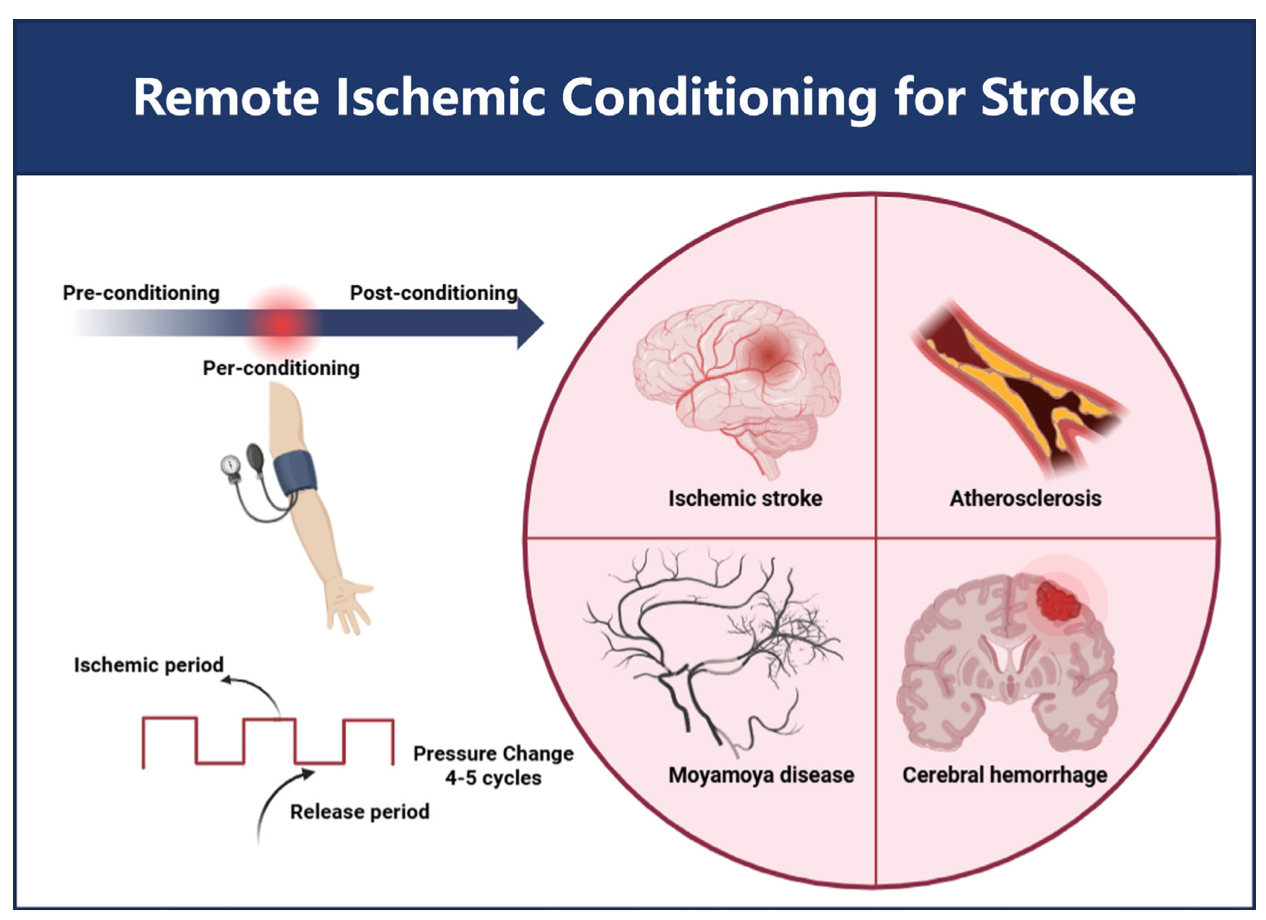
In a new window | Download PPT
Figure 1. Schematic diagram of the usage of remote ischemia conditioning and the applicable diseases.
Hemorrhagic stroke includes intracranial cerebral hemorrhage (ICH) and aneurysmal subarachnoid hemorrhage (aSAH). The pathogenesis of ICH is mainly related to the rupture of blood vessels within the brain, which can be caused by factors such as hypertension, cerebral amyloid angiopathy, or vascular malformations (Naidech et al., 2011). The bleeding leads to increased intracranial pressure, compression of surrounding brain tissue, and damage to brain cells. aSAH is caused by the rupture of an aneurysm in the subarachnoid space, resulting in sudden severe headache, potential brain damage, and increased intracranial pressure (Sharma et al., 2020). The potential role of RIC in hemorrhagic stroke may be related to its modulation of inflammatory responses (May et al., 2021). Following hemorrhagic injury, the inflammatory response exacerbates cerebral edema and brain damage. RIC can attenuate the inflammatory response by affecting immune cell function and the release of inflammatory mediators, thus improving hemorrhagic stroke prognosis. At the same time, RIC may also promote hematoma absorption and the subsidence of cerebral edema, further reducing intracranial pressure and protecting brain tissue (He et al., 2023). Specific breakthrough therapies for hemorrhagic stroke have been elusive. Therefore, there is an urgent need for better protective measures to effectively reduce the incidence and improve the prognosis of stroke patients.
RIC is a convenient, simple, non-invasive, and effective treatment that can be applied to patients with cerebrovascular diseases. However, the application of RIC in clinical settings faces some challenges. While RIC is convenient, simple, and non-invasive, its implementation in real-world scenarios may be limited by factors such as patient compliance, the need for appropriate equipment and trained personnel, and the lack of standardized protocols. Additionally, the optimal timing, frequency, and duration of RIC for different types of stroke and patient populations remain to be determined. In this paper, the clinical research progress of RIC as a brain protection strategy for cerebrovascular diseases in the past two years is reviewed, and the opportunities and challenges of RIC research in the future are prospected.
Clinical trial research progress
Several previous studies have confirmed that RIC combined with drug therapy is a safe and effective method to improve neurological dysfunction after stroke (Krag et al., 2021; Li et al., 2024). Researchers continue to conduct large-scale studies to explore the best protocols, indications, and potential mechanisms for RIC combined with drugs or intravascular stroke therapy to achieve breakthroughs in stroke prevention and treatment. Here, we review and summarize the progress of clinical research over the past two years to fully understand RIC's application in stroke treatment (Table 1).
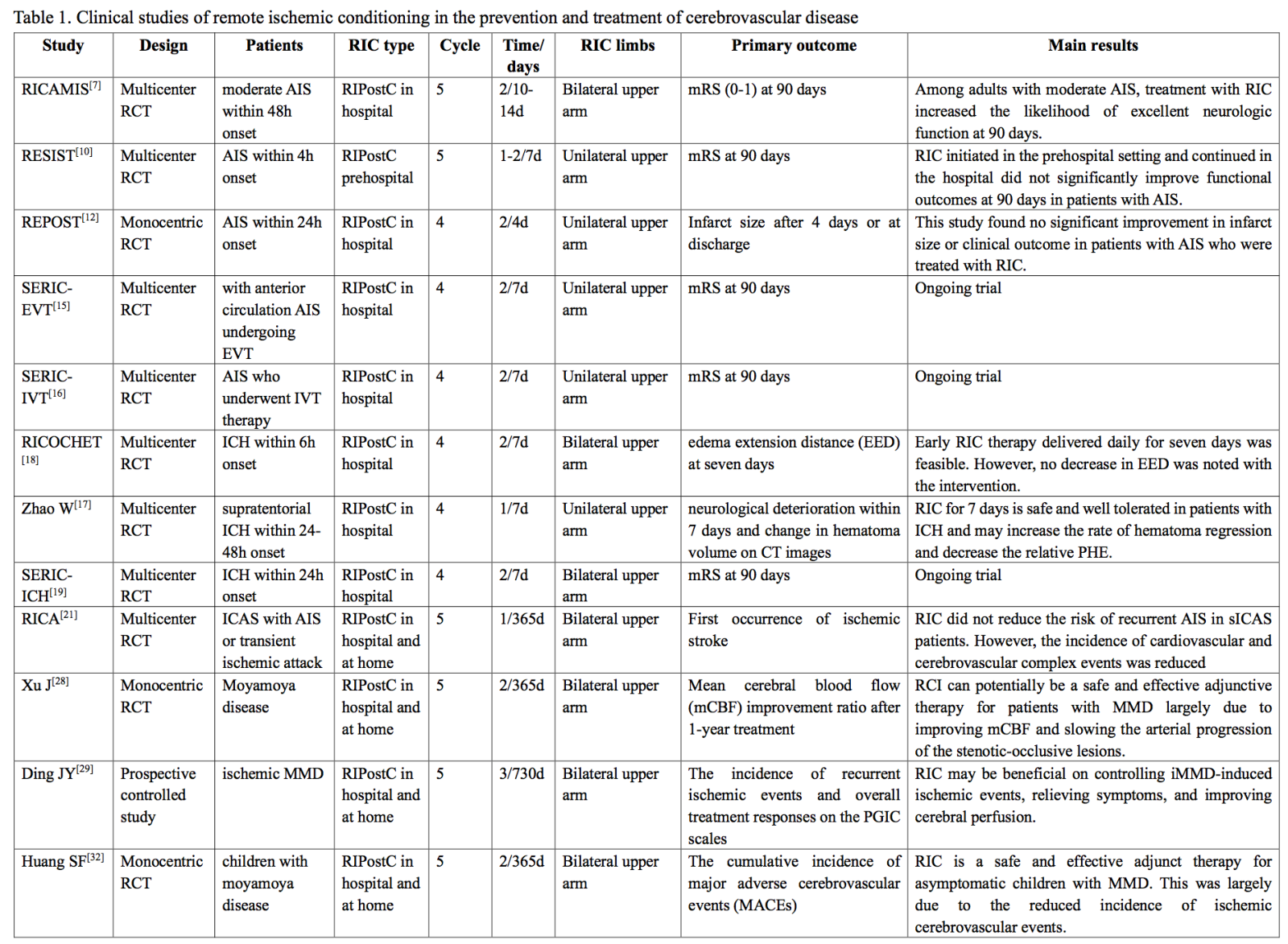
RIC - remote ischemic conditioning; AIS - acute ischemic stroke; RCT - randomized controlled trial; IVT intravenous thrombolysis; EVT - endovascular treatment; ICH - intracranial cerebral hemorrhage; aSAH - aneurysmal subarachnoid hemorrhage; sICAS - symptomatic intracranial arterial stenosis; MMD - Moyamoya disease; PHE - early perihematoma edema; EED - edema extension distance; ESOC - European Stroke Organization Conference; SERI-ICH - Safety and efficacy of remote ischemic conditioning for intracerebral hemorrhage; RICAMIS - Remote ischemic conditioning for acute moderate ischemic stroke; RESIST - Remote ischemic conditioning for acute stroke; REPOST - Reperfusion and endovascular treatment in posterior circulation stroke; RICH-2 - Remote ischemic conditioning for intracerebral hemorrhage phase II study; RICOCHET - Remote ischemic conditioning to reduce perihematoma edema in patients with intracerebral hemorrhage; RICA - Remote ischemic conditionng in acute stroke
Ischemic Stroke
Acute Ischemic Stroke
The Remote Ischemic Conditioning for Acute Moderate Ischemic Stroke (RICAMIS), a large-sample, multicenter randomized controlled trial (RCT) conducted in China, provided further evidence for the efficacy of RIC in patients with acute ischemic stroke (AIS) (Chen et al., 2022). The study included 1,893 patients with acute moderate ischemic stroke within 48 hours, with National Institute of Health Stroke Scale (NIHSS) scores ranging from 6 to 16. Patients in the intervention group received RIC twice daily for 10-14 days, while the control group received the best medical management according to the guidelines. The results showed that the RIC group had better outcomes at 90 days. Further subgroup analysis of RICAMIS showed that efficacy was dose-dependent over time in patients with acute moderate ischemic stroke, with increased RIC treatment days associated with improved functional outcomes (Cui et al., 2024).
Similarly, another study included AIS patients who did not receive intravenous thrombolysis (IVT) or endovascular treatment (EVT) within 48 hours of ictus and had NIHSS scores ranging from 1 to 15. Patients in the intervention group received RIC treatment once a day for seven days. The results showed that compared with the control group and conventional drug therapy, RIC combined therapy for AIS is effective and safe and can alleviate neurological deficits in AIS patients (Wang et al., 2022). Of course, studies on RIC treatment of AIS have also had mixed results. The Remote Ischemic Conditioning for Acute Stroke (RESIST) trial is a large, multicenter RCT study from Denmark that evaluates the effect of prehospital initiation and in-hospital continuation of RIC treatment on functional outcomes in patients with acute stroke (Blauenfeldt et al., 2023). The study included 1,500 patients with prehospital stroke symptoms lasting less than four hours, and patients in the intervention group received RIC treatment twice a day for seven days. The results showed that RIC intervention did not improve the proportion of good 90-day outcomes for either ischemic or hemorrhagic stroke. The study included patients whose onset was less than four hours, and half of the patients received treatment within the first 60 minutes after stroke onset, achieving treatment results during the hyperacute phase. There was no significant benefit of RIC in the hyperacute phase. In addition, the RIC intervention used in this study was unilateral and may not be as effective as bilateral upper limb interventions. Post-subgroup analysis of the RESIST trial showed that RIC was associated with improved functional outcomes at 90 days in patients with AIS due to small cerebral vascular disease who maintained good treatment compliance, suggesting that RIC intervention efficacy was also affected by stroke subtypes (Blauenfeldt et al., 2024). This also suggests that RIC therapy may be effective for some causes of stroke.
In the Reperfusion and Endovascular Treatment in Posterior Circulation Stroke (REPOST) trial, a randomized controlled study of AIS patients with onset within 24 hours, the intervention group received RIC treatment twice daily for four days in the hospital, and results showed that RIC intervention did not significantly reduce cerebral infarction size or improve clinical outcome (Landman et al., 2023). Due to the low patient inclusion rate in this study, there was also a baseline difference in NIHSS scores between the two groups, with patients having relatively small average infarct size and 19% of patients not seeing infarct lesions on magnetic resonance imaging. These ultimately lead to potentially biased results.
Although RIC has been demonstrated to trigger endogenous defense mechanisms and have neuroprotective effects, its effects may be mild and only benefit a specific subgroup of AIS patients (Liang et al., 2018). This may be because different subtypes of stroke have different pathophysiological mechanisms. For example, AIS due to small-vessel disease differs from AIS caused by large-vessel atherosclerosis in the nature and extent of vascular lesions. Small-vessel disease mainly affects small vessels deep in the brain, resulting in localized brain tissue ischemia, whereas large-vessel atherosclerosis may result in widespread brain tissue ischemia. RIC may have a more significant effect on ischemic injury caused by small-vessel disease by promoting the establishment of collateral circulation and improving cerebral hemodynamics (Saito et al., 2024). In a subgroup analysis of the RESIST trial, RIC had an improved 90-day functional prognosis in patients with AIS caused by small-vessel vascular disease, possibly because RIC could better ameliorate localized cerebral ischemia induced by small-vessel vascular disease (Blauenfeldt et al., 2024). In addition, RIC, as a nonspecific treatment, may be more effective in intervening in specific pathological processes. For example, for AIS caused by rupture of atherosclerotic plaques, RIC may work by regulating the inflammatory response and stabilizing plaques, whereas for AIS caused by other reasons, such as cardiogenic embolism, the effect of RIC may be relatively weaker. This is because the mechanism of action of RIC has a higher degree of fit with the pathological process of atherosclerosis and can more effectively target this pathological process for intervention. Finally, individual patient differences may also play a role. Some patients may have a better foundation of collateral circulation, and RIC treatment can further promote the establishment and improvement of collateral circulation, thus obtaining better therapeutic effects. In contrast, other patients may not experience the apparent effects of RIC treatment due to poor vascular conditions. In addition, the patient's age, gender, and genetic background may also impact RIC efficacy. Two large multi-center clinical trials (NCT04980625 and NCT04977869) are currently underway, which may provide the most reliable and authentic evidence for future RIC combined with IVT or EVT therapy (Guo et al., 2023; Abuduxukuer et al., 2023).
We note that current RIC studies have focused on the acute stroke phase, while relatively few studies have been conducted in the chronic phase. This may be because AIS patients face high mortality and disability rates in the acute phase, and thus, there is an urgent need for effective treatments. RIC, as a potential neuroprotective strategy, can be initiated rapidly in the acute phase to provide patients with timely interventions to improve brain injury and functional prognosis in the acute phase. In contrast, in the chronic phase, treatment goals are mainly to prevent recurrence, improve functional recovery, and enhance quality of life, and the need for RIC is relatively less urgent. The therapeutic effect of RIC may be time-dependent, and RIC treatment in the acute phase can better fulfill its neuroprotective role. In the chronic phase, brain tissue injury may be relatively stable, and the therapeutic effect of RIC may not be noticeable or require a more extended treatment period to become apparent. Research in the chronic phase also faces many difficulties and challenges. It is relatively difficult to assess treatment effects in the chronic phase, requiring long-term follow-up and multidimensional observational indicators that may be difficult to realize in actual studies.
Atherosclerotic diseases
Intracranial atherosclerotic stenosis is one of the most important reasons for AIS and its recurrence. The best medical management remains the standard therapy for patients with AIS secondary to intracranial atherosclerotic stenosis, especially for secondary prevention management. RIC has been investigated as an adjuvant treatment in patients with intracranial atherosclerotic stenosis to prevent recurrent stroke. Results indicate that RIC, if applied twice daily for 6 to 12 months, could significantly reduce stroke recurrence (Hou et al., 2022).
To further determine the effects of RIC in preventing stroke recurrence in patients with intracranial atherosclerotic stenosis, the Remote Ischemic Conditioning in Acute Stroke (RICA) trial was conducted to evaluate the effectiveness of chronic RIC in preventing symptomatic intracranial arterial stenosis (sICAS) stroke recurrence (Hou et al., 2022). The study lasted six years and eventually included 1,517 patients with sICAS who received RIC once daily for one year. The results showed no difference in the survival distribution between the two groups at the time of the first ischemic stroke. However, the cumulative incidence of stroke (ischemic or hemorrhagic), transient ischemic attack, or myocardial infarction in the RIC intervention group was significantly lower than that in the control group. These results suggest that chronic RIC can reduce the incidence of cardiovascular and cerebrovascular events in sICAS patients. In addition, a post-hoc analysis of the RICAMIS trial showed that RIC intervention was more effective for large atherosclerotic stroke (Cui et al., 2023). These studies not only provide high-level medical evidence for RIC in the field of stroke neuroprotective treatment but also provide new strategies and directions for treating and preventing cerebrovascular diseases.
Moyamoya disease
Moyamoya disease (MMD) is a chronic cerebrovascular disease characterized by progressive stenosis or occlusion of the end of the internal carotid artery and abnormalities of the basal reticular blood vessels of the brain (Scott et al., 2009). The mechanism of MMD has not been fully elucidated, and no cure or treatment exists. Numerous studies demonstrated that RIC intervention can stimulate angiogenesis, enhance collateral circulation, and protect neurovascular units, thereby improving cerebral blood flow (Jiang et al., 2023; Nyquist et al., 2019). RIC may also enhance tolerance to ischemia-reperfusion injury by reducing the duration and extent of hyperperfusion, modulating the inflammatory environment in cerebral ischemic areas, and altering the immune response to ischemia (Li et al., 2021; Burda et al., 2023). As a result, several clinical studies have recently begun exploring the efficacy and safety of RIC in treating MMD. A one-year prospective randomized controlled study enrolled 18 patients with MMD who had not undergone a vasectomy and received RIC therapy once a day (Xu et al., 2022). The primary outcome was the rate of improvement in median cerebral artery mean cerebral blood flow. The one-year follow-up showed that RIC improved cerebral blood flow and delayed the progression of narrow occlusive artery lesions. This suggests that RIC may be a safe and effective adjuvant therapy for patients with MMD. Ding et al. (2020) explored the safety and effectiveness of RIC in improving the sequelae of ischemic MMD. The study included a total of 30 MMD patients who received long-term RIC therapy three times a day. Clinical outcomes at 0.5, 1, and 2 years of follow-up after the ischemic event included stroke recurrence frequency, patient global impression of change (PGIC), and cerebral perfusion. The results suggested that RIC intervention reduced the incidence of recurrent ischemic events, alleviated clinical symptoms, and improved cerebral blood perfusion.
Clinically, children aged 5-9 years are at a high incidence age of MMD, and the clinical prognosis of children with MMD is still poor even after surgical reconstruction of blood circulation (Baba et al., 2008; Piao et al., 2015). Therefore, a recent randomized controlled study evaluated the safety and efficacy of RIC for pediatrics (Huang et al., 2024). MMD in 23 pediatric MMD patients with no history of revascularization surgery who were treated with RIC twice daily for one year and followed for three years. The primary outcome was the cumulative incidence of adverse cerebrovascular events. The results show that RIC is a safe and effective adjunctive treatment for asymptomatic MMD children, which can reduce the incidence of ischemic cerebrovascular events. The results of this study show a breakthrough in clinical treatment decision-making for asymptomatic MMD in children.
Although the application of RIC in the treatment of MMD is still in the research stage, preliminary results suggest that RIC has the potential to be a safe and effective adjunctive treatment, both before and after surgery. Future studies will investigate the optimal application and long-term effects of RIC in treating MMD.
Intracerebral hemorrhage
The physiopathologic mechanisms of intracerebral hemorrhage are similar to those of cerebral infarction, and intriguingly, studies have found that RIC can enhance hematoma resolution and improve neurological outcomes through the polarization of anti-inflammatory macrophages in preclinical ICH models (McDonough et al., 2020). Recently, new advances have been made in the safety and efficacy of RIC intervention in the resolution of hematoma and cerebral edema in patients with clinical cerebral hemorrhage.
An increase in early perihematoma edema (PHE) after intracerebral hemorrhage is associated with a poorer functional prognosis. In a multicenter RCT study, Remote Ischemic Conditioning for Intracerebral Hemorrhage Phase II Study (RICH-2), 40 subjects who developed supratentorial intracerebral hemorrhage within 24-48 hours of onset were randomly assigned to receive drug therapy combined with remote ischemic preconditioning for seven consecutive days or to receive drug therapy alone (Zhao et al., 2022). This study preliminarily suggested that RIC intervention in patients with intracerebral hemorrhage was safe and well tolerated and may improve hematoma resolution and reduce relative PHE. However, the effects of RIC on absolute hematoma and PHE volume and functional outcomes in this patient population require further investigation. In contrast, the Remote Ischemic Conditioning to Reduce Perihematoma Edema in Patients with Intracerebral Hemorrhage (RICOCHET) clinical trial aimed to evaluate the effect of RIC on perihematoma edema in ICH patients (Kakarla et al., 2024). The study included 60 patients with ICH whose symptom onset was less than six hours and hematoma volume was less than 60 mL. The primary outcome measure was the edema extension distance (EED). Patients in the intervention group received early RIC therapy twice a day for seven days. However, the results showed that the intervention did not reduce the edema extension distance. The RICH-2, a multicenter, prospective, randomized, sham-controlled, outcome-blinded parallel-group trial, reported its results at the European Stroke Organization Conference 2024. The results showed that RIC for seven consecutive days did not improve functional outcomes in patients with acute supratentorial ICH who did not undergo surgical therapy. In addition, the Safety and Efficacy of Remote Ischemic Conditioning for Intracerebral Hemorrhage (SERIC-ICH) is a multicenter RCT study conducted in China to evaluate whether combined RIC intervention in the acute phase of intracerebral hemorrhage can improve patient functional outcomes (Guo et al., 2024). We look forward to groundbreaking results from the SERIC-ICH study.
There may still be some controversy about the effectiveness of RIC on ICH, which may be related to the following reasons. Although RIC can influence some of the secondary injuries associated with ICH to a certain degree by regulating the inflammatory response, its impact on the mechanical factors of hemorrhagic injuries, such as increased intracranial pressure, is limited. This may be one reason why the overall effect of RIC on ICH is not significant. Secondly, there are differences in the design of clinical trials. Different clinical trials vary in patient selection, RIC treatment protocols, and observation indexes, which may lead to inconsistent results. For example, in the RICH-2 study, patients enrolled were those with episodic intracerebral hemorrhage (ICH) within 24-48 hours of onset. In contrast, patients enrolled in the RICOCHET study were patients with ICH within six hours of onset, and the RIC regimens and key observational markers differed between the two (Zhao et al., 2022; Kakarla et al., 2024). These differences may impact the accurate assessment of RIC efficacy in ICH, and the timing and duration of RIC therapy may also be crucial factors. In the early stage of hemorrhagic injury, the inflammatory reaction and edema of brain tissue may be more pronounced; at this time, RIC treatment may be more effective in regulating the inflammatory reaction and reducing cerebral edema. At the late stage of hemorrhagic injury, the damage to the brain tissue may have been more fixed, and the effect of RIC treatment may be relatively weak. Additionally, the duration of RIC treatment may also impact the therapeutic effect. The appropriate treatment duration may help maintain the therapeutic effect of RIC, while too short or too long a treatment duration may lead to poor effects or adverse reactions.
RIC Opportunities and challenges
Opportunities
For AIS, multiple clinical trials have revealed the feasibility and safety of RIC. Although preliminary results regarding the beneficial outcomes of RIC are unclear, there are some clues to the potential beneficial effects of RIC. Ongoing clinical trials remain promising. In addition, RIC has less impact on patients with early recanalization but is useful for patients with more prolonged treatment and delayed or failed recanalization therapy. For example, the narrow treatment window of IVT or EVT means that RIC treatment before recanalization may not be sufficient to achieve the desired therapeutic effect. Therefore, it is best to start RIC treatment on an emergency vehicle before admission if possible. When RIC is used after vascular opening, large-scale clinical trials are needed to investigate further the cycle, frequency, and pressure of RIC combined with IVT or EVT. Finally, two large multicenter clinical trials (NCT04980625 and NCT04977869) are currently underway that may provide the most reliable and authentic evidence for future RIC combined with IVT or EVT therapy (Guo et al., 2023; Abuduxukuer et al., 2023).
Based on the current research progress, we found that RIC intervention has potential efficacy in ischemic stroke, hemorrhagic stroke, intracranial atherosclerosis, and MMD. However, we still need high-level evidence-based medical evidence to drive clinical translation. This is especially needed for hemorrhagic stroke and MMD, as such studies are still in the early stages of exploration. The current study only suggests that RIC may be effective, but more research is needed to confirm this.
In addition, patient selection may be a key factor in accessing RIC's therapeutic potential, and the impact of stroke comorbidities on RIC's beneficial effects remains unclear. A study of remote ischemia adaptation therapy in elderly patients with symptomatic intracranial artery stenosis over the age of 80 years showed that 180 consecutive days of therapy twice/day was safe and feasible, and it was effective in reducing the recurrence rate of stroke. Therefore, in elderly patients, the frequency and duration of treatment can be increased appropriately to enhance the therapeutic effect (Meng et al., 2015). Most RIC clinical studies completed are pilot studies that provide preliminary data only. Therefore, larger clinical trials are needed for subgroup analysis to understand the potential impact of comorbidities such as diabetes, atherosclerosis, hypertension, and age, for example, on the benefits of RIC treatment. Therefore, in ischemic stroke patients, patients with comorbidities need to be included in clinical trials, such as stroke patients with hypertension, diabetes, and obesity, to assess whether RIC intervention is effective or whether the presence of these risk factors will offset the beneficial effects of RIC intervention.
Challenges
The setting of the RIC treatment regimen remains a crucial factor influencing the outcome and patient compliance (Bromage et al., 2017). Given the protective time window of one RIC treatment and the clinical impact of chronic RIC in patients with cerebrovascular disease, studying the clinical benefits of chronic or repeated daily RIC may be a more effective approach (Wills et al., 2021). As previously mentioned in the RICA study, chronic RIC intervention can reduce the incidence of cardiovascular events in patients with sICAS. However, unlike once-daily medications, RIC surgery uses a pneumatic cuff or device. It lasts 30 minutes or more when the patient is unable to perform other activities. As a result, some patients' compliance may also be reduced, which does not reflect the true efficacy of RIC intervention.
To further improve RIC compliance, portable and smart devices should be developed, but more importantly, RIC protocols should be optimized to speed up processes. Further studies are needed to determine if RIC treatment time can be reduced to five minutes or less and if RIC administered once every four days or once a week is as effective as once or twice a day. Therefore, it is difficult to determine the best solution. In RIC animal studies of myocardial or cerebral infarction, there is a lack of specific molecular serum biomarkers to monitor the biological effects of RIC and evaluate clinical efficacy.
The underlying mechanisms of RIC involve multiple pathways, including humoral, neurological, immune, and inflammatory pathways, which involve numerous genes and proteins. However, the exact molecules by which RIC triggers downstream pathophysiological changes to protect organs and tissues remain unknown. Without identifying specific molecules associated with RIC protection, it may be difficult to determine the best course of action. The optimal RIC regimen for different diseases and populations may vary, making the task even more challenging.
Conclusions
In summary, though both animal and clinical studies of RIC are progressing, the lack of specific molecular biomarkers hinders the identification of optimal protocols. Although chronic RIC appears to be the most promising protocol based on available evidence, early initiation in the target population in the acute phase and patient adherence to chronic RIC thereafter are important for achieving desirable outcomes. However, as a viable, safe, and non-invasive treatment, RIC can be applied in various clinical settings without the need for a professional. However, much work remains to be done before RIC is widely accepted.
Ethical statement and patient consent
Not applicable.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgment
This work was supported by the National Key R&D Program of China (2022YFC3602401), the Beijing Natural Science Foundation (JQ22020), and the National Natural Science Foundation of China (82274401).
References
Lu yang1
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Wenbo Zhao1
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China.
Changhong Ren2
2Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University.
Xunming Ji3
3Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Corresponding author:
Xunming Ji
Email: jixm@ccmu.edu.cn

In a new window | Download PPT
Figure 1. Schematic diagram of the usage of remote ischemia conditioning and the applicable diseases.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7018 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA