Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Neuroprotective potential of exercise preconditioning in stroke
Time:2017-12-11
Number:11554
Mohammad Rashedul Islam1, Michael F. Young1, Christiane D. Wrann1
Author Affiliations


- 1Massachussetts General Hospital and Harvard Medical School, 149 13th Street, Charlestown, MA 02129, USA
Conditioning Medicine, 2017. 1(1):27-34.
Abstract
Stroke is one of leading causes of mortality and morbidity in the world with limited availability of therapeutic intervention. Exercise has been shown to improve stroke functional outcome in different preclinical and clinical setup. Exercise preconditioning induced neuroprotection in preclinical stroke models is believed to be mediated through its ability to restore brain vasculature and blood brain barrier integrity, promote neurogenesis, and help fight against neuroinflammation and excitotoxicity. In this short review, we will summarize the molecular mechanisms of exercise preconditioning described in preclinical stroke studies. We will also discuss the neuroprotective effects of pre-ischemic exercise.
Keywords: Stroke, exercise, preconditioning, cognition, physical activity
Abstract
Stroke is one of leading causes of mortality and morbidity in the world with limited availability of therapeutic intervention. Exercise has been shown to improve stroke functional outcome in different preclinical and clinical setup. Exercise preconditioning induced neuroprotection in preclinical stroke models is believed to be mediated through its ability to restore brain vasculature and blood brain barrier integrity, promote neurogenesis, and help fight against neuroinflammation and excitotoxicity. In this short review, we will summarize the molecular mechanisms of exercise preconditioning described in preclinical stroke studies. We will also discuss the neuroprotective effects of pre-ischemic exercise.
Keywords: Stroke, exercise, preconditioning, cognition, physical activity
Introduction
Stroke is the fifth leading cause of death and most common cause of adult disability in the USA (Kochanek, Murphy et al. 2014). About 795,000 people in the United States suffer from stroke each year among which 140,000 Americans die of stroke (Mozaffarian, Benjamin et al. 2016). Unfortunately, due to difficulty in differentiating etiology and a limited therapeutic time window, the only FDA approved treatment for ischemic stroke, tissue-plasminogen activator, is available to 3-5% of all stroke patients (Fonarow, Smith et al. 2011, Roger, Go et al. 2012). Brain injury following stroke occurs by a complex interplay of multiple pathways (Doyle, Simon et al. 2008, Sierra, Coca et al. 2011). Despite extensive research and effort in both the preclinical and clinical setup, the development of effective neuroprotectants has largely been unsuccessful (Mozaffarian, Benjamin et al. 2016, Traystman 2010). As a result, there is an immense need for therapies that could prevent or ameliorate stroke-induced brain injury.
The use of the brain's endogenous mechanisms to protect itself against stroke injury through preconditioning will complement pharmacological treatments. Preconditioning is a procedure in which brief episodes of a noxious stimulus below the threshold of damage are applied to the target organ, which in return induces a robust protection against subsequent damaging injuries. Preconditioning in animal models has been used to induce resistance against the injury of ischemic stroke, trauma and different neurodegenerative diseases using a diverse range of stimuli, such as ischemia (Zhou, Li et al. 2004, Speetzen, Endres et al. 2013), hypoxia (Fan, Hu et al. 2011), hyperbaric oxygen (Cheng, Ostrowski et al. 2011), hypothermia (Nishio, Yunoki et al. 2000), hyperthermia (Xu, Aibiki et al. 2002), exposure to neurotoxins, and different pharmacological agents (Pinto, Simao et al. 2014, Cai, Yang et al. 2017). Preconditioning can have beneficial effects on different organs of the body. However, it has gained the highest clinical interest in brain injury due to the severity of stroke disability and limited regenerative properties of neurons.
Exercise, which can be considered a mild stressor (Arumugam, Gleichmann et al. 2006, Morton, Kayani et al. 2009) and thus follow the prototypical preconditioning stimulus, has beneficial effects on brain health and cognitive function (Cotman, Berchtold et al. 2007, Mattson 2012, Voss, Vivar et al. 2013). Exercise induced neuroprotection includes multiple targets, such as the blood brain barrier (BBB) and neurovascular unit maintenance (Ding, Li et al. 2006), cerebral inflammation reduction (Ding, Young et al. 2005,Barrientos, Frank et al. 2011 ), neurogenesis (Brandt, Maass et al. 2010), and neuronal apoptosis inhibition (Liebelt, Papapetrou et al. 2010) (Figure 2). This neuroprotective potential has generated a large interest in exercise as preconditioning in stroke treatment. In this review, we will discuss exercise as a preconditioning stimulus in stroke, review the proposed molecular mechanisms and discuss its clinical potential.

In a new window | Download PPT
Figure 2: The beneficial effects of preconditioning on stroke progression.
The progression of classical ischemic stroke involves excitotoxicity, ROS production, inflammation, and tissue infarction. Preconditioning, specifically with exercise, helps reduce the typical deleterious effects of stroke. This includes improved blood flow (to the region of infarct) as well as upregulation of certain cellular pathways involved in glutamate transport to minimize the effects of excitotoxicity.
ROS, reactive oxygen species; EAAT 2, excitatory amino acid transporter; ERK1/2, extracellular signal-regulated kinases; HSP-70, heat shock protein 70; GLT-1, glutamate transporter 1.
Exercise induced neuroprotection in ischemic stroke
Exercise preconditioning in animal models
Previously reported studies have shown the beneficial effects of exercise on stroke- induced brain injuries in animal models, nicely reviewed in Zhang et al. (Zhang, Bai et al. 2012)). The ischemic middle cerebral artery occlusion model in rats and mice was primarily used for these studies. The paradigms for exercise prior to stroke included either forced treadmill running (He, Wang et al. 2014) or voluntary free-wheel running (Kalogeraki, Pielecka-Fortuna et al. 2016) both of which showed a protective effect. At least 2 or 3 weeks of preischemic exercise is necessary to obtain a neuroprotective effect (Jia, Hu et al. 2009, Liebelt, Papapetrou et al. 2010). Exercise preconditioning derived neuroprotection has been shown to affect various systems including neurogenesis and angiogenesis stimulation, remodeling cerebral vasculature, modulating excitatory signaling, decreasing inflammatory markers and maintaining blood brain barrier integrity (Figure 1).
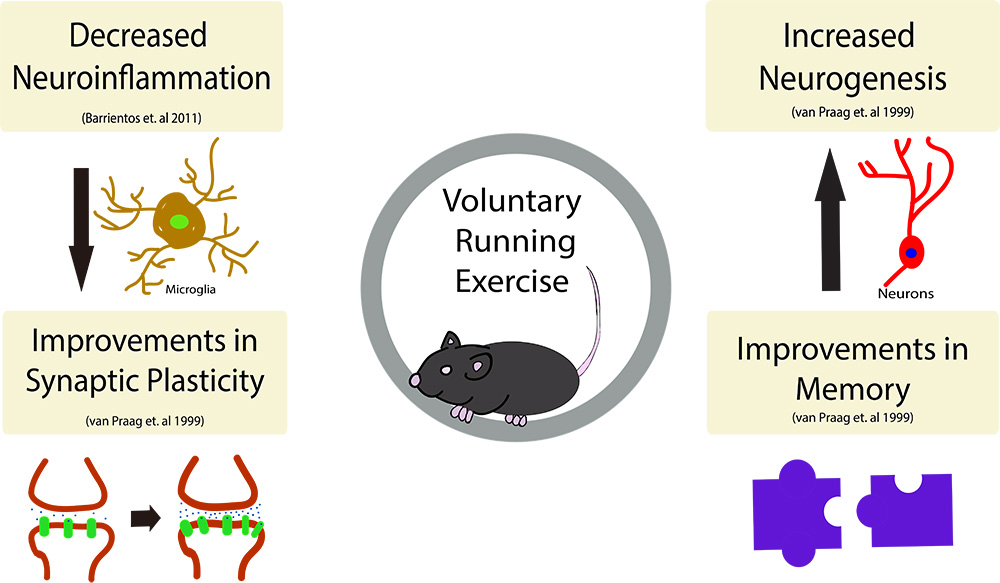
In a new window | Download PPT
Figure 1: The beneficial effects of exercise on the brain.
Exercise has been shown to have many beneficial effects on the brain, including (but not limited to), increased neurogenesis in the dentate gyrus of the hippocampus as well as improvements in spatial learning and memory. Further, running activity decreases neuroinflammation in the brain and subsequently has been shown to decreased the overall number of microglia in the brain. Synaptic plasticity, the strengthening of synapses over time, has also been shown to take place following an exercise regimen.
Exercise preconditioning and neurogenesis
Stroke induces neurogenesis in the subventricular zone and in the subgranular zone in adult rodents (Ming and Song 2005, Xiong, Mahmood et al. 2010). Recent reports have also suggested the presence of stroke-induced neurogenesis in the human brain based on the presence of cells that express markers associated with newborn neurons in the ischemic penumbra (Jin, Wang et al. 2006). These neuronal progenitors can migrate to the site of injury, possibly aiding the repair. However, in the hippocampus it had been shown that the newborn neurons generated post-stroke show an abnormal morphology or fail to integrate into the circuit (Niv, Keiner et al. 2012, Woitke, Ceanga et al. 2017). Previous animal studies showed that exercise can induce hippocampal neurogenesis (van Praag, Kempermann et al. 1999, Brown, Cooper-Kuhn et al. 2003, Bednarczyk, Aumont et al. 2009) and improves contextual, spatial and temporal function by reorganizing the newly formed neuron circuitry (Vivar, Peterson et al. 2016, Sah, Peterson et al. 2017). Interestingly, running exercise post-MCAO in mice increased hippocampal neurogenesis and the use of spatial strategies. Despite this, it did not improve latency to find the hidden platform in the Morris water maze compared to non-runners with MCAO, nor did it change the morphology of newborn neurons (Woitke, Ceanga et al. 2017). However, blocking the proliferation of the progenitor cells reduces ischemic tolerance in the brain (Maysami, Lan et al. 2008). In summary, we suggest that exercise-induced neurogenesis might be a possible mechanism that helps restore neuronal circuitry and thus improve functional outcome in post stroke recovery.
Exercise preconditioning helps to maintain BBB integrity
Disruption of blood brain barrier (BBB) is an important pathological hallmark in ischemic stroke, negatively affecting the cerebral microenvironment. The BBB is a dynamic structure that results from the interplay of effective tight junctions, trans-endothelial transport systems, enzymes, and the regulation of leukocyte permeation (Abbott and Friedman 2012). Exercise preconditioning has been shown to improve different structural and functional components of the BBB. Treadmill pretraining reduced cerebral edema (Shamsaei, Khaksari et al. 2015) by downregulating the aquaporin-4 transporter in a rat ischemic stroke model (He, Wang et al. 2014). 30-minute exercise preconditioning for three weeks has been shown to improve basal lamina by strengthening collagen IV (Davis, Mahale et al. 2007). Exercise preconditioning has also been shown to improve BBB function by upregulating TIMP-1 and inhibiting MMP-9 overexpression (Guo, Cox et al. 2008, Naderi, Alimohammadi et al. 2017). Preischemic exercise restores BBB function through the extracellular signal regulated kinases (ERK) pathway (Guo, Cox et al. 2008).
Exercise preconditioning effect on cerebral vasculature
Vascular remodeling is important to improve stroke outcome. Exercise in general has been shown to promote cerebral small vessel formation, increase capillary density, induce angiogenesis, and enhance both cerebrovascular integrity and neogenetic microvessel markers. In rat ischemic models, exercise preconditioning has shown improved cerebral blood flow (CBF) during reperfusion (Zhang, Jia et al. 2010) and an additional study proposed that this was through regulation of endothelin-1 (ET-1) expression (Zhang, Zhang et al. 2014). Expression of Netrin-1 and its receptors deleted in colon cancer (DCC) and uncoordinated gene 5B (Unc5B), known mediators of neural and vascular activities, are also regulated in the exercise preconditioning in vascular activity (Liu, Huang et al. 2011). Vigorous exercise training, i.e. treadmill running 5 days/week for 8-19 weeks, improved the NOS-dependent vascular reactivity of cerebral arterioles and reduced infarct volume following MCAO (Arrick, Yang et al. 2014). Vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) both play a vital role in cerebral vasculature angiogenesis which, in turn, is essential for vascular remodeling following ischemic stroke. Physical exercise has been shown to increase levels of both VEGF and IGF1 (Carro, Nuñez et al. 2000, Cotman, Berchtold et al. 2007, Tang, Xia et al. 2010) in the brain. Exercise preconditioning helps the brain resist ischemic stress by increasing glucose uptake and metabolism, resulting in increased ATP production following stroke; this promotes neuronal survival and cerebral tissue viability (Dornbos, Zwagerman et al. 2013). Based on the above studies, we can say that exercise preconditioning improves CBF, vascular activity and helps in vascularization via angiogenesis following induced ischemic stroke in animal models.
Exercise preconditioning targeting neuronal death
The ischemic core consists of necrotic tissue whereas the penumbral region surrounding the core shows signs of apoptosis. Proper intervention into the apoptotic program in the penumbra can provide neuroprotection. Heat shock protein (HSP-70) and ERK-mediated signaling pathways have been shown to be involved in ischemia-induced apoptosis (Zhang, Wu et al. 2011). Exercise preconditioning in rat stroke models diminishes neuronal injury by upregulating HSP-70 and ERK 1/2 (Liebelt, Papapetrou et al. 2010). Similarly, small heat shock protein (HSP-20), which protects neurons and glia against ischemia-induced apoptosis, and Matrix Metallopeptidase 9 (MMP-9) increased significantly upon exercise preconditioning and was associated with improved stroke outcomes in rats (Chaudhry, Rogers et al. 2010, Lin, Chang et al. 2015). Exercise preconditioning elevates midkine (MK) levels, a neurotropic factors that possesses anti-apoptotic and angiogenic activity (Muramatsu 2010), and thus provides a neuroprotective and regenerative role in cerebral ischemia (Otsuka, Sakakima et al. 2016). Exercise also reduces expression of the apoptosis associated gene bcl-X and neuronal death protein in the rat hippocampus (Tong, Shen et al. 2001). The hippocampus, a part of the brain important for learning and memory, is vulnerable to ischemic insults (Albasser, Amin et al. 2012). Previous studies suggested transient forebrain and global ischemia causes neuronal injury and loss in the CA1 region of hippocampus (Olsson, Wieloch et al. 2003, Ouyang, Voloboueva et al. 2007). Exercise preconditioning rescued ischemia-induced hippocampal CA1 neuronal degeneration in rat common carotid artery stroke model and thus prevented memory deficit (Shamsaei, Erfani et al. 2015). Exercise prior to ischemic insult prevented hippocampal neuronal loss in CA1 and CA3 regions through BAX/BCL-2 ratio reduction and caspase-3 activation (Aboutaleb, Shamsaei et al. 2015, Aboutaleb, Shamsaei et al. 2016).
Exercise preconditioning in excitatory system
Excitotoxicity, due to excessive excitatory neurotransmitter glutamate release, is considered the primary reason of neuronal death in stroke (Lai, Zhang et al. 2014). Pre- ischemic exercise has been shown to decrease glutamate release (Zhang, Wu et al. 2010) and inhibit the expression of glutamate receptors, metabotropic glutamate receptor 5 (mGluR5) and N-methyl-D-aspartate receptor subunit type 2B (NR2B), following MCAO (Zhang, Jia et al. 2010, Zhang, Bai et al. 2012)). Another study in rats showed that exercise preconditioning increases glutamate transporter-1 (GLT-1), also known as EAAT2, and thus exerts its neuroprotective potential by re-uptaking excessive glutamate after stroke possibly through extracellular signal- regulated kinase 1/2 (ERK1/2). Rats that underwent exercise preconditioning using treadmill running prior to MCAO had increased activity of superoxide dismutase (SOD) and decreased concentration of MDA, a marker of oxidative damage, suggesting exercise precondition decreases brain oxidative damage following ischemia reperfusion injury (Feng, Zhang et al. 2014). Another study on rats with three-week treadmill running demonstrated that preischemic exercise preconditioning alleviates neuronal oxidative damage by suppressing 4-hydroxy-2- nonenal-modified proteins, 8-hydroxy-2′-deoxyguanosine, and the levels of superoxide dismutase (SOD) following ischemic stroke (Hamakawa, Ishida et al. 2013).
Exercise preconditioning and inflammation
Different preclinical data have suggested that inflammation contributes to brain injury during ischemic stroke models (Wang, Tang et al. 2007, Barrientos, Frank et al. 2011). Various inflammatory mediators released by ischemic brain cells exacerbate the deleterious effects of ischemic brain injury (Mizuma and Yenari 2017). Several studies have shown that the ischemic reperfusion induced inflammatory response occurs through TLR4 mediated pathways, reviewed in Yang et al. (Yang, Xiang et al. 2010). Exercise has been shown to provide neuroprotection by regulating TLR4/NF-κB signaling pathway and thereby inhibiting central and peripheral inflammatory cascades during cerebral ischemic/reperfusion injury (Zhu, Ye et al. 2016). In summary, exercise preconditioning ameliorates the inflammatory response in ischemic stroke.
Exercise preconditioning in clinical studies
The beneficial effect of post stroke exercise has been well document in various preclinical (Zhang, Deng et al. 2013, Pan, Jiang et al. 2017, Stradecki-Cohan, Youbi et al. 2017) and clinical studies and meta-reviews (Belfiore, Miele et al. 2017, Oberlin, Waiwood et al. 2017, Robertson, Marzolini et al. 2017). In a few clinical studies, pre-stroke physical activity has been shown to be beneficial. However, there are fewer clinical studies that have assessed the effect of pre-stroke physical activity as a determinant for outcome. In a clinical study with 362 patients, lower severity and a better short-term stroke outcome were observed for patients who remained weakly to moderately physically active prior to cerebral ischemic stroke occurrence (Deplanque, Masse et al. 2006, Deplanque, Masse et al. 2012). In another clinical trial with 265 patients, which represents a subset of patients with first-ever stroke, enrolled into the ExStroke Pilot Trial, providing further evidence that physical activity prior to stroke was associated with a less severe stroke and better long-term outcome (Krarup, Truelsen et al. 2008). Self-reported higher physical activity prior to ischemic stroke was associated with functional advantages in a three- month follow-up as part of the Ischemic Stroke Genetics Study (Stroud, Mazwi et al. 2009). Higher-level pre-ischemic physical activity has also been shown to be correlated with smaller final infarct volume (Ricciardi, Lopez-Cancio et al. 2014). Maessen et al. report that lifelong exercise training is associated with increased tolerance against endothelial I/R, which could be cardio- and neuroprotective (Maessen, van Mil et al. 2017).
In humans, exercise increases cerebral blood volume in the hippocampal dentate gyrus and improves cognitive function in adult humans (Pereira, Huddleston et al. 2007). Physical activity, especially aerobic exercise, is also related to increased gray matter volume, increased hippocampal volume, and decreased cognitive impairment in aging (Colcombe, Erickson et al. 2006, Erickson, Raji et al. 2010, Erickson, Raji et al. 2010). Overall, better functional outcome and severity was observed in ischemic stroke with pre-ischemic physical activity in human study, but the molecular mechanism behind exercise preconditioning in stroke has not been explored in detail. The neurotrophin brain-derived neurotrophic factor (BDNF) has been reported to be elevated during exercise both in preclinical (Neeper, Gómez-Pinilla et al. 1995, Kobilo, Liu et al. 2011) and clinical studies (Rasmussen, Brassard et al. 2009, Erickson, Voss et al. 2011) and it is believed to be a mediator of neurogenesis (Pencea, Bingaman et al. 2001), hence it can be one of the molecular mediators for exercise preconditioning in ischemic stroke outcome. Another study reports that serum level of vascular endothelial growth factor (VEGF), an important neurotrophin, is increased significantly after ischemic stroke in patients with pre- ischemic physical activity (Lopez-Cancio, Ricciardi et al. 2017). These clinical studies strongly suggest that pre-stroke physical activity has been shown to be beneficial for the severity and the outcome following stroke. However, most studies could not exclude the beneficial effects of the higher fitness were due to improved overall health pre-stroke. Longitudinal studies with matched cohorts will be required to dissect these effects.
Conclusion
In conclusion, exercise preconditioning has been proven to be beneficial in both preclinical and clinical studies. Elucidating the mechanisms underlying exercise preconditioning- induced neuroprotection in stroke will provide us with the opportunity to explore new treatment strategies for stroke patients.
Acknowledgements
C.D.W. was supported by the NIH (NS087096), a NeuroBehavior Laboratory Pilot Project Research Award from the Harvard NeuroDiscovery Center (HNDC) and the Hassenfeld Cardiovascular Scholar Award. The authors declare no competing financial interests.
References
Mohammad Rashedul Islam
Massachussetts General Hospital and Harvard Medical School, 149 13th Street, Charlestown, MA 02129, USA
Michael F. Young
Massachussetts General Hospital and Harvard Medical School, 149 13th Street, Charlestown, MA 02129, USA
Christiane D. Wrann
Massachussetts General Hospital and Harvard Medical School, 149 13th Street, Charlestown, MA 02129, USA
Corresponding Author:
Christiane D. Wrann
Email: cwrann@mgh.harvard.edu

In a new window | Download PPT
Figure 1: The beneficial effects of exercise on the brain.
Exercise has been shown to have many beneficial effects on the brain, including (but not limited to), increased neurogenesis in the dentate gyrus of the hippocampus as well as improvements in spatial learning and memory. Further, running activity decreases neuroinflammation in the brain and subsequently has been shown to decreased the overall number of microglia in the brain. Synaptic plasticity, the strengthening of synapses over time, has also been shown to take place following an exercise regimen.

In a new window | Download PPT
Figure 2: The beneficial effects of preconditioning on stroke progression.
The progression of classical ischemic stroke involves excitotoxicity, ROS production, inflammation, and tissue infarction. Preconditioning, specifically with exercise, helps reduce the typical deleterious effects of stroke. This includes improved blood flow (to the region of infarct) as well as upregulation of certain cellular pathways involved in glutamate transport to minimize the effects of excitotoxicity.
ROS, reactive oxygen species; EAAT 2, excitatory amino acid transporter; ERK1/2, extracellular signal-regulated kinases; HSP-70, heat shock protein 70; GLT-1, glutamate transporter 1.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 11554 | 57 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA