Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Ischaemic conditioning of the heart: From academic curiosity to clinical application
Time:2017-12-20
Number:10957
Mervyn Chan1, Sauri Hernández-Reséndiz1,2, Valeria Paradies2, Derek J Hausenloy1,2,3,4,5,6,7
Author Affiliations


- 1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore
- 2National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore
- 3National Heart Research Institute Singapore, National Heart Centre Singapore
- 4Yong Loo Lin School of Medicine, National University Singapore, Singapore
- 5The Hatter Cardiovascular Institute, University College London, London, UK
- 6The National Institute of Health Research University College London Hospitals Biomedical Research Centre, London, UK
- 7Barts Heart Centre, St Bartholomew’s Hospital, London, UK
Conditioning Medicine, 2017. 1(1):17-26.
Abstract
Ischaemic heart disease (IHD) is one of the leading causes of death and disability worldwide. There remains a need therefore, to discover novel therapies for protecting the myocardium from the detrimental effects of acute ischaemia/reperfusion injury (IRI). Over 30 years ago, the concept of ‘ischaemic preconditioning’ (IPC), in which the myocardium is protected from acute IRI when subjected to brief cycles of non-lethal ischaemia and reperfusion, was first introduced. After reperfusion, IPC remains the most powerful endogenous cardioprotective strategy for reducing myocardial infarct size. Over the years, the concept of IPC has evolved into ischaemic postconditioning (IPost) and remote ischaemic conditioning (RIC), strategies which have facilitated the translation of ischaemic conditioning into the clinical setting. In this article, we provide a historical overview of IPC and the heart, we trace its evolution into IPost and RIC, and we discuss the therapeutic potential of ischaemic conditioning for improving clinical outcomes in patients with IHD.
Keywords: Cardioprotection, Ischaemia, Reperfusion, Myocardial Infarction, Ischaemic Conditioning, Coronary Artery Bypass Graft Surgery
Abstract
Ischaemic heart disease (IHD) is one of the leading causes of death and disability worldwide. There remains a need therefore, to discover novel therapies for protecting the myocardium from the detrimental effects of acute ischaemia/reperfusion injury (IRI). Over 30 years ago, the concept of ‘ischaemic preconditioning’ (IPC), in which the myocardium is protected from acute IRI when subjected to brief cycles of non-lethal ischaemia and reperfusion, was first introduced. After reperfusion, IPC remains the most powerful endogenous cardioprotective strategy for reducing myocardial infarct size. Over the years, the concept of IPC has evolved into ischaemic postconditioning (IPost) and remote ischaemic conditioning (RIC), strategies which have facilitated the translation of ischaemic conditioning into the clinical setting. In this article, we provide a historical overview of IPC and the heart, we trace its evolution into IPost and RIC, and we discuss the therapeutic potential of ischaemic conditioning for improving clinical outcomes in patients with IHD.
Keywords: Cardioprotection, Ischaemia, Reperfusion, Myocardial Infarction, Ischaemic Conditioning, Coronary Artery Bypass Graft Surgery
Introduction
Ischaemic heart disease (IHD) is one of the leading causes of death and disability worldwide. This underscores the need for novel therapeutic strategies for protecting the myocardium against the detrimental effects of acute ischaemia/reperfusion injury (IRI). In this regard, the phenomenon of ‘ischaemic conditioning’, which describes the powerful cardioprotection induced by subjecting the heart to cycles of brief non-lethal ischaemia and reperfusion (Murry et al., 1986), has the therapeutic potential to protect the myocardium from acute IRI and improve clinical outcomes in patients with IHD.
In this article, we provide a historical overview of the origins of ischaemic conditioning as an endogenous cardioprotective strategy with the discovery of ischaemic preconditioning (IPC) over 30 years ago, we trace its evolution over the years into ischaemic postconditioning (iPost) and remote ischaemic conditioning (RIC), and we discuss its therapeutic potential to improve clinical outcomes in IHD patients. We only provide an overview in this article, and focus mainly on the clinical aspects of ischaemic conditioning - for comprehensive reviews on IPC, IPost and RIC, please see the following reviews (Yellon and Downey, 2003; Hausenloy, 2013 ; Bulluck and Hausenloy, 2015).
Ischaemic Preconditioning
“…..we proposed that multiple brief ischemic episodes might actually protect the heart from a subsequent sustained ischemic insult.” Murry et al 1986
In 1986, Reimer et al. first made the surprising observation that subjecting the canine heart to four cycles of 10-minute cycles of ischaemia and reperfusion attenuated the cumulative decrease in myocardial ATP levels, when compared to the reduction in myocardial ATP levels observed in hearts subjected to 40 minutes of sustained ischaemia - this suggested that intermittent ischaemic and reperfusion may decrease ATP depletion and increase catabolite wash-out (Reimer et al., 1986). On this background, in 1986, the same authors performed a seminal study to determine the cardioprotective efficacy of cycles of ischaemia and reperfusion on myocardial infarct (MI) size in canine hearts (Murry et al., 1986). They subjected canine hearts to four 5-minute cycles of acute myocardial ischaemia and reperfusion by occlusion and reflow of the left circumflex artery (LCx) immediately prior to 40 minutes of sustained LCx occlusion followed by 3 days of reperfusion (Murry et al., 1986). They found that this IPC protocol resulted in a 75% reduction in MI size following 40 minutes of sustained ischaemia, when compared to control hearts (Murry et al., 1986). Since the discovery of IPC by Murry et al in 1986, IPC has been shown to confer ubiquitous cardioprotection in every animal species tested including man; it has resulted in nearly 9000 publications; and after reperfusion, it remains the most powerful strategy for reducing MI size (Kloner et al., 1998; Hausenloy and Yellon, 2016).
In a new window | Download PPT
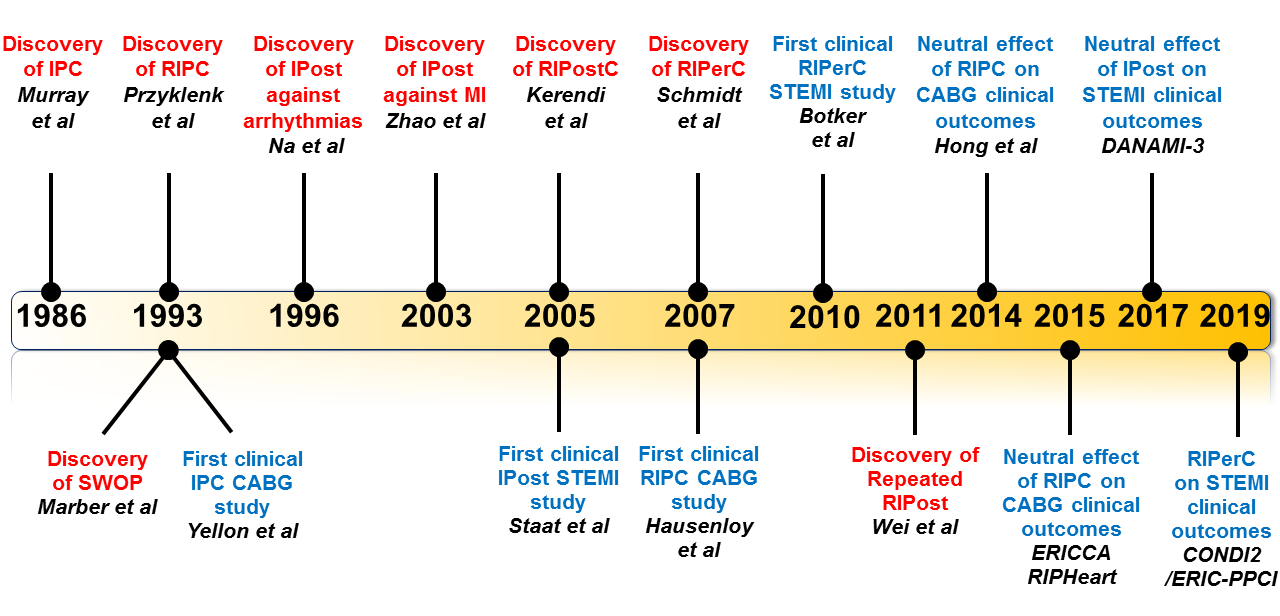
Figure 1: Time-line of major discoveries (red) and major clinical studies (blue) in the field of ischaemic conditioning. CABG, coronary artery bypass graft; IPC, Ischaemic preconditioning; RIPC, Remote ischaemic preconditioning; IPost, Ischaemic postconditioning; RIPerC, Remote Ischaemic perconditioning; RIPostC, Remote ischaemic postconditioning; STEMI, ST-segment elevation myocardial infarction.
Experimental IPC studies
A standard IPC stimulus is known to elicit 2 windows of cardioprotection; the first window begins immediately following the IPC stimulus, and lasts 2-3 hours (termed “classical IPC” or “acute IPC”), following which it disappears; and the second window which appears 12-24 hours later, and lasts 48-72 hours (known as the ‘Second Window Of Protection’ [SWOP] or “delayed IPC”) (Kuzuya et al., 1993; Marber et al., 1993). Despite intensive investigation over the last 30 years, the mechanisms underlying both classical and delayed IPC cardioprotection remain incompletely understood. However, elucidation of the multiple complex signalling pathways mediating IPC cardioprotection, has provided important insights into the pathophysiology underlying acute myocardial IRI, and has identified numerous cytoprotective signal transduction pathways within the cardiomyocyte. Only a brief overview of some of the major signalling pathways underlying IPC can be reviewed here, given the large number of signalling cascades and mechanisms which have been linked to IPC. For comprehensive reviews of the mechanisms and signalling pathways underlying IPC please see (Yellon and Downey, 2003; Hausenloy, 2013; Heusch, 2015).
In response to the IPC stimulus, autacoids (such as acetylcholine, opioids, endothelin, adenosine and bradykinin) generated by the cardiomyocyte in response to cycles of brief non-lethal ischaemia and reperfusion, bind to their receptors on the plasma membrane, and recruit the activation of a number of signal cascades, both prior to ischaemia, and at the time of reperfusion. These include the Reperfusion Injury Salvage Kinase (RISK, comprising Akt and Erk1/2) (Hausenloy and Yellon, 2007), the cGMP-PKG, and the Survivor Activating Factor Enhancement (SAFE, comprising TNF-1α and STAT-3) pathways. These pro-survival signalling cascades have been shown to converge on the mitochondria to inhibit the opening of the mitochondrial permeability transition pore (MPTP), a critical mediator of cell death following acute myocardial IRI. With regards to SWOP, the IPC stimulus has been shown to activate a number of transcription factors such as activator protein-1 (AP-1), HIF-1α, NF-κB, Nrf2, and STAT1/3, which in turn mediate the transcription and synthesis of de novo mediators of cardioprotection 12 to 24 hours after the IPC stimulus, such as iNOS, COX-2, heat shock proteins, aldose reductase, and MnSOD (Hausenloy and Yellon, 2010; Hausenloy et al., 2011).
In a new window | Download PPT
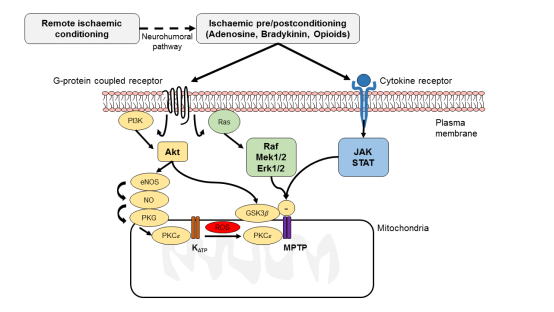
Figure 2: This figure depicts some of the major signalling pathways within the cardiomyocyte underlying ischaemic conditioning (ischaemic pre- and postconditioning as well as remote ischaemic conditioning). Pre- and post-conditioning generates such as autacoids (adenosine, bradykinin and opioids) which bind to G-protein coupled receptors and activate pro-survival signalling pathways – the Reperfusion Injury Salvage Kinase (RISK) pathway (involves Akt and Erk1/2), the cGMP-protein kinase G (PKG) pathway and the Survivor Activator Factor Enhancement (SAFE) pathway (involves TNF-1α and JAK-STAT). Remote ischaemic conditioning generates a cardioprotective signal in an organ or tissue away from the heart, which is then relayed to the myocardium via a neuro-hormonal pathway, where it activates signalling pathways within the cardiomyocyte common to ischaemic pre- and postconditioning. These pro-survival signalling pathways recruit mediators such as endothelial nitric oxide synthase (eNOS), glycogen synthase kinase (GSK)-3, protein kinase C-ε (PKC-ε) and the mitochondrial ATP-dependent potassium channel (KATP), which inhibit the opening of the mitochondrial permeability transition pore (MPTP).
Clinical IPC studies
Evidence of IPC as an endogenous cardioprotective phenomenon in humans has been demonstrated in two different settings: ‘Warm-up angina’ - this refers to the phenomenon in which a patient with stable IHD is able to exercise for a longer duration following an episode of angina (Williams et al., 2014); and ‘Pre-infarct angina’ - this refers to the cardioprotective effects of anginal episodes occurring in the 1-2 days preceding an acute myocardial infarction (AMI): these result in smaller MI size, and better clinical outcomes (Eitel et al., 2015).
Harnessing IPC cardioprotection as a therapeutic intervention has been more challenging given the necessity to apply the IPC stimulus prior to the index ischaemic event. However, in 1993, Yellon et al. demonstrated in a small proof-of-concept study in patients undergoing coronary artery bypass graft (CABG) surgery, that intermittent clamping and unclamping of the aorta to induce two cycles of 3-minutes ischaemia and 2-minutes reperfusion, preserved myocardial levels of ATP, following a sustained 10-minute acute ischaemic insult (achieved by cross-clamping the aorta). Since this landmark study, a number of clinical studies and a subsequent meta-analysis have confirmed that IPC administered to CABG patients can reduce peri-procedural myocardial injury, prevent ventricular arrhythmias, and shorten intensive care unit stay (Walsh et al., 2008). However, despite its overwhelming efficacy, the invasive nature of the IPC stimulus (from clamping the aorta), and the need to administer the IPC stimulus before the index ischaemic insult, has hindered its application in clinical settings of acute myocardial IRI such as AMI. This limitation was overcome by the discovery of IPost by Zhao et al. in 2003.
Ischaemic Postconditioning
“…salvage of the coronary endothelium and cardiomyocyte can be achieved when the heart is postconditioned by cycles of briefly interrupted perfusion during the early moments of reflow.” Zhao et al. 2003
The discovery of IPost by Zhao et al. in 2003, that cardioprotection on par with IPC could be obtained by interrupting the first few minutes of reperfusion with intermittent episodes of acute myocardial ischaemia, allowed the cardioprotective intervention to be applied at the onset of reperfusion, making it possible to be administered to AMI patients undergoing reperfusion therapy (Zhao et al. 2003). The authors found that applying three 30-second cycles of intermittent left anterior descending (LAD) occlusion and reflow at the onset of reperfusion following 60 minutes of LAD ligation, reduced MI size by 44% in canine hearts. Interestingly, the idea of modifying reperfusion as a strategy to reduce MI size had been introduced nearly 20 years earlier in the 1980s as ‘gentle’ or ‘gradual’ reperfusion. Furthermore, the actual term ‘postconditioning’ was first used in 1996 by Na et al. to describe the phenomenon through which intermittent reperfusion, induced by ventricular premature beats (Na et al., 1996), prevented reperfusion-induced ventricular fibrillation in ischaemic feline hearts. All things considered, the concept of IPost has succeeded in revitalizing efforts to target myocardial reperfusion injury as a therapeutic strategy for reducing MI size (Heusch, 2004).
Experimental IPost studies
When IPost was first described, it was believed that its cardioprotective effects were due to it attenuating the detrimental effects of acute myocardial IRI, with the observation of less myocardial oedema and inflammation, preservation of endothelial function, and reduced MI size. However, subsequent studies have demonstrated that IPost activates many of the signalling pathways within the cardiomyocyte common to IPC. For more comprehensive reviews of the mechanisms underlying IPost cardioprotection please see the following articles (Tsang et al., 2004; Hausenloy, 2009; Ovize et al., 2010; Hausenloy, 2013).
Clinical IPost studies
It took only 2 years after the discovery of IPost in the experimental setting, for Staat et al. to translate this cardioprotective strategy into the clinical setting (Staat et al., 2005). In this landmark small proof-of-concept clinical study, it was shown in STEMI patients treated by direct stenting, that serial inflations/deflations of a coronary angioplasty balloon upstream of the stent, to interrupt coronary reflow with four 1-minute cycles of ischaemia and reperfusion within the infarct-related coronary artery, reduced MI size by 36% (measured by total CK). The discovery that IPost could reduce MI size in the clinical setting, redirected attention to reperfusion injury, as a target for cardioprotection. Until that point, the existence of reperfusion injury (defined as the cardiomyocyte death which paradoxically occurs on establishing coronary reflow following a sustained episode of acute myocardial ischaemia) (Piper et al., 1998; Yellon and Hausenloy, 2007), had been surrounded by controversy. However, the fact that an intervention, in this case IPost, could be applied at the onset of reperfusion and reduced MI size, provided the strongest evidence for the existence of reperfusion injury in man (Yellon and Opie, 2006).
Since this landmark clinical study, the results of subsequent clinical studies of IPost in STEMI patients have been mixed, with positive, neutral and even negative studies (Staat et al., 2005; Ma et al., 2006; Yang et al., 2007; Thibault et al., 2008; Lonborg et al., 2010; Araszkiewicz et al., 2014). Although several meta-analyses have confirmed the efficacy for IPost reducing MI size (Favaretto et al., 2014; Touboul et al., 2015), not all have been positive (Khalili et al., 2014). Furthermore, two large clinical trials of IPost in STEMI patients have also been published, but both were neutral. Hahn et al. failed to demonstrate IPost (four 1 minute inflations/deflation of coronary angioplasty balloon following PPCI) improving ST-segment resolution when compared to control in a 700 STEMI patient study (Hahn et al., 2013), and in the 460 STEMI patient LIPSIA trial, Eitel et al. also found no difference between IPost (four 1 minute inflations/deflation of coronary angioplasty balloon following PPCI) and control with respect to MRI-determined MI size and clinical outcomes at 6 months (death, re-infarction, and new congestive heart failure) (Eitel et al., 2015).
The reasons for the discordant results of IPost in STEMI remain unclear, but they may relate to several factors: patient selection (STEMI patients most likely to benefit are those presenting with fully occluded coronary artery and treated by direct stenting), and delivery of the IPost protocol (the most effective protocol should be four 60-seconds angioplasty inflations/deflations, delivered upstream of the deployed stent to avoid potential coronary thromboembolism) (Hausenloy et al., 2017a). As such, IPost in the setting of STEMI is an invasive cardioprotective strategy which requires stringent patient selection and careful delivery of the IPost protocol to be effective.
The largest clinical study of IPost to date, the multi-centre DANAMI 3-iPOST trial, tested the effect of IPost on major clinical endpoints in 1,234 STEMI patients treated by PPCI (Engstrom et al., 2017). Unfortunately, it too, found no difference between IPost (four 30 seconds inflations/deflation of coronary angioplasty balloon following PPCI) and control with respect to clinical outcomes (all-cause death, re-infarction, and hospitalisation for heart failure), after an extended median follow-up of 3.8 years. However, this trial was likely underpowered (the primary event rate was lower than expected), may have used a suboptimal IPost stimulus (four 30-second angioplasty balloon inflations/deflations instead of standard four 60-second protocol), and included patients who were reperfused not using direct stenting. As such, whether IPost can improve clinical outcomes following STEMI remains unclear.
IPost has also been investigated in patients undergoing cardiac bypass surgery, a clinical setting in which the heart is subjected to acute global IRI as the patient is put on to and taken off cardiopulmonary bypass. IPost was delivered by applying three 30-second cycles of aorta clamping and unclamping, with the patient coming off bypass, and was shown to reduce peri-operative myocardial injury in children and patients undergoing cardiac surgery (Luo et al., 2007). Given the invasive nature of this IPost protocol and the potential risk of thromboembolic complications from manipulating an atherosclerotic aorta, the translation of IPost for adult patients undergoing cardiac surgery may be challenging. In contrast, IPost may have greater therapeutic potential in children undergoing corrective cardiac surgery for congenital heart disease, where the risk of thromboembolism is substantially lower.
Remote Ischaemic Conditioning
“…preconditioning may be mediated by factor(s) activated, produced, or transported throughout the heart during brief ischemia/reperfusion.” Przyklenk et al. 1993
The fact that both IPC and IPost require an invasive intervention to be administered directly to the aorta (in the case of IPC or IPost in CABG patients) or heart (in the case of IPost in STEMI patients) has hampered their application in the clinical setting. Therefore, the discovery of RIC, which allows the cardioprotective intervention to be applied non-invasively away from the heart has greatly facilitated the translation of ischaemic conditioning into the clinical setting (Birnbaum et al., 1997; Oxman et al., 1997).
In 1993, Przyklenk et al. made the intriguing observation that applying an IPC stimulus to the LCx artery reduced MI size induced by ligation in the LAD artery, suggesting that the cardioprotection can be transferred between different regions of the heart. This concept of cardioprotection from afar, was then extended to organs and tissues remote from the heart, giving rise to the concept of inter-organ protection against acute IRI (Hausenloy and Yellon, 2008). Two key developments have greatly facilitated the translation of RIC into the clinical setting: (1) The observation that RIC could applied to the hind-limb of animals (using a tourniquet to induce ischaemia and reperfusion) (Birnbaum et al., 1997; Oxman et al., 1997), and the subsequent finding that RIC could be non-invasively applied to human volunteers and patients by simply inflating a standard blood pressure cuff placed on the upper arm or thigh to induce cycles of brief ischaemia and reperfusion (Kharbanda et al., 2002); (2) The finding that RIC stimulus could be applied at various time-points to induce cardioprotection: prior to the index acute myocardial ischaemic event (remote ischaemic preconditioning [RIPC]) (Przyklenk et al. 1993), after the onset of acute myocardial ischaemia but prior to reperfusion (remote ischaemic perconditioning [RIPerC] (Schmidt et al., 2007), and at the onset of reperfusion (remote ischaemic postconditioning [RIPost]) (Kerendi et al., 2005).
Experimental RIC studies
The actual mechanism through which applying a RIC stimulus to an organ or tissue remote from the heart confers cardioprotection is not known. The current paradigm suggests that the RIC stimulus mediates the cardioprotective signal to the heart via a neurohormonal pathway, although the exact details remain unclear (Hausenloy and Yellon, 2008). The evidence of a transferable cardioprotective factor (hormonal pathway) being generated in response to RIC to the remote organ or tissue include the following: (1) reperfusion was shown to be required for RIC to kidney to be effective, suggesting the wash-out of a cardioprotective factor (Gho et al., 1996); (2) transfusion of blood from a RIC-treated rabbit to a naïve animal was shown to limit MI size (Dickson et al., 1999); (3) limb RIC has been shown in small animals and humans to generate a cardioprotective factor in plasma diasylate which has been shown to limit MI size in naïve small animal hearts subjected to acute IRI (Wang et al., 2008; Shimizu et al., 2009). The identity of the cardioprotective factor(s) mediating RIC cardioprotection remain unknown, although studies suggest that it is a peptide of <30 kDa in size (Lang et al., 2006; Serejo et al., 2007) – experimental studies have proposed a number of potential candidates including calcitonin gene related peptide (Wolfrum et al., 2005), miRNA144 (Li et al., 2014), sodium nitrite (Rassaf et al., 2014), SDF-1 (Davidson et al., 2013) amongst others.
The evidence of a neural pathway mediating the RIC cardioprotective signal from the remote organ or tissue to the heart include the following: (1) the ganglionic blocker, hexamethonium, was demonstrated to block RIC cardioprotection (Gho et al., 1996); (2) resection of the femoral and/or sciatic nerves to the hind-limb was shown to block limb RIC cardioprotection (Ding et al., 2001; Lim et al., 2010); (3) resection of the vagal nerve to the heart was shown to block limb RIC cardioprotection (Basalay et al., 2012; Mastitskaya et al., 2012) ; (4) stimulation of sensory nerves in the remote organ or tissue using a variety of methods (trauma, capsaicin, electro-acupuncture or transcutaneous electrical nerve stimulation) was shown to either induce cardioprotection and/or generate a cardioprotective plasma diasylate which limited MI size in naïve small animal hearts subjected to acute IRI (Redington et al., 2012; Redington et al., 2013; Merlocco et al., 2014); (5) Limb RIC in diabetic patients with upper limb sensory peripheral neuropathy did not generate a cardioprotective plasma diasylate, when compared to diabetic patients with no upper limb sensory peripheral neuropathy (Jensen et al., 2012). These findings suggest that the transferrable cardioprotective factor(s) generated by the RIC stimulus are downstream of the neural pathway. Further work is needed to identify the actual factor(s) responsible for RIC cardioprotection in order to provide a biomarker to assess the efficacy of RIC protocols and to provide a pharmacological strategy for cardioprotection.
Clinical RIC studies
The first proof-of-concept study to test the effects of limb RIC in the clinical setting was by Gunaydin et al., in 2000, where they investigated the feasibility of applying lower limb RIC (two cycles of 3-minutes ischaemia and 2-minutes reperfusion) in 8 patients undergoing CABG surgery (Gunaydin et al., 2000). In 2007, we were the first to demonstrate the cardioprotective efficacy of RIC in the clinical setting, showing that upper limb RIC (four 5-minute cycles of ischaemia and reperfusion applied by inflating and deflating a blood pressure placed on the upper arm) administered prior to CABG surgery reduced perioperative myocardial injury (as evidenced by a 43% reduction in Troponin T release), when compared to control in a small 57 patient proof-of-concept study. Subsequent studies confirmed the cardioprotective effect of limb RIC in this clinical setting (Cheung et al., 2006; Hausenloy and Yellon, 2007; Venugopal et al., 2009; Thielmann et al., 2010; Wagner et al., 2010; Kottenberg et al., 2012; Thielmann et al., 2013; Candilio et al., 2015), although not all studies have been positive. A number of meta-analyses have confirmed the cardioprotective efficacy of limb RIC in cardiac surgery, in terms of reducing perioperative myocardial injury (cardiac biomarker release). Follow-up of a cohort of 329 CABG patients randomised to either limb RIC (three 5 minutes cycles of upper arm cuff inflations/deflations) or control found that all-cause mortality was reduced by 73% at a median follow-up of 1.54 years following RIC when compared to control, although this study was not powered to detect an effect of limb RIC on clinical outcomes (Thielmann et al., 2013). However, 3 subsequent large clinical studies investigating the effect of limb RIC on perioperative myocardial injury and clinical outcomes have been neutral.
In 1,280 cardiac surgery patients, Hong et al, found no effect with limb RIC (applied at two time-points, prior to surgery and coming off bypass) on and primary composite endpoint (death, myocardial infarction, arrhythmia, stroke, coma, renal failure respiratory failure, cardiogenic shock, gastrointestinal complication and multi-organ failure) when compared to control (Hong et al., 2014). Two further large clinical studies, have failed to demonstrate any effect of limb RIC in patients undergoing cardiac surgery. In the ERICCA trial, we found no effect of limb RIC (four 5-minute cycles of arm cuff inflations/deflations) on the primary combined endpoint of cardiac death, revascularisation, stroke and either non-fatal MI or perioperative myocardial injury (measured by serum Troponin release) in 1,612 patients undergoing cardiac surgery (CABG plus or minus valve surgery) (Hausenloy et al., 2015a). Similarly, the RIPHeart trial in 1385 patients undergoing cardiac surgery (CABG, valve or aortic surgery) also failed to find any cardioprotective effect with limb RIC in terms of less peri-operative myocardial injury (serum Troponin release) or the primary clinical endpoint (cardiac death, stroke, acute kidney injury and non-fatal MI) at the time of hospital discharge (Meybohm et al., 2015).
The reasons for the discordant results of limb RIC in cardiac surgery patients remain unclear, but may relate to several factors: the limb RIC protocol had not been adequately optimised for cardioprotection in either the experimental or clinical settings, the type of surgery (limb RIC may only be effective in patients undergoing CABG surgery only), co-medications (propofol (Kottenberg et al., 2012) or nitrates (Candilio et al., 2015) administered during cardiac surgery may have interfered with the limb RIC cardioprotective effect).
Another clinical setting in which limb RIC has been investigated as a cardioprotective strategy is planned PCI for stable and unstable coronary artery disease. In this setting, the acute myocardial injury is multi-factorial (side branch occlusion, coronary embolization, dissection and so on) and does not include classical acute IRI. The results of relatively small proof-of-concept clinical studies have been mixed (Iliodromitis et al., 2006; Hoole et al., 2009; Ahmed et al., 2013; Davies et al., 2013; Luo et al., 2013; Prasad et al., 2013; Lavi et al., 2014; Liu et al., 2014; Xu et al., 2014; Zografos et al., 2014; Moretti et al., 2015).
In patients presenting with a STEMI, a condition of classical acute myocardial IRI, the application of limb RIC to either the arm or leg prior to or even at the onset of PPCI, has been shown in several proof-of-concept clinical studies to reduce MI size (assessed by serum cardiac biomarker, myocardial nuclear imaging and cardiac MRI) and preserve left ventricle (LV) ejection fraction (Botker et al., 2010; Rentoukas et al., 2010; Crimi et al., 2013; Sloth et al., 2014; Eitel et al., 2015; Hausenloy et al., 2015b; Yellon et al., 2015; Liu et al., 2016). Only one recent study has been neutral, but this may have been due to the non-standardised and unorthodox limb RIC protocol used in this study, which comprised variable numbers of cycles of RIC (5 to 11 five-minute cycles of lower limb cuff inflations/deflations) initiated prior to PPCI, and continued to the end of the PCI procedure (Verouhis et al., 2016). Extended follow-up of a cohort of STEMI patients treated by limb RIC reported a 51% reduction in the combined clinical endpoint of all-cause mortality, non-fatal MI, transient ischaemic attack or stroke and hospitalisation for heart failure, when compared to control, although this study was not originally designed to test the effect of limb RIC on these clinical endpoints (Sloth et al., 2014). In this regard, the 5,200 STEMI CONDI2/ERIC-PPCI patient prospective study is currently investigating whether limb RIC (four 5-minute inflations and deflations of a cuff placed on the upper arm administered prior to PPCI) can reduce cardiac death and hospitalisation for heart failure at 12 months - the results for this study are expected in the Summer of 2019. Therefore, of all the ischaemic conditioning strategies, limb RIC has the most promise for improving clinical outcomes in STEMI patients.
New developments in RIC
The majority of experimental and clinical studies have investigated the effect of a single limb RIC stimulus against a single index acute IRI episode. However, Wei et al have shown that applying repeated episodes of limb RIC (daily for 1 month), could prevent adverse post-AMI LV remodelling, suggesting that prolonged episodes of limb RIC may have more chronic beneficial effects (Wei et al., 2011). The mechanisms underlying this chronic cardioprotective effect is not clear but may be due to it attenuating ongoing oxidative stress, apoptosis and inflammation. Repeated or chronic limb RIC is currently being tested in 2 clinical studies (DREAM trial [NCT01664611] and CRIC-RCT trial [NCT01817114]) of STEMI patients investigating whether repeated limb RIC (four 5-minute cycles of arm cuff inflations/deflations daily for 1 month) can prevent adverse post-AMI LV remodelling.
Challenges in translating ischaemic conditioning: Future directions
Despite extensive efforts over the last 30 years since ischaemic conditioning was first discovered, the path to translating cardioprotection into the clinical setting for patient benefit has been both challenging and disappointing. The reasons for this are manifold, and have been discussed extensively in the literature (Kloner and Rezkalla, 2004). In brief, they can be divided into: (1) lack of rigorous testing of the novel cardioprotective therapies in the experimental setting using reproducible and clinically-relevant animal models (Lecour et al., 2014); and (2) poorly designed clinical studies – this relates to patient selection, timing and dosing of the cardioprotective therapy, the clinical setting, failure to take into account co-morbidities and co-medications which interfere with cardioprotection, incorrect endpoint for assessing cardioprotection and so on (Hausenloy, 2013). Therefore, increased efforts are required to improve the pre-clinical assessment of novel cardioprotective therapies to ensure that only those which show the most robust and reproducible infarct-limiting effects are taken into the clinical setting for testing. This may be achieved through multicentre randomised blinded studies using a network of research centres of small and large animals to test the efficacy and the reproducibility of novel cardioprotective therapies before clinical testing, as put into action by the National Institute of Health CAESAR network (Lefer and Bolli, 2011; Jones et al., 2015). Furthermore, new therapeutic targets and signalling pathways need to be discovered both within and outside the cardiomyocyte (platelets, fibroblasts, endothelial cells, microvasculature inflammatory cells, pericytes and so on) (Hausenloy et al., 2017b). Similarly, innovative cardioprotective strategies should be pursued, including combination therapy directed to multiple targets, an approach which is likely to be more effective than a single-targeted approach (Hausenloy et al., 2017b).
Conclusions
After reperfusion, ischaemic conditioning remains the most powerful intervention for reducing MI size. Following its discovery over 30 years ago, ischaemic preconditioning has evolved into ischaemic postconditioning and remote ischaemic conditioning, advances which have greatly facilitated the translation of ischaemic conditioning into the clinical setting for patient benefit. Despite this, the clinical translation of this cardioprotective strategy from the experimental setting, has been challenging, and improvements are needed in both the pre-clinical assessment of novel cardioprotective therapies, and the design of clinical cardioprotection studies. Of the ischaemic conditioning strategies, limb RIC holds the most promise for STEMI patients treated by PPCI, and large clinical studies are underway to investigate whether this non-invasive virtually cost-free treatment can reduce cardiac death and hospitalisation for heart failure following AMI.
Acknowledgements
Derek Hausenloy was supported by the British Heart Foundation (FS/10/039/28270), Duke-National University Singapore Medical School, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021), and the National Research Foundation (NRF) Singapore (NRF-CRP13-2014-05). This article/publication is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
Mervyn Chan
1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore
Sauri Hernández-Reséndiz
1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore;
2National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore
Valeria Paradies
2National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore
Derek J Hausenloy
1Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore;
2National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore;
3National Heart Research Institute Singapore, National Heart Centre Singapore;
4Yong Loo Lin School of Medicine, National University Singapore, Singapore;
5The Hatter Cardiovascular Institute, University College London, London, UK;
6The National Institute of Health Research University College London Hospitals Biomedical Research Centre, London, UK;
7Barts Heart Centre, St Bartholomew’s Hospital, London, UK
Corresponding author
Derek J Hausenloy
Email:derek.hausenloy@duke-nus.edu.sg
In a new window | Download PPT
Figure 1: Time-line of major discoveries (red) and major clinical studies (blue) in the field of ischaemic conditioning. CABG, coronary artery bypass graft; IPC, Ischaemic preconditioning; RIPC, Remote ischaemic preconditioning; IPost, Ischaemic postconditioning; RIPerC, Remote Ischaemic perconditioning; RIPostC, Remote ischaemic postconditioning; STEMI, ST-segment elevation myocardial infarction.
In a new window | Download PPT
Figure 2: This figure depicts some of the major signalling pathways within the cardiomyocyte underlying ischaemic conditioning (ischaemic pre- and postconditioning as well as remote ischaemic conditioning). Pre- and post-conditioning generates such as autacoids (adenosine, bradykinin and opioids) which bind to G-protein coupled receptors and activate pro-survival signalling pathways – the Reperfusion Injury Salvage Kinase (RISK) pathway (involves Akt and Erk1/2), the cGMP-protein kinase G (PKG) pathway and the Survivor Activator Factor Enhancement (SAFE) pathway (involves TNF-1α and JAK-STAT). Remote ischaemic conditioning generates a cardioprotective signal in an organ or tissue away from the heart, which is then relayed to the myocardium via a neuro-hormonal pathway, where it activates signalling pathways within the cardiomyocyte common to ischaemic pre- and postconditioning. These pro-survival signalling pathways recruit mediators such as endothelial nitric oxide synthase (eNOS), glycogen synthase kinase (GSK)-3, protein kinase C-ε (PKC-ε) and the mitochondrial ATP-dependent potassium channel (KATP), which inhibit the opening of the mitochondrial permeability transition pore (MPTP).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10957 | 38 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA