Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Remote ischemic conditioning in ST-segment elevation myocardial infarction: an update
Time:2018-09-02
Number:8021
Jun Chong1, Heerajnarain Bulluck2,3, En Ping Yap4, Andrew F.W. Ho5,6, William A. Boisvert7, Derek J. Hausenloy1,2,4,6,8,9
Author Affiliations


- 1Barts Heart Centre, St Bartholomew’s Hospital, London, United Kingdom.
- 2Hatter Cardiovascular Institute, London, United Kingdom.
- 3Norfolk and Norwich University Hospital, Norwich, UK.
- 4National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 5Department of Emergency Medicine, Singapore General Hospital.
- 6Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore.
- 7Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA
- 8The National Institute of Health Research, University College London Hospitals Biomedical Research Centre, London, United Kingdom.
- 9Yong Loo Lin School of Medicine, National University Singapore, Singapore.
Conditioning Medicine, 2018. 1(5):233-242.
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often results are among the leading causes of death and disability in the world. As such, novel strategies are required to protect the heart against the detrimental effects of acute ischemia/reperfusion injury (IRI), in order to reduce myocardial infarct (MI) size and prevent the onset of HF. The endogenous cardioprotective strategy of remote ischemic conditioning (RIC), in which cycles of brief ischemia and reperfusion are applied to a tissue or organ away from the heart, has been reported in experimental studies to reduce MI size in animal models of acute IRI. In the clinical setting, RIC can be induced by simply inflating and deflating a cuff placed on the upper arm or thigh to induce brief cycles of ischemia and reperfusion, a strategy which has been shown to reduce MI size in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI). The results of the ongoing CONDI2/ERIC-PPCI trial are eagerly awaited, and will provide definitive answers with regards to the cardioprotective effect and clinical outcome benefits of RIC in STEMI.
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often results are among the leading causes of death and disability in the world. As such, novel strategies are required to protect the heart against the detrimental effects of acute ischemia/reperfusion injury (IRI), in order to reduce myocardial infarct (MI) size and prevent the onset of HF. The endogenous cardioprotective strategy of remote ischemic conditioning (RIC), in which cycles of brief ischemia and reperfusion are applied to a tissue or organ away from the heart, has been reported in experimental studies to reduce MI size in animal models of acute IRI. In the clinical setting, RIC can be induced by simply inflating and deflating a cuff placed on the upper arm or thigh to induce brief cycles of ischemia and reperfusion, a strategy which has been shown to reduce MI size in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI). The results of the ongoing CONDI2/ERIC-PPCI trial are eagerly awaited, and will provide definitive answers with regards to the cardioprotective effect and clinical outcome benefits of RIC in STEMI.
Introduction and background
Ischemic heart disease is the leading cause of morbidity and mortality in the world. This reflects the increased prevalence of cardiovascular risk factors including cigarette smoking, diabetes, hypertension and hypercholesterolemia. These conditions also predispose patients to peripheral vascular disease, cerebrovascular disease and renal disease, adding to disease complexity of the cardiovascular patient.
Up to 25% of ST-segment elevation myocardial infarcts (STEMI) are fatal (Lambert, et al., 2016). Survivors of the acute coronary thrombotic occlusion depend on timely revascularization with thrombolysis or primary percutaneous coronary intervention (PPCI) to abate infarction-induced lethal arrhythmias and cardiac arrest in the short term. In the long term, reduced ischemic time leads to smaller infarct size. However, despite timely PPCI or thrombolytic therapy, there remains significant morbidity and mortality following STEMI.
The ischemic insult to the myocardium is twofold: (1) at the time of coronary occlusion, and (2) at the time of reperfusion secondary to reperfusion injury. Reperfusion injury can be responsible for up to 50% of final infarct size (Hausenloy, 2013). Pump failure secondary to non-viable infarcted myocardium is one of the long-term sequelae of STEMI. The ensuing heart failure syndrome involves deleterious activation of the renin-angiotensin-aldosterone system and peripheral vasoconstriction, leading to sodium and water retention (with worsening heart failure) and left ventricular remodeling (hypertrophy, dilation and impaired cardiac function) in a patient already burdened with multiple morbidities as outlined.
To reduce the risk of short- and long-term complications of infarction, sophisticated and efficient systems are in place in many countries for prompt recognition and treatment of STEMI through thrombolysis or PPCI. Strategies employed have included ambulance initiation of thrombolysis and the development of designated centers providing direct PPCI services to respective catchment areas, bypassing the emergency department, with reduction in “pain-to-balloon” and “pain-to-thrombolysis” time. However, despite these measures, morbidity and mortality following PPCI or thrombolytic therapy remain significant.
Attenuating myocardial reperfusion injury, the cardiomyocyte death which occurs on reperfusing ischemic myocardium, is a potential therapeutic target for reducing infarct complications such as cardiac death and re-hospitalization for heart failure (HHF). Accessible and effective clinical interventions are required to address reperfusion injury and reduce associated complications. In this regard, remote ischemic conditioning (RIC) has been shown to reduce perioperative myocardial injury in patients undergoing coronary artery bypass graft (CABG) surgery in small studies, but the beneficial effects of RIC have not been reproduced in large clinical outcome studies. In contrast, RIC remains a promising cardioprotective strategy in STEMI patients undergoing PPCI. In this article, we review the therapeutic potential for RIC as a cardioprotective strategy for reducing MI size and improving clinical outcomes post-PPCI.
Remote ischemic conditioning – cardioprotection from a distance
Przyklenk et al. (1993) were the first to describe the cardioprotective phenomenon of remote ischemic preconditioning, where 4×5-minute cycles of occlusion and reflow to the circumflex coronary artery reduced MI size in a canine heart induced by 45-minute occlusion and 3 hrs reperfusion of the left anterior descending artery (Przyklenk et al.,1993). This study suggested that cardioprotection could be transferred from one coronary artery territory to another through ischemic preconditioning (Przyklenk et al.,1993). This concept was then extended to the remote organ, the kidney, by McClanahan et al., who showed that 10-minute occlusion and reflow in the renal artery could reduce MI size induced by 30-minute ligation and 3 hrs reperfusion of the left main coronary artery (McClanahan et al., 1993). Oxman et al. demonstrated that RIC could be applied non-invasively, using a tourniquet applied to the hindlimb (Oxman et al., 1997), a key finding in the translation of RIC into the clinical setting.
Two key properties of RIC have facilitated its translation into the clinical setting (see Figure 1 for a timeline of translation of RIC from experimental to clinical studies):
(1) Feasibility: In an experimental animal MI model, the RIC stimulus could be applied to the hindlimb to protect the heart against acute IRI (Oxman et al., 1997; Birnbaum & Hale, 1997). The RIC stimulus can be delivered non-invasively in human volunteers by inflating a blood pressure cuff on the upper arm to induce cycles of brief ischemia-reperfusion (Kharbanda & Mortensen, 2002). Hence, most clinical studies have applied RIC using cycles of brief ischemia-reperfusion in the upper or lower limb (limb RIC).
(2) Flexibility: In ischemic preconditioning (IPC), the protective stimulus has to be applied prior to ischemia and in ischemic postconditioning (IPost), at the onset of reperfusion to the heart directly. RIC can be applied at any time (before, after the onset of, or at the end of ischemia) to a remote organ or tissue.
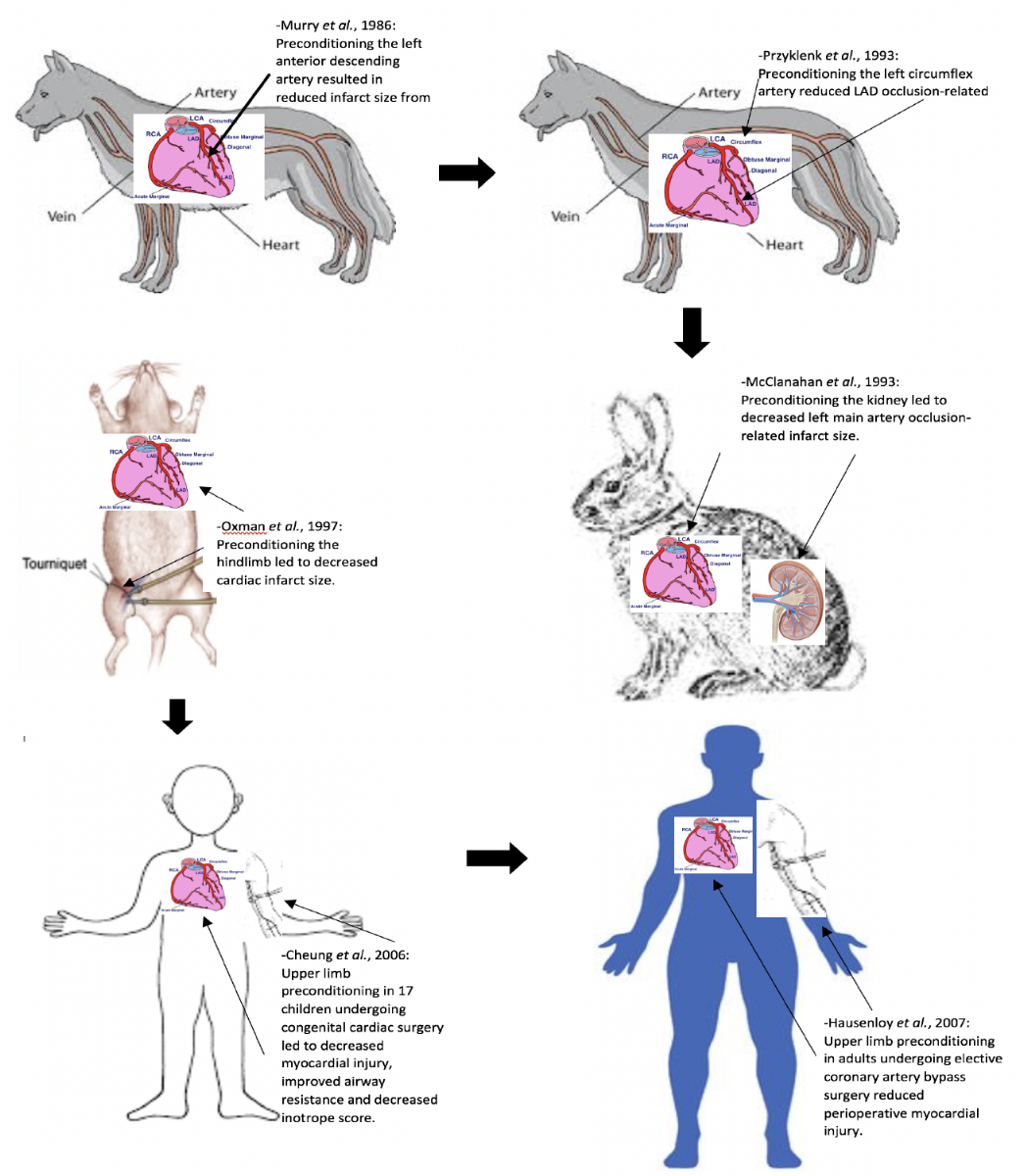
In a new window | Download PPT
Figure 1: This figure shows the timeline of translation of RIC from experimental to clinical studies.
Mechanisms underlying RIC cardioprotection
The actual mechanistic pathways underlying RIC cardioprotection are not known, but it has been established that a neurohormonal pathway links the distal organ or tissue to the heart. The pathway is believed to involve the release of local autacoids stimulating the sensory afferent neural pathway in the remote organ or tissue, resulting in the production of circulating transferrable blood-borne factor(s) conferring cardioprotection. Current evidence suggests the factor is thermolabile and hydrophobic, and is between 3.5 and 30 kDa. There is likely a complex interaction of signaling pathways in response to RIC, linking to the regulation of various cellular functions, including the acute phase response, immune response, hemostasis and lipid transport (Sivaraman & Hausenloy, 2015).
A number of candidate molecules have been suggested to be the blood-borne cardioprotective mediator of RIC, including opioid (Dickson et al., 2001), adenosine (Leung & Wang, 2014), bradykinin (Schoemaker, 2000), erythropoietin, calcitonin gene-related peptide, stromal derived factor 1-alpha (SDF1-α) (Davidson & Selvaraj, 2013), hypoxia inducible factor 1-alpha (HIF1-α) and nanoparticles called exosomes produced by cells (Giricz & Varga, 2014). Adenosine, bradykinin and calcitonin gene-related peptide (CGRP) may activate afferent neural pathways within the remote preconditioned organ to confer cardioprotection (Hausenloy & Yellon, 2008). Activation of protein kinase C appears to be an important step in humoral cardioprotection in rats (Serejo et al., 2007).
Naloxone appears to block the cardioprotective effect of RIC in rats (Patel et al., 2002). Endogenous opioids generated by remote preconditioning may be a humoral factor conferring cardioprotection (Patel et al., 2002). It has been proposed that endocannabinoids generated by intestinal ischemia may activate CB2 endocannabinoid receptors on the myocardium in cardioprotection (Hajrasouliha et al., 2008). Remote ischemic preconditioning (RIPC) appears to suppress the inflammatory response and activate an anti-inflammatory, anti-apoptotic gene transcription profile (Konstantinov et al., 2004; Konstantinov et al., 2005; Peralta et al., 2001). Further investigation of the relevance to cardioprotection is required. KATP channels of the myocardial sarcolemma and mitochondria have been implicated in IPC cardioprotection (Yellon, 2003). Ligand receptor binding at the cell surface activates signal transduction pathways which open mitochondrial KATP channels. The generation of mitochondrial reactive oxygen species then mediates cardioprotection by either activating pro-survival kinases (Yellon, 2003) or inhibiting mitochondrial permeability transition pore (mPTP) opening (Costa et al., 2006). ĸ-opioid agonist induces mPTP opening (Zhang et al., 2006). Remote rat limb preconditioning can mediate cardioprotection through ĸ-opioid receptor blockade (Zhang et al., 2006). 8-sulphophenyl theophylline (8-SPT), a non-specific adenosine receptor antagonist, could block the cardioprotective effect of RIC performed on the rabbit kidney if administered prior to preconditioning (Pell et al., 1998) and also after preconditioning (Takaoka et al., 1999). Elevated adenosine levels in carotid artery blood of rabbits subjected to preconditioning suggests that myocardial adenosine receptor binding is a key step in the mechanism of preconditioning (Takaoka et al., 1999). Free radical scavenger was able to block the cardioprotective effect of RIC (Weinbrenner et al., 2004), implicating signaling reactive oxygen species as a mediator of RIC cardioprotection. Transection of the femoral nerve before application of the RIC stimulus blocked cardioprotection (Lim & Yellon, 2010; Steensrud & Li, 2010). Ding and Zhang noted that brief renal artery occlusion was associated with increased afferent renal nerve activity, and nerve transection also blocked RIC-induced cardioprotection (Ding & Zhang, 2001). Direct stimulation of the sensory nerve of the remote organ or tissue has been reported to reproduce the cardioprotective effect of RIC (Dong et al., 2004; Merlocco & Redington, 2014; Redington & Disenhouse, 2013). Stimulation of cutaneous sensory nerves, using either topical application of capsaicin (Redington & Disenhouse, 2012) or surgical skin incision (Ren & Wang, 2004; Gross et al., 2011), has been reported to mimic RIC cardioprotection.
Clinical application of remote ischemic conditioning
Cardiac bypass surgery as a clinical setting for cardioprotection
The first clinical setting for RIC to be tested in was cardiac bypass surgery, in which the heart is subjected to a global ischemic insult when put onto cardiopulmonary bypass, followed by global reperfusion injury (acute IRI) when taken off cardiopulmonary bypass (Venugopal & Ludman, 2009). Direct handling of the heart, coronary embolization, and the inflammatory response to cardiopulmonary bypass can all contribute to perioperative myocardial injury (PMI), which can be quantified by measuring serum cardiac enzymes (creatine kinase MB isoenzyme, troponin T and I) (Croal & Hillis, 2006; Wang & Stewart, 2013), and can be detected as late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging (CMR) (Selvanayagam & Porto, 2005). The presence of PMI has been associated with worse clinical outcomes post-cardiac surgery (Croal & Hillis, 2006; Wang & Stewart, 2013).
The first attempt to clinically apply RIC involved only eight patients, in a study in which remote limb preconditioning failed to affect CK-MB in elective patients undergoing cardiac surgery (Gunaydin et al., 2000). This study was underpowered; CK-MB was measured 5 minutes after declamping the aorta; cuff inflation to 300 mmHg was used; and an inadequate RIPC protocol was used with two cycles of 3-minute upper limb ischemia followed by 2-minute reperfusion (Gunaydin et al., 2000).
Cheung et al. were the first to successfully apply RIPC clinically (Cheung et al., 2006). They reported that an RIPC protocol using four 5-minute cycles of lower limb ischemia was able to reduce myocardial injury, improve airway resistance, and decrease inotrope score in 17 children undergoing congenital cardiac surgery (Cheung et al., 2006).
Hausenloy et al. (2007) demonstrated that RIPC, using three 5-min cycles of upper limb ischemia, was able to reduce myocardial injury (43% reduction in serum troponin T released over 72 hours) in adult patients undergoing elective coronary artery bypass grafting surgery. RIPC using limb ischemia has also been reported to be cardioprotective in the setting of repair of abdominal aortic aneurysm (AAA) elective surgery (Ali et al., 2007). Ali et al. demonstrated that invasive lower limb ischemia using two 10-minute episodes of iliac artery occlusion was able to reduce myocardial injury (27% reduction in serum troponin I released over the perioperative period) and preserve renal function during elective AAA surgical repair (Ali et al., 2007).
The results of several recent meta-analyses have confirmed the cardioprotective effects of RIC cardiac bypass surgery in attenuating perioperative myocardial injury (Haji Mohd Yasin & Herbison, 2014; Healy & Khan, 2014). There have, however, been several neutral studies (Karuppasamy & Chaubey, 2011; Young & Dalley, 2012; Rahman & Mascaro, 2010) including at least one very large study (McCrindle & Clarizia, 2014).
RIPHeart study (Meybohm et al., 2015): 1403 adults undergoing elective cardiac surgery with cardiopulmonary bypass under general anesthesia with intravenous propofol were randomized to upper-limb RIPC or sham intervention. No significant differences between the RIPC group and the sham-RIPC group were seen in the level of troponin, the duration of mechanical ventilation, the length of stay in the intensive care unit, the length of hospital stay, incidence of new onset atrial fibrillation, or the incidence of postoperative delirium.
ERICCA study (Hausenloy et al., 2015a): 1612 patients undergoing elective on-pump CABG with or without valve surgery were randomly assigned to RIPC or sham conditioning. There was no standardization of anesthetic management and perioperative care. The combined primary endpoint was death from cardiovascular causes, nonfatal myocardial infarction, need for coronary revascularization, or stroke at 12 months from randomization. RIPC was not shown to improve clinical outcomes in patients undergoing elective on-pump CABG with or without valve surgery.
The reasons for this discrepancy may relate to: patient/clinical factors (CABG vs. valve surgery, stable vs. unstable patients); timing of the limb RIC protocol (before vs. after surgical incision); blinding to the RIC protocol (proper vs. limited blinding); the intensity of the RIC protocol (3 vs. 4 cycles of limb RIC and inflation of cuff to 200 mmHg vs. 15 mmHg above systolic blood pressure); and the presence of confounding factors.
Propofol and volatile anaesthetic agents – potential confounding factors
Attenuation of RIC has been noted when propofol anaesthesia has been used (Bautin et al., 2014), and the use of propofol, rather than volatile anaesthesia, appears to be a common factor in studies that failed to protect with RIC in CABG (Heusch, 2013; Zangrillo et al., 2015). The American College of Cardiology Foundation and the American Heart Association Task Force on Practice Guidelines have recommended the use of volatile anaesthetics in surgical patients with increased cardiovascular risk (Fleisher et al., 2008). Whether RIC provides additional cardioprotection to the use of volatile anaesthetic in patients undergoing cardiac surgery is uncertain, with clinical studies showing mixed results.
Diabetes may attenuate RIC cardioprotection through neurohumoral pathways
Single-dose RIC does not appear to offer much cardioprotection in diabetic patients (Xu et al., 2014; Baranyai et al., 2015; Epps & Smart, 2016; Lejay et al., 2016) and its mechanisms are not well understood. About 60% to 70% of people with diabetes mellitus will eventually develop the complication of diabetic peripheral neuropathy (Boulton et al., 2005). In many of these patients, sensory C fibers mediate the cardioprotective effect of RIC and are damaged (Green et al., 2010), and this may be an important contributor to the attenuation of RIC cardioprotection (Saxena et al., 2010; Jensen et al., 2012). It may be necessary to exclude patients with diabetic neuropathy or sensory neuropathy from future clinical trials in RIC. In addition, diabetes affects the intracellular signaling pathways that are crucial for endogenous cardioprotection. These include: PI3K/Akt/glycogen synthase kinase 3-beta (PI3K/Akt/GSK3-β) signaling pathway, phosphorylation of ERK1/2 (extracellular signal-regulated protein kinases 1 and 2), generation and release of nitric oxide, ATP-sensitive potassium channels, and oxidative stress generation (Chen et al., 2012; Baumgardt et al., 2016; Wang & Zhao, 2016). This may further contribute to the attenuation of RIC cardioprotection.
PPCI as a clinical setting for RIC cardioprotection
Timely myocardial reperfusion by PPCI is the most effective therapy for limiting MI size and preserving LV systolic function in patients presenting with STEMI. Restoration of coronary blood flow in the occluded artery results in myocardial reperfusion injury, which may be amenable to cardioprotection by IPost and RIC (Bulluck & Hausenloy, 2015).
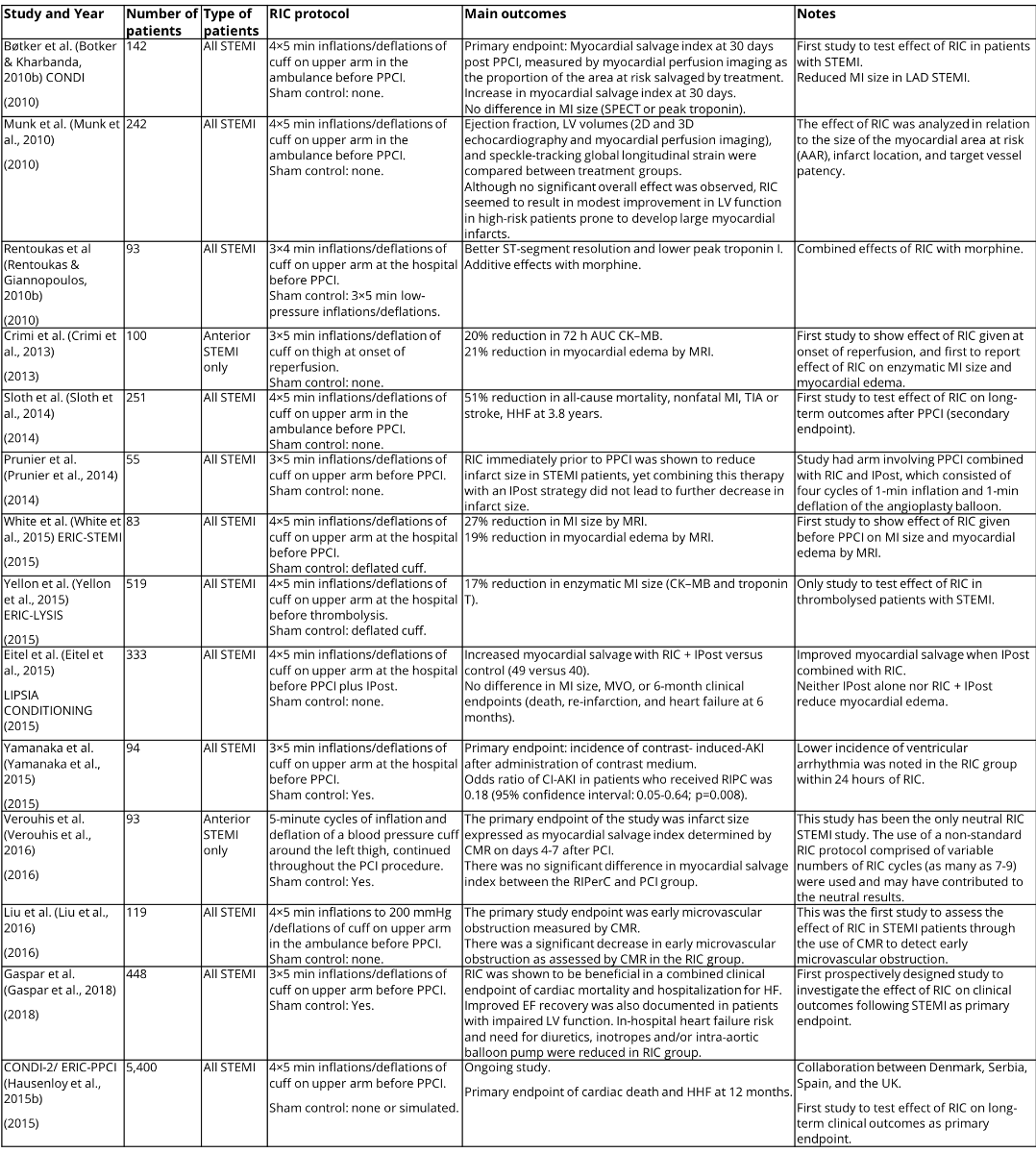
In several proof-of-concept studies, limb RIC appeared to be effective when administered by paramedics in the ambulance (Botker & Kharbanda, 2010a), on hospital arrival prior to PPCI (Rentoukas & Giannopoulos, 2010a; White & Frohlich, 2014), and even at the onset of reperfusion with PPCI (Crimi & Pica, 2013). Please see 1 for a summary of major clinical studies in STEMI patients.
Bøtker and Kharbanda (2010b) in the CONDI trial were the first group to test the effect of RIC in patients with STEMI. The study involved 142 STEMI patients with the primary endpoint of myocardial salvage index at 30 days post-PPCI, measured by myocardial perfusion imaging as the proportion of the area at risk salvaged by treatment. They found an increase in myocardial salvage index at 30 days with no difference in MI size measured by SPECT or in peak troponin. Reduced MI size was found, however, in LAD STEMI.
Crimi et al. (2013) then assessed the effect of RIC on 100 anterior STEMI patients and found a 20% reduction in 72-hour AUC CK–MB and a 21% reduction in myocardial edema by MRI. This was the first study to show the effect of RIC given at onset of reperfusion, and the first to report the effect of RIC on enzymatic MI size and myocardial edema.
It has been observed that the beneficial effect of RIPC can be inhibited by the opioid receptor blocker naloxone (Patel et al., 2002). Rentoukas and Giannopoulos (2010b) sought to assess the enhancement of the cardioprotective effect of RIC by opioids by having 3 arms in their study: an RIC-only group, an RIC and morphine group, and a control group. In paired comparisons between groups, the RIC and morphine group performed better than the control group in terms of both ST-segment reduction and peak troponin I, whereas the differences in outcomes between the RIC-only group and the control group did not reach statistical significance.
Munk et al. ( 2010) analyzed the effect of RIC in relation to the size of the myocardial area at risk (AAR), infarct location, and target vessel patency in a study involving 242 STEMI patients. Ejection fraction, LV volumes (2D and 3D echocardiography and myocardial perfusion imaging), and speckle-tracking global longitudinal strain were compared between treatment groups. Although no significant overall effect was observed, RIC seemed to result in modest improvement in LV function in high-risk patients prone to develop large myocardial infarcts.
Sloth et al. (2014) performed a study involving 251 STEMI patients. The RIC intervention was 4×5-min inflations/deflations of cuff on upper arm in the ambulance before PPCI without sham control. This showed a 51% reduction in all-cause mortality, nonfatal MI, TIA or stroke, and HHF at 3.8 years, and was the first study to test the effect of RIC on long-term outcomes after PPCI as a secondary endpoint.
Prunier et al. (2014) had a study arm involving PPCI combined with RIC and IPost which consisted of four cycles of 1-min inflation and 1-min deflation of the angioplasty balloon in their study consisting of 55 STEMI patients. RIC immediately prior to PPCI was shown to reduce infarct size in STEMI patients, yet combining this therapy with an IPost strategy did not lead to a further decrease in infarct size.
In the study by White et al. (2015), 197 patients with ST-segment elevation myocardial infarction with TIMI (thrombolysis in myocardial infarction) flow grade 0 were randomly assigned to receive RIC (four 5-min cycles of upper-arm cuff inflation/deflation) or control (uninflated cuff placed on upper arm for 40 min) protocols prior to PPCI. This study aimed to determine whether RIC could reduce myocardial infarct (MI) size, assessed by cardiac MRI, in patients presenting with ST-segment elevation myocardial infarction. The primary study endpoint was MI size assessed by cardiac MRI in 83 subjects on days 3 to 6 after admission. RIC was found to reduce MI size by 27%, when compared with the MI size of control subjects. At 24 hours, high-sensitivity troponin T was lower with RIC. RIC also reduced the extent of myocardial edema measured by T2-mapping CMR, and lowered mean T2 values. This precluded the use of CMR edema imaging to accurately estimate the area at risk. When using coronary angiography jeopardy scores to estimate the area at risk, RIC was found to significantly improve the myocardial salvage index. This study demonstrated that RIC performed in patients with STEMI treated by PPCI reduced MI size, increased myocardial salvage, and reduced myocardial edema when performed prior to PPCI.
Yellon et al. (2015) performed the ERIC LYSIS study, which is the only study to test effect of RIC in thrombolysed patients with STEMI. Five-hundred nineteen STEMI patients were randomized to 4×5 min inflations/deflations of cuff on upper arm at the hospital before thrombolysis or sham control with deflated cuff application. There was a 17% reduction in enzymatic MI size (CK–MB and troponin T) in the RIC group.
In the LIPSIA CONDITIONING study by Eitel et al. (2015) involving 333 STEMI patients, improved myocardial salvage was seen when IPost was combined with RIC. Neither IPost alone nor RIC + IPost reduced myocardial edema. No differences in MI size, MVO, or 6 month clinical endpoints (death, re-infarction, and heart failure at 6 months) were seen.
Renal outcomes were assessed by Yamanaka et al. (2015) in a 94-STEMI patient study with the primary endpoint being incidence of contrast-induced AKI after administration of contrast medium. The odds ratio of CI-AKI in patients who received RIPC was 0.18 (95% confidence interval: 0.05-0.64; p=0.008). Lower incidence of ventricular arrhythmia was also noted in the RIC group within 24 hours of RIC.
The study by Verouhis et al. (2016) has been the only neutral study of RIC in STEMI. The use of a non-standard RIC protocol comprised of variable numbers of RIC cycles (as many as 7-9) may have contributed to the neutral results. The primary endpoint of the study was infarct size expressed as myocardial salvage index determined by CMR on days 4-7 after PCI. There was no significant difference in myocardial salvage index between the RIPerC and PCI group.
Liu et al. (2016) performed the first study to assess the effect of RIC in STEMI patients through the use of CMR to detect early microvascular obstruction. The primary study endpoint was early microvascular obstruction measured by CMR. There was a significant decrease in early microvascular obstruction as assessed by CMR in the RIC group.
Most recently, Gaspar et al. found that RIC administered prior to PPCI improved clinical outcomes following STEMI with reduced rates of HHF (Gaspar et al., 2018). This is the first prospectively designed study to investigate the effect of RIC on clinical outcomes following STEMI as a primary endpoint. RIC was shown to be beneficial in a combined clinical endpoint of cardiac mortality and hospitalization for HF. Improved EF recovery was also documented in patients with impaired LV function. In-hospital heart failure risk and need for diuretics, inotropes and/or intra-aortic balloon pump were reduced in the RIC group.
In a large European multicenter study, the CONDI2/ERIC-PPCI trial (ClinicalTrials.gov identifier: NCT02342522) is investigating whether RIC initiated prior to PPCI can reduce the rates of cardiac death and hospitalization for heart failure at 12 months, the primary endpoint. It is a prospective, randomized-controlled trial of 5200 STEMI patients undergoing PPCI. Patients have been randomized to either RIC or sham control. Secondary endpoints include: (i) rates of cardiac death and heart failure hospitalization at 30 days; (ii) rates of all-cause death, coronary revascularization, re-infarction, and stroke at 30 days and at 12 months; (iii) TIMI flow post-PPCI; (iv) ST-segment resolution on ECG taken at 90 minutes; (v) enzymatic MI size as assessed from a 48-h area-under-the-curve (AUC) high-sensitive troponin T (hsTrop-T) using blood samples collected at 0, 6, 12, 24, and 48 hours in a sub-study; and (vi) MI size as measured by cardiac magnetic resonance (CMR) scan performed at 6 months in a sub-study. It is well established that RIC can reduce MI size in STEMI patients who have received PPCI. It is not known whether this beneficial effect translates to improved clinical outcomes. The results of the CONDI2/ERIC-PPCI study, which will be available in summer 2019, will establish whether limb RIC, as a non-invasive low-cost intervention, can improve long-term clinical outcomes in STEMI patients treated with PPCI.
Challenges and future directions
Several post-hoc analyses of RIC in STEMI studies have shed further insights. In the post-hoc analysis by Pryds et al. (2016a) assessing the influence of pre-infarction angina and coronary collateral blood flow (CCBF) on the effectiveness of RIC in STEMI patients, pre-infarction angina was found not to modify RIC efficacy in STEMI patients undergoing PPCI. CCBF to the infarct-related artery seemed to affect the cardioprotective efficacy of RIC, with mean myocardial salvage index (MSI) increased in patients with CCBF versus without CCBF in the RIC with PPCI group. Pryds et al. (2016b) also found, in a separate post-hoc analysis, that RIC as adjunct to PPCI attenuated the detrimental effect of healthcare system delay on myocardial salvage in patients with STEMI. In patients with healthcare system delay >120 min, RIC with PPCI increased median MSI compared with PPCI alone, suggesting that the cardioprotective effect of RIC increases with the duration of ischemia. Sloth et al. (2015) found no significant difference in the effectiveness of RIC in subgroups of cardiovascular risk factors, lipid and glucose levels, and medication use in their post-hoc analysis. Sloth et al. (2016) also performed a post-hoc analysis addressing the issue of cost-effectiveness of RIC in STEMI patients. They found that after 4 years of follow-up, mean cumulative cardiovascular medical care costs were lower in the RIC group than in the control group, while mean major adverse cardiac and cerebrovascular event-free survival time was 0.30 years higher in the RIC than in the control group. These results suggest that RIC in STEMI appears to be a cost-effective treatment strategy in patients with STEMI.
Daily RIC following STEMI
Chronic renal failure patients undergoing hemodialysis are subjected to repeated episodes of acute myocardial ischemia resulting in myocardial stunning and chronic LV systolic impairment (Crowley, 2013). Limb RIC has been reported to attenuate ST-segment depression and prevent myocardial stunning in these patients (Crowley, 2013).
One experimental study has demonstrated that performing limb RIC daily for a period of 28 days prevented adverse post-MI LV remodeling in the rat heart (Wei & Xin, 2011). This approach has been tested in the clinical setting in two studies.
DREAM (Vanezis et al., 2018): This trial assessed the role of daily RIC in improving left ventricular ejection fraction (LVEF) recovery in patients with reduced LVEF (<45%) after STEMI treatment with PPCI. Patients were recruited from four UK hospitals and randomized to receive either 4 weeks of daily RIC or sham conditioning commencing on day 3 post-PPCI. The primary endpoint was the improvement in LVEF over 4 months assessed by cardiac MRI. Seventy-three patients (38 cases, 35 controls) completed the study. There was no difference in the improvement in LVEF over 4 months between the treatment and control groups. No differences were seen in the secondary outcome measures of changes in infarct size and left ventricular end-diastolic and systolic volumes, major adverse cardiac and cerebral events, mean Kansas City Cardiomyopathy Questionnaire scores, or changes in N-terminal pro-brain natriuretic peptide levels. Daily RIC starting on day 3 and continuing for 4 weeks following P-PCI for STEMI did not improve LVEF as assessed by CMR after 4 months when compared with a matched control group. The failure to begin RIC immediately prior to PPCI may have contributed to the neutral results of this study, and in this regard, the ongoing CRIC-RCT trial (NCT01817114), which is initiating RIC prior to PPCI and administering RIC daily for one month, may provide further insights.
Table 1 - Major clinical studies of RIC in STEMI
Summary and conclusions
RIC provides an easily applied and very effective endogenous strategy for reducing MI size following acute IRI. Despite extensive studies, the actual mechanistic pathway underlying RIC cardioprotection remains unclear; although a neurohormonal pathway is believed to be critical, the exact interplay between the neural and hormonal pathway is yet to be determined, and the identity of the cardioprotective humoral factors remains unknown. RIC has been successfully tested in a number of clinical settings including CABG surgery, elective PCI and more recently and most promisingly in STEMI patients undergoing PPCI. The translation of RIC into patient benefit has been elusive for CABG surgery patients, and this failure may be attributed to insufficient information on the optimum RIC protocol, the effects of co-medications of RIC cardioprotection such as propofol anesthesia and use of nitrates, and the presence of age and co-morbidities such as diabetes. RIC has the most promise for STEMI patients undergoing PPCI. The results of the CONDI2/PPCI study, due in Summer 2019, are eagerly awaited and will provide the definitive answer as to whether RIC can improve clinical outcomes and change clinical practice.
Disclosures/conflicts
The authors declare they have no conflicts of interest.
Acknowledgements
This project was funded by NIH grant R01HL81863 to WAB. DJH is supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021) and the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action.
References
Jun Chong1
1Barts Heart Centre, St Bartholomew’s Hospital, London, United Kingdom.
Heerajnarain Bulluck 2,3
2Hatter Cardiovascular Institute, London, United Kingdom.
3Norfolk and Norwich University Hospital, Norwich, UK.
En Ping Yap 4
4National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
Andrew F.W. Ho5,6
5Department of Emergency Medicine, Singapore General Hospital.
6Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore.
William A. Boisvert7
7Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA.
Derek J. Hausenloy1,2,4,6,8,9
1Barts Heart Centre, St Bartholomew’s Hospital, London, United Kingdom.
2Hatter Cardiovascular Institute, London, United Kingdom.
4National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
6Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore.
8The National Institute of Health Research, University College London Hospitals Biomedical Research Centre, London, United Kingdom.
9Yong Loo Lin School of Medicine, National University Singapore, Singapore.
Corresponding author:
Professor Derek J. Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg
Jun Chong and Heerajnarain Bulluck contributed equally to this article.

In a new window | Download PPT
Figure 1: This figure shows the timeline of translation of RIC from experimental to clinical studies.
Table 1 - Major clinical studies of RIC in STEMI

Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8021 | 36 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA