International bi-monthly journal of cell signaling, tissue protection, and translational research.
Influence of continuous electrical stimulation on development of human cardiomyocytes from induced pluripotent stem cells
Damián Hernández1,2,3, Rodney Millard4,5, Priyadharshini Sivakumaran1,3, Anne M Kong1, Geraldine M Mitchell1,2,6, Alice Pébay2,3, Robert K Shepherd4,5, Gregory J Dusting2,3, Shiang Y Lim1,2
Author Affiliations


- 1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia
- 2Departments of Medicine and Surgery, University of Melbourne, East Melbourne, Victoria, Australia
- 3Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, Victoria, Australia
- 4Bionics Institute, East Melbourne, Victoria, Australia
- 5Medical Bionics Department, University of Melbourne, Parkville, Victoria, Australia
- 6Faculty of Health Sciences, Australian Catholic University, Fitzroy, Victoria, 3065, Australia
Abstract
Regeneration of cardiac tissue remains an ideal approach to restore cardiac function after myocardial infarction. The ability of human induced pluripotent stem cells (iPSCs) to differentiate into bona fide cardiomyocytes also provides a platform for cardiac disease modeling, drug discovery and pharmacological safety testing of new drugs. One of the major limitations for the use of cardiomyocytes derived from iPSCs is that they resemble fetal cardiomyocytes and are immature compared to adult cardiomyocytes. Considering that the developing heart grows in an electric field, we investigated whether electrical stimulation can promote maturation of cardiomyocytes derived from human iPSCs. Two-dimensional cultures of immature cardiomyocytes at day 22 post-differentiation were subjected to continuous electrical stimulation at 200 mV/mm for 7 days using a custom-made electrical stimulator. This long-term electrical stimulation significantly increased the percentage of cardiomyocytes with organized sarcomeres and promoted alignment of cardiomyocytes parallel to the electric field. Electrical stimulation also decreased the circularity index of cardiomyocytes suggesting a more rod-like morphology. In conclusion, long-term continuous electrical stimulation promotes maturation of cardiomyocytes derived from human iPSCs. Mature cardiomyocytes can better recapitulate the pathophysiological conditions of the human heart for more accurate disease modeling and drug testing. Mature cardiomyocytes can also provide a substrate for cardiac regeneration and repair by tissue engineering in the future.
Keywords: induced pluripotent stem cells, cardiomyocytes, electrical stimulation, maturation
Abstract
Regeneration of cardiac tissue remains an ideal approach to restore cardiac function after myocardial infarction. The ability of human induced pluripotent stem cells (iPSCs) to differentiate into bona fide cardiomyocytes also provides a platform for cardiac disease modeling, drug discovery and pharmacological safety testing of new drugs. One of the major limitations for the use of cardiomyocytes derived from iPSCs is that they resemble fetal cardiomyocytes and are immature compared to adult cardiomyocytes. Considering that the developing heart grows in an electric field, we investigated whether electrical stimulation can promote maturation of cardiomyocytes derived from human iPSCs. Two-dimensional cultures of immature cardiomyocytes at day 22 post-differentiation were subjected to continuous electrical stimulation at 200 mV/mm for 7 days using a custom-made electrical stimulator. This long-term electrical stimulation significantly increased the percentage of cardiomyocytes with organized sarcomeres and promoted alignment of cardiomyocytes parallel to the electric field. Electrical stimulation also decreased the circularity index of cardiomyocytes suggesting a more rod-like morphology. In conclusion, long-term continuous electrical stimulation promotes maturation of cardiomyocytes derived from human iPSCs. Mature cardiomyocytes can better recapitulate the pathophysiological conditions of the human heart for more accurate disease modeling and drug testing. Mature cardiomyocytes can also provide a substrate for cardiac regeneration and repair by tissue engineering in the future.
Keywords: induced pluripotent stem cells, cardiomyocytes, electrical stimulation, maturation
Introduction
Cell culture models using human cardiomyocytes are useful for studying cardiac diseases and for drug testing. Whereas primary adult cardiomyocytes isolated from patients would be the ideal choice for such in vitro assays, the scarcity of healthy and diseased human heart samples and the difficulties in maintaining primary cardiomyocytes in culture have prevented their routine application for high throughput studies. Pluripotent stem cells, especially induced pluripotent stem cells (iPSCs), which are generated by reprogramming adult somatic cells (Takahashi and Yamanaka, 2006; Yu et al., 2007), can provide an abundant source of functional human cardiomyocytes for high throughput studies required for drug development. This process generates autologous patient-specific stem cells that can subsequently produce cardiomyocytes on a large scale with potential to provide patient-specific drug responses. However, cardiomyocytes derived from pluripotent stem cells are known to be immature and exhibit foetal-like morphology and electrophysiological properties (Keung et al., 2014; Bedada et al., 2016). This immaturity has limited their application in cardiac disease modeling, drug screening and cardiac repair. For example, immature cardiomyocytes have pro-arrhythmic properties characterized by spontaneous beating, shorter action potential, prominent phase 4-like depolarization, depolarized resting membrane potential, smaller Ca2+ transient amplitude, and lower upstroke rate (Liao et al., 2010). Current in vitro approaches to promote maturation of cardiomyocytes include culturing for months (Lundy et al., 2013), hormonal treatment with the thyroid hormone tri-iodo-L-thyronine (Yang et al., 2014b), mechanical loading (Nguyen et al., 2013; Mihic et al., 2014), and electrical stimulation for various durations (Chan et al., 2013; Lieu et al., 2013; Nunes et al., 2013; Hirt et al., 2014; Eng et al., 2016; Richards et al., 2016; Ruan et al., 2016).
Electrical signals are known to play an important role during embryogenesis and development of the heart. Previous studies have reported the ability of externally applied electrical stimulation to promote cardiac differentiation of different cell types (Hernandez et al., 2016). However, how electrical stimulation affects cardiomyocytes derived from human pluripotent stem cells remains unclear (Chan et al., 2013; Lieu et al., 2013; Nunes et al., 2013; Hirt et al., 2014; Eng et al., 2016). Most studies have used 3-dimensional cultures of immature cardiomyocytes, either as spheroids (Chan et al., 2013; Eng et al., 2016; Richards et al., 2016) or as engineered heart tissues (Nunes et al., 2013; Hirt et al., 2014; Ruan et al., 2016; Ronaldson-Bouchard et al., 2018), to demonstrate effects of electrical stimulation on maturation (Table 1). Three-dimensional cultures mimic the cardiac microenvironment of real myocardium. They also allow maturation of cardiomyocytes partly due to increased mechanical stretch, which induces better cell alignment (McDonald et al., 1972); Veerman et al., 2015; Ruan et al., 2016). In order to study the effect of electrical stimulation in isolation, we examined the effect of long-term electrical stimulation on maturation of 2-dimensional cultured cardiomyocytes derived from human iPSCs using a custom-made electrical stimulation system (Figure 1A).
Table 1: Effect of electrical stimulation on the maturation of human cardiomyocytes derived from pluripotent stem cells.
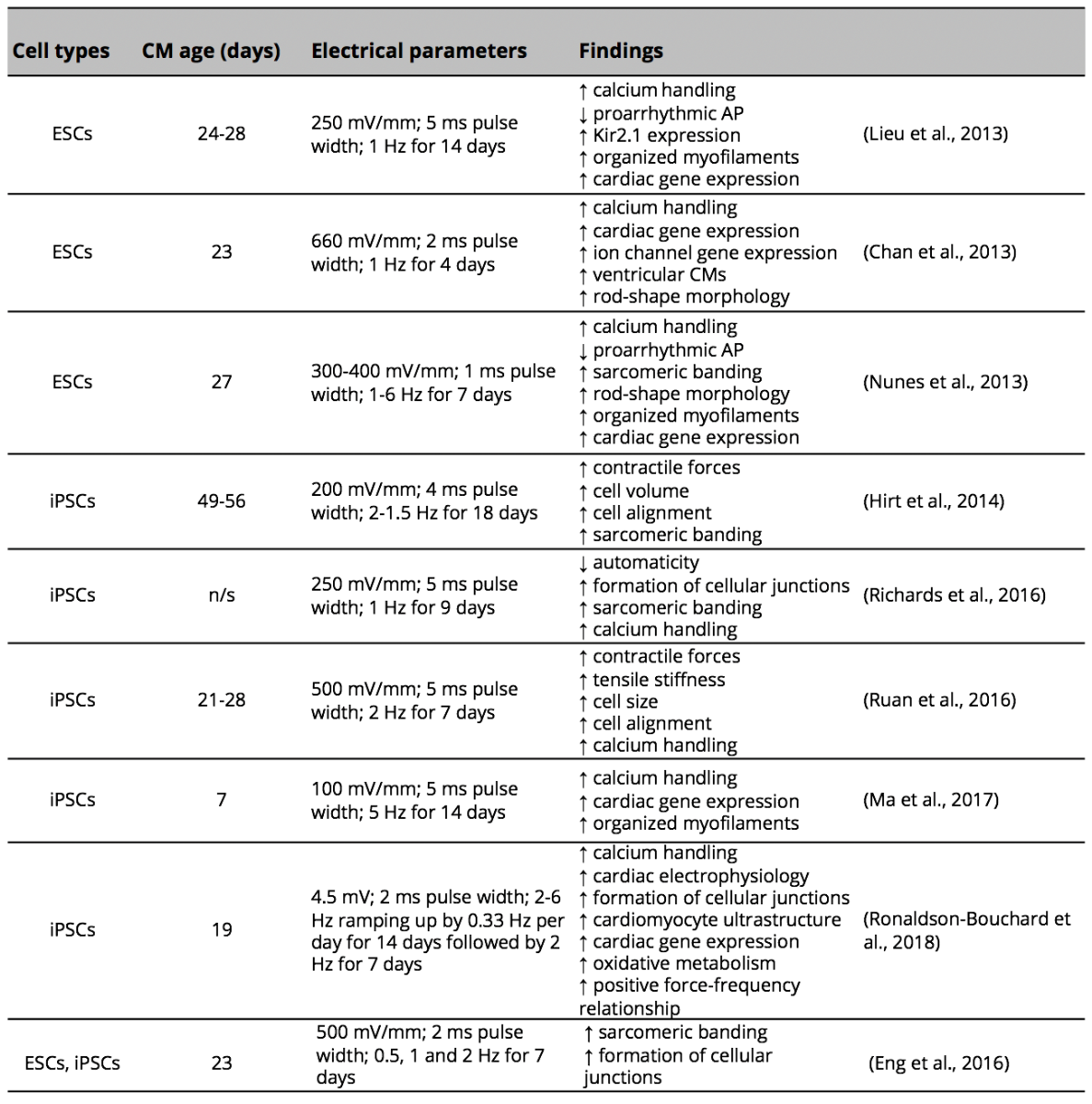
CM (cardiomyocytes); AP (action potential); ESC (embryonic stem cells); iPSCs (induced pluripotent stem cells); n/s (not specified).
Materials and Methods
Human iPSC culture and cardiac differentiation
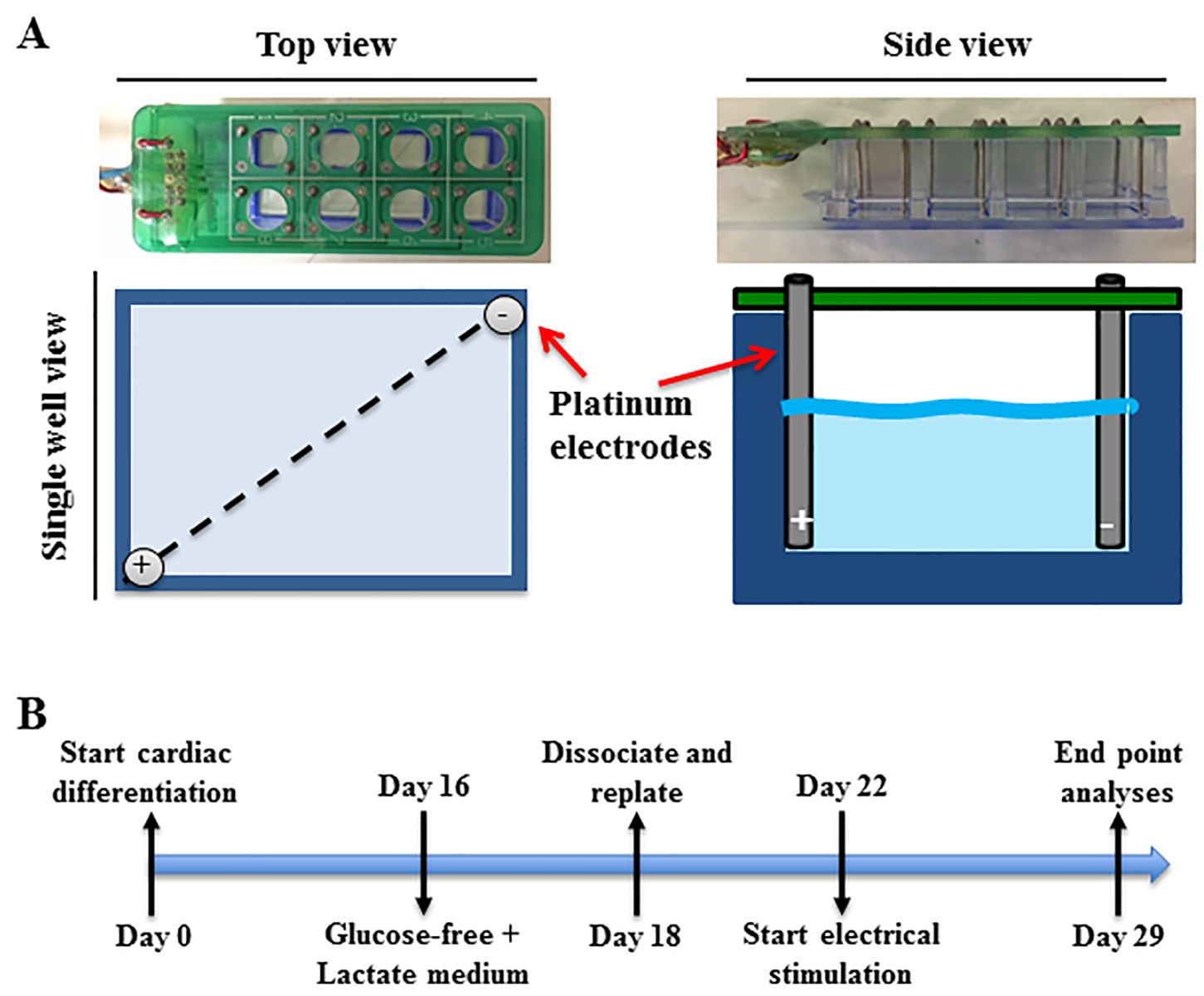
The CERA007c6 iPSC line used here was previously generated using skin fibroblasts from a 36-year-old healthy male by the episomal method as described previously (Hernandez et al., 2016). Human iPSCs were maintained and propagated on vitronectin-coated plates in TeSR-E8 medium (Stem Cell Technologies, Vancouver, Canada) according to the manufacturer’s protocol. To promote cardiac differentiation, iPSCs maintained on feeder-free culture were dissociated into single cells with TrypLE (Thermo Fisher Scientific, VIC, Australia) and seeded onto a Matrigel (Corning, Tewksbury, MA, USA) coated plate at a density of 1 x 105 cells/cm2 in TeSR-E8 medium supplemented with 10 µM Y-27632 (Tocris Bioscience, Bristol, UK). After 24 hours, at day 0 of cardiac differentiation, iPSC were treated with TeSR™-E8™ medium containing Matrigel (hESC-qualified Matrix, 1:60 dilution) and 10 μM of CHIR99021 (Cayman Chemical, Ann Arbor, MI, USA) for 24 hours. Subsequently, media were replaced with RPMI 1640 basal medium containing B-27 without insulin supplement (Thermo Fisher Scientific, VIC, Australia) and 100 ng/mL of Activin A (R&D Systems, Minneapolis, MN, USA). After 24 hours, media were replaced with RPMI 1640 basal medium containing B-27 without insulin supplement, 10 ng/mL of BMP4 (Peprotech, Rocky Hill, NJ, USA), 10 ng/mL basic fibroblast growth factor (Merck Millipore, Burlington, MA, USA) and 5 μM IWP2 (Tocris Bioscience, Bristol, UK) for 4 days. Following this, cells were cultured in RPMI 1640 basal medium containing B-27 supplement (Thermo Fisher Scientific, VIC, Australia). The medium was changed every 2-3 days (Figure 1B).
Long-term electrical stimulation
At day 16 post-differentiation, cardiomyocytes derived from human iPSCs were washed twice with phosphate-buffered saline and cultured in glucose-free medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 4 mM lactate (Sigma-Aldrich, St. Louis, MO, USA) for 2 days to enrich for cardiomyocytes. Cardiomyocytes were then dissociated using Accutase (Stem Cells Technologies, Vancouver, Canada) for 5 minutes at 37 °C, centrifuged at 200 g for 5 minutes, and pre-plated on non-coated plates for 20 minutes to further enrich for cardiomyocytes. The unattached cells were then collected and re-plated onto Matrigel (1:20 dilution) coated 8-well chamber slides at 90,000 cells/cm2 and cultured in RPMI 1640 basal medium containing B-27 supplement for 4 days before being subjected to electrical stimulation for 7 days (Figure 1B) using a custom made electrical stimulator. The electrical stimulator consisted of 16 platinum electrodes fitted into an 8-well chamber slide (BD Falcon, MA, USA) (Figure 1A). Each well contained an anode and cathode that were connected to an electrical stimulator that generated a charge-balanced biphasic current pulse with an electric field of 200 mV/mm at 1 Hz frequency and 1 ms pulse width. Electrical stimulation was for the most part continuous, with the only interruption being no more than 10 minutes when media were changed every second day. Control cells were subjected to the same procedure but without electrical stimulation.
Immunocytochemistry
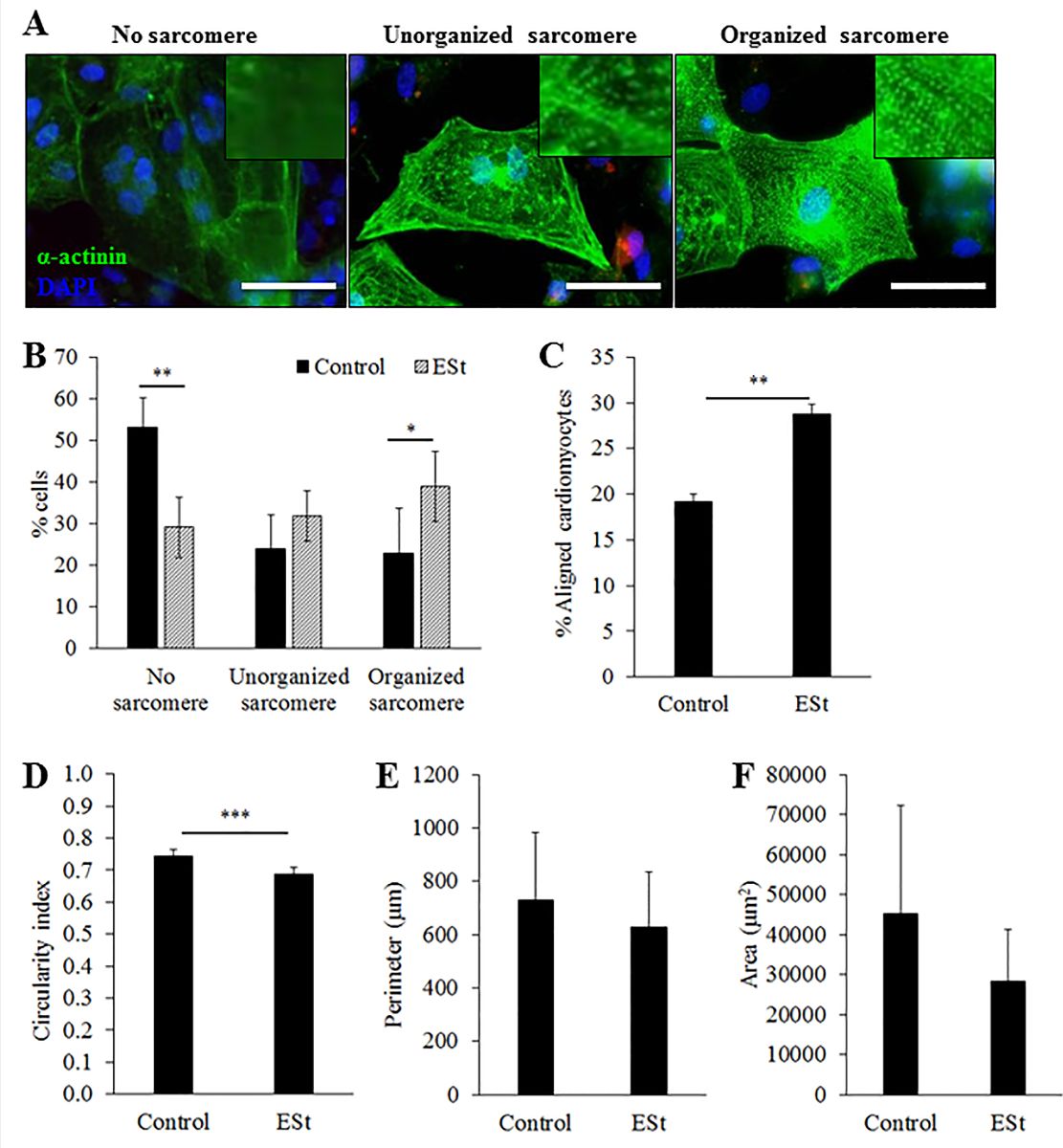
At the end of the electrical stimulation, cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. After blocking with serum-free blocking solution (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min, cells were incubated with a primary antibody against sarcomeric α-actinin (25 µg/mL, mouse monoclonal IgG; Sigma-Aldrich, St. Louis, MO, USA) and counterstained with 4’,6-diamidino-2-phenylindole (1 µg/mL; Thermo Fisher Scientific, Waltham, MA, USA) for nuclear staining. Images were taken using a BX-61 Olympus fluorescence microscope (Tokyo, Japan) and cell morphology was analysed using Image J software (National Institute of Health, Bethesda. MD, USA). Using the “Polygon selection” tool, the perimeter of cells positive for α-actinin was manually traced. The shape of the cardiomyocytes was quantified using the perimeter, area and circularity measurement functions of Image J. The more rounded the cells the closer the circularity index was to 1, whereas the more elongated the cells were the closer the circularity index was to 0. The sarcomeric structure of cardiomyocytes immunostained with α-actinin was classified into three categories: 1) no distinct sarcomere, 2) disorganized sarcomere, and 3) organized sarcomere (Figure 2A). A total 135 and 413 cardiomyocytes were measured from 3 independent experiments in control and electrical stimulated groups, respectively. Cell orientation was calculated as an angle of the direction of cell nuclei (the longest diameter) against the electric field (a reference line from electrode to electrode). The cells with angle ± 20 degree from the electric field were defined as aligned cells.
Real time quantitative PCR (RT-qPCR)
RNA was extracted from cells using TriReagent (Thermo Fisher Scientific, Waltham, MA, USA) followed by RNA precipitation with chloroform and isopropanol (Sigma-Aldrich, St. Louis, MO, USA). cDNA was synthesised using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). qPCR was carried out using TaqMan Universal master mix, the 7900HT Fast Real-Time PCR system, and TaqMan gene expression assays (Applied Biosystems, Foster City, CA, USA) for 18S (Hs99999901_s1), ACTC1 (Hs01109515_m1), TNNT2 (Hs01109515_m1), TNNI3 (Hs00165957_m1), MYH6 (Hs01101425_m1), MYH7 (Hs00165276_m1), MYL2 (Hs00166405_m1), MYL7 (Hs01085598_g1), CACNA1C (Hs00167681_m1), SCN2A (Hs01109877_m1), HCN2 (Hs00606903_m1), HCN4 (Hs00975492_m1), KCNJ2 (Hs01876357_s1), GJA1 (Hs00748445_s1), NPPA (Hs00383230_g1), and RYR2 (Hs00892883_m1). All readings were performed in duplicate. Relative quantitation was calculated by applying the comparative CT method (2-ΔΔCt), whereby the mRNA expression levels were normalized against the level of the housekeeping human gene 18s (ΔCt) with the level of candidate genes in control samples used as the reference (ΔΔCt).
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism software with Student’s t-test. Values of P < 0.05 were considered statistically significant.
Results
Optimization of the electrode array system for long-term electrical stimulation
The custom-made electrode array was suitable for continuous long-term stimulation inside the humidified 5% CO2 incubator at 37 ºC. The array consisted of two main parts. The first part was the electrode array base and the other part was the electrode. The electrode array base was a pre-printed circuit board that organized the 8-pairs of biocompatible platinum electrodes and connected through cables to the electrical stimulator located outside the incubator (Figure 1A). Noble metals such as platinum, gold, iridium, palladium and rhodium have been commonly used for electrical stimulation of cells and tissues for their relative resistance to corrosion (Merrill et al., 2005). However, some noble metals like gold do exhibit some corrosion and evidence of long-term toxic effects (Merrill et al., 2005). Platinum electrodes are biocompatible with cochlear implants and are used with different cells and tissues (Chouard and Pialoux, 1995; Merrill et al., 2005). Our platinum electrodes appeared to be non-cytotoxic and efficient for 7 days of electrical stimulation of cardiomyocytes derived from iPSCs. The humidity inside the incubator can alter the pre-printed circuit flow of the electrode array base by bridging electrodes through condensed water droplets. To avoid this phenomenon, a conformal coating was applied to the pre-printed board as this coating is biocompatible and resistant to different conditions such as temperature and moisture.
In a new window | Download PPT
Figure 1: Electrical stimulator and experimental timeline. (A) An electrode array containing a panel of 16 platinum electrodes that fit into an 8-well chamber slide, with each well containing a pair of electrodes. (B) A schematic of the experimental protocol from cardiac differentiation of human induced pluripotent stem cells to long-term electrical stimulation of the derived cardiomyocytes. Control cells were subjected to the same procedure but without electrical stimulation.
Effect of chronic electrical stimulation on morphology of cardiomyocytes derived from human iPSCs
Morphological analysis was performed after 7 days of continuous electrical stimulation. Electrical stimulation significantly increased the percentage of cardiomyocytes with organized sarcomeres when compared to controls (39 ± 8% vs. 23 ± 11%, p < 0.05, n = 3, Figure.2B), and increased the percentage of cardiomyocytes aligned to the electrical field (29 ± 1% vs. 19 ± 1%, p < 0.05, n = 3, Figure 2C). Electrically stimulated cardiomyocytes that stained positive for the cardiac sarcomeric α-actinin showed a significant reduction in their circularity index compared to control (0.69 ± 0.02 vs. 0.74 ± 0.02, p < 0.05, n = 3, Figure 2D), indicating a more rod-shape morphology. However, cell size measured by perimeter (Figure. 2E) and area (Figure. 2F) was not significantly affected by electrical stimulation for 7 days.
In a new window | Download PPT
Figure 2: Morphology and alignment of cardiomyocytes after 7 days of electrical stimulation. (A) Representative images of the three categories of sarcomere organization of cardiomyocytes. Scale bar = 50 µm. (B) Percentage of cardiomyocytes in each category of sarcomere organization. (C) Percentage of aligned cardiomyocytes. (D-F) Circularity (D), perimeter (E) and surface area (F) of cardiomyocytes with or without electrical stimulation (n = 3 independent experiments). Data are expressed as mean ± SEM. ** p < 0.01 and *** p < 0.001 by Student’s t-test. ESt = electrical stimulation.
Gene expression of cardiomyocytes derived from human iPSC after long-term electrical stimulation
To examine the maturation level of iPSC-cardiomyocytes, the expression of marker genes was examined at the end of the electrical stimulation period. The mRNA expressions of cardiac contractile muscle proteins (ACTC1, TNNT2, TNNI3, MYH6, MYH7, MYL2 and MYL7, Figure 3A), ion channels (CACNAIC, SCN2A, HCN2, HCN4 and KCNJ2, Figure 3B), the gap junctional protein (GJA1, Figure 3C), natriuretic peptide A (NPPA, Fig. 3C), and cardiac ryanodine receptor-2 (RYR2, Figure. 3C) were not changed significantly in electrically stimulated cardiomyocytes when compared to control. Moreover, the gene ratio of beta myosin heavy chain (MYH7) to alpha myosin heavy chain (MYH6) was similar between groups (0.76 ± 0.11 in control versus 0.65 ± 0.08 in electrical stimulated cells, p > 0.05, n = 4).

In a new window | Download PPT
Figure 3: Gene expression of cardiac markers and ion channels in cardiomyocytes after 7 days of electrical stimulation. mRNA expression of cardiac contractile muscle proteins (A), ion channels (B), as well as gap junctional protein, natriuretic peptide A, and ryanodine receptor-2 (C) in cardiomyocytes with or without electrical stimulation (n = 4 independent experiments). Data are expressed as mean ± SEM. ESt = electrical stimulation.
Discussion
This study demonstrates that long-term electrical stimulation of cardiomyocytes derived from human iPSCs induces a shift in phenotype development from an immature towards an adult-like cardiomyocyte by promoting cell alignment, sarcomere organization, and elongation of the cardiomyocytes. This positive effect of electrical stimulation on the maturation of cardiomyocytes derived from human iPSCs is in agreement with previous studies employing different duration and parameters of electrical stimulation to promote maturation of young and immature cardiomyocytes derived from pluripotent stem cells (Chan et al., 2013; Lieu et al., 2013; Nunes et al., 2013; Eng et al., 2016) (Table 1).
During cardiac development, cardiomyocytes undergo a series of structural changes before acquiring their mature adult phenotype. Cardiac maturation relies on a balance of intrinsic and extrinsic mechanical loads that regulate cell size, protein synthesis, sarcomere organization, contractile activity, as well as cell-cell and cell-extracellular matrix interaction (Yang et al., 2014a; Zhu et al., 2014). The maturity of cardiomyocytes derived from pluripotent stem cells can be assessed by characterizing their morphology. While the cell size has an important role in impulse propagation, the maximal rate of action potential depolarization, and total contractile force, the shape has an important role in the excitation-contraction coupling of cardiomyocytes (Spach et al., 2004; Yang et al., 2014a). Adult cardiomyocytes are rod-shaped with a length to width ratio of 7 to 9.5, and are aligned relative to cardiac tissue (Gerdes et al., 1992). In the present study, electrically stimulated cardiomyocytes have more elongated rod-shape morphology than non-stimulated cardiomyocytes indicated by a modest but significant reduction in the circularity index. However, the cell size measured by perimeter and area was not changed among groups. These results are supported by previous studies that have shown an increased number of cardiomyocytes derived from human embryonic stem cells (Chan et al., 2013; Nunes et al., 2013; Eng et al., 2016) and iPSCs (Hirt et al., 2014; Eng et al., 2016) with an elongated rod-shape morphology after long-term electrical stimulation. Studies using 3-dimensional tissue culture conditions also reported an increase in cardiomyocyte alignment (Nunes et al., 2013; Hirt et al., 2014; Ruan et al., 2016), which would be expected by the 3-dimenional topographic structure regardless of the presence of electrical stimulation. Our study using 2-dimensional cultured cardiomyocytes derived from human iPSCs supports the positive effect of long-term electrical stimulation in promoting alignment of cardiomyocytes to the electric field.
The contractile apparatus differs between immature and adult cardiomyocytes. The sarcomere is the functional unit for cardiomyocyte contraction. Therefore, the expression of sarcomeric proteins such as cardiac troponin T, cardiac troponin I, α-actinin and myosin heavy chain can be assessed to determine the degree of maturation of cardiomyocytes (Yang et al., 2014a). As cardiomyocytes develop, the sarcomere becomes more organized and sarcomere length increases to facilitate generation of force (Lundy et al., 2013). Here, we found that 7 days of electrical stimulation significantly increased the percentage of cardiomyocytes with an organized sarcomere when compared to controls. Similarly, improved sarcomere banding with electrical stimulation was reported in cardiomyocytes derived from human embryonic stem cells and iPSCs in a 3-dimensional culture format (Hirt et al., 2014; Eng et al., 2016; Richards et al., 2016).
Maturation of cardiomyocytes derived from pluripotent stem cells has been shown to be accompanied by increased expression of genes involved in sarcomere structures such as sarcomeric structural proteins α-actinin (ACTC1), cardiac troponin T (TNNT2), troponin I (TNNI3) and myosin regulatory light chain-2 (MYL2) (Lieu et al., 2013; Schwan and Campbell, 2015; Ma et al., 2017), as well as those involved in calcium handling (ryanodine receptor and sarco/endoplasmic reticulum calcium-ATPase) (Ivashchenko et al., 2013; Lundy et al., 2013), ion channels such as calcium voltage-gated channel subunit alpha-1C (CACNA1C) (Ivashchenko et al., 2013; Lundy et al., 2013), sodium voltage-gated channel subunit alpha-5 (SCN5A) (Ivashchenko et al., 2013), potassium voltage-gated channel subfamily J member 2 (KCNJ2) (Lieu et al., 2013; Nunes et al., 2013), and hyperpolarization-activated cyclic nucleotide-gated 4 (HCN4) (Lundy et al., 2013). On the other hand, changes in gene expression of other cardiac markers such as MYH6, MYH7, HCN4 and GJA1 as cardiomyocytes derived from iPSCs acquire a more mature phenotype in culture varies across studies (Ivashchenko et al., 2013; Kamakura et al., 2013; Lundy et al., 2013; Nunes et al., 2013; Ma et al., 2017). These variations can be attributable to the heterogeneity of cardiac subtypes and differences in maturation protocols. Although the profile of gene expression in mature cardiomyocytes is not completely defined, no difference was noted in the present study and further analysis at the protein level is warranted. Future studies could also test the effect of electrical stimulation with different combinations of electrical fields (500-1000 mV/mm), as well as frequency and durations. Supporting the use of different frequency of electrical stimulation to promote maturation of cardiomyocytes are recent studies reporting a positive effect when electrical stimulation was at higher frequencies. In these studies, electrical stimulation at frequencies of 2 to 6 Hz, but not at 1 Hz, significantly promoted the maturation of cardiomyocytes derived from pluripotent stem cells as demonstrated by a higher degree of sarcomere organization, rod-shape morphology, improved calcium handling, higher expression of cardiac genes and gap junctions, as well as increased maximum diastolic potential and reduction of cardiac automaticity (Lieu et al., 2013; Nunes et al., 2013; Eng et al., 2016; Ronaldson-Bouchard et al., 2018). Furthermore, Lieu et al. also showed that the gene expression of the inward rectifier potassium channel Kir2.2 was significantly higher in cardiomyocytes derived from embryonic stem cells when electrically stimulated for 14-21 days but not after 7 days of stimulation (Lieu et al., 2013).
In conclusion, we have demonstrated that continuous electrical stimulation for 7 days at 1 Hz significantly increased the percentage of cardiomyocytes with organized sarcomeres, promoted alignment of cardiomyocytes in parallel to the electric field, and induced a more rod-like morphology. These results indicate that electrically-stimulated cardiomyocytes have a more mature morphology than non-stimulated cardiomyocytes. More mature cardiomyocytes would better recapitulate the human physiological phenotype and pharmacological response, and allow for more faithful assessment of novel cardioprotective agents.
Acknowledgements
This work was carried out with support from the National Health and Medical Research Council of Australia (NHMRC 1024817), Friedreich’s Ataxia Research Alliance, and Stafford Fox Medical Research Foundation. Alice Pébay is an Australian Research Council Future Fellow (FT140100047). The O’Brien Institute Department, St Vincent’s Institute of Medical Research, Bionics Institute and the Centre for Eye Research Australia receive Operational Infrastructure Support from the Victorian State Government’s Department of Innovation, Industry and Regional Development. We thank Owen Burns (Bionic Institute) for manufacturing the well-based electrode arrays.
Conflicts of Interest
None.
References
Damián Hernández1,2,3
1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia.
2Departments of Medicine and Surgery, University of Melbourne, East Melbourne, Victoria, Australia.
3Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, Victoria, Australia.
Rodney Millard4,5
4Bionics Institute, East Melbourne, Victoria, Australia.
5Medical Bionics Department, University of Melbourne, Parkville, Victoria, Australia.
Priyadharshini Sivakumaran1,3
1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia.
3Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, Victoria, Australia.
Anne M Kong1
1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia.
Geraldine M Mitchell1,2,6
1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia.
2Departments of Medicine and Surgery, University of Melbourne, East Melbourne, Victoria, Australia.
6Faculty of Health Sciences, Australian Catholic University, Fitzroy, Victoria, 3065, Australia.
Alice Pébay2,3, Robert K Shepherd4,5
4Bionics Institute, East Melbourne, Victoria, Australia.
5Medical Bionics Department, University of Melbourne, Parkville, Victoria, Australia.
Gregory J Dusting2,3,*
2Departments of Medicine and Surgery, University of Melbourne, East Melbourne, Victoria, Australia.
3Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, Victoria, Australia.
Shiang Y Lim1,2,*
1O’Brien Institute Department, St Vincent´s Institute of Medical Research, Fitzroy, Victoria, Australia.
2Departments of Medicine and Surgery, University of Melbourne, East Melbourne, Victoria, Australia.
Corresponding author:
Shiang Y. Lim
Email: maxlim@unimelb.edu.au or mlim@svi.edu.au
*These authors contributed equally to this article.
In a new window | Download PPT
Figure 1: Electrical stimulator and experimental timeline. (A) An electrode array containing a panel of 16 platinum electrodes that fit into an 8-well chamber slide, with each well containing a pair of electrodes. (B) A schematic of the experimental protocol from cardiac differentiation of human induced pluripotent stem cells to long-term electrical stimulation of the derived cardiomyocytes. Control cells were subjected to the same procedure but without electrical stimulation.
In a new window | Download PPT
Figure 2: Morphology and alignment of cardiomyocytes after 7 days of electrical stimulation. (A) Representative images of the three categories of sarcomere organization of cardiomyocytes. Scale bar = 50 µm. (B) Percentage of cardiomyocytes in each category of sarcomere organization. (C) Percentage of aligned cardiomyocytes. (D-F) Circularity (D), perimeter (E) and surface area (F) of cardiomyocytes with or without electrical stimulation (n = 3 independent experiments). Data are expressed as mean ± SEM. ** p < 0.01 and *** p < 0.001 by Student’s t-test. ESt = electrical stimulation.
In a new window | Download PPT
Figure 3: Gene expression of cardiac markers and ion channels in cardiomyocytes after 7 days of electrical stimulation. mRNA expression of cardiac contractile muscle proteins (A), ion channels (B), as well as gap junctional protein, natriuretic peptide A, and ryanodine receptor-2 (C) in cardiomyocytes with or without electrical stimulation (n = 4 independent experiments). Data are expressed as mean ± SEM. ESt = electrical stimulation.
Table 1: Effect of electrical stimulation on the maturation of human cardiomyocytes derived from pluripotent stem cells.

CM (cardiomyocytes); AP (action potential); ESC (embryonic stem cells); iPSCs (induced pluripotent stem cells); n/s (not specified).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 12839 | 92 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA