Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Rehabilitation and conditioning therapies in basic and translational research for ischemic stroke
Time:2018-11-08
Number:10642
Michael Jiang1, Zheng Zachory Wei1, Ling Wei1,2, Shan Ping Yu1,3
Author Affiliations


- 1Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA 30322.
- 2Department of Neurology, Emory University School of Medicine, Atlanta, GA 30322.
- 3Center for Visual and Neurocognitive Rehabilitation, Atlanta Veterans Affairs Medical Center, Decatur, GA 30033.
Conditioning Medicine, 2018. 1(6):294-305.
Abstract
Functional deficits including behavioral and motor impairments are a significant cause of morbidity amongst stroke survivors, but therapies addressing neuronal repair remain unsatisfactory. Rodent models of stroke have provided some insights into the endogenous mechanisms underlying spontaneous recovery due to neuronal plasticity and have provided potential therapeutic targets for improving functional recovery. The complexity and heterogeneity of stroke have yielded a variety of rehabilitative approaches to promote functional recovery, including exposure to intrinsic and extrinsic stimuli, as well as exposure to enriched environments. In recent years, conditioning therapy, especially postconditioning, has emerged as a comprehensive method to induce neuroprotection, neuroplasticity and brain recovery. Many rehabilitative strategies are based on activity-dependent endogenous mechanisms. Specifically, common rehabilitative therapies involve the establishment or strengthening of unmasked or redundant cortical connections following stroke. Ischemic pre- and postconditioning as well as intensive, repetitive, and controlled exercise may result in morphological and functional improvements via neurotrophic supports, neurogenesis, angiogenesis, arteriogenesis, and synaptogenesis. Basic and clinical research has demonstrated beneficial results of rehabilitation and conditioning for improved outcomes after stroke and ischemic disorders. It is expected that, by leveraging activity-dependent endogenous neurovascular plasticity/remodeling, future investigations will help to enhance the efficacy and efficiency of protective/regenerative therapies and promote their clinical translation for stroke patients.
Keywords: stroke, rehabilitation, conditioning, regeneration, functional recovery, clinical translation.
Abstract
Functional deficits including behavioral and motor impairments are a significant cause of morbidity amongst stroke survivors, but therapies addressing neuronal repair remain unsatisfactory. Rodent models of stroke have provided some insights into the endogenous mechanisms underlying spontaneous recovery due to neuronal plasticity and have provided potential therapeutic targets for improving functional recovery. The complexity and heterogeneity of stroke have yielded a variety of rehabilitative approaches to promote functional recovery, including exposure to intrinsic and extrinsic stimuli, as well as exposure to enriched environments. In recent years, conditioning therapy, especially postconditioning, has emerged as a comprehensive method to induce neuroprotection, neuroplasticity and brain recovery. Many rehabilitative strategies are based on activity-dependent endogenous mechanisms. Specifically, common rehabilitative therapies involve the establishment or strengthening of unmasked or redundant cortical connections following stroke. Ischemic pre- and postconditioning as well as intensive, repetitive, and controlled exercise may result in morphological and functional improvements via neurotrophic supports, neurogenesis, angiogenesis, arteriogenesis, and synaptogenesis. Basic and clinical research has demonstrated beneficial results of rehabilitation and conditioning for improved outcomes after stroke and ischemic disorders. It is expected that, by leveraging activity-dependent endogenous neurovascular plasticity/remodeling, future investigations will help to enhance the efficacy and efficiency of protective/regenerative therapies and promote their clinical translation for stroke patients.
Keywords: stroke, rehabilitation, conditioning, regeneration, functional recovery, clinical translation.
Introduction
Stroke is a heterogeneous disease resulting from a reduction in blood supply to the brain. Ischemic stroke accounts for >80% of stroke cases in humans. Stroke is a major public health concern as it is the fifth leading cause of death and the leading cause of disability in the United States (Go et al., 2014). Brain tissue is sensitive to loss of energy and nutrient supply, and these often result in permanent damage to neurons and the disruption of existing brain circuitry. Neurons sustain numerous insults during the acute phase of a stroke, leading to excitotoxicity and selective neuronal loss (Baron et al., 2013). While on-time reperfusion of ischemic tissue can reduce cell death and improve recovery, activation of the inflammatory system and reactive oxygen species often causes significant secondary injury along with reperfusion. In the hours to days that follow, these injurious insults and sustained depressed perfusion to the affected regions result in further apoptotic cell death (Ahmad and Graham, 2010; Sims and Muyderman, 2010). While stroke is often associated with significant morbidity, many patients experience mild to moderate recovery with and without intervention (Kong et al., 2011).
The brain has been appreciated for its remarkable ability to adapt, and it exhibits plasticity and regeneration under both healthy and diseased states (Li et al., 2008; Li et al., 2010; Winner et al., 2011). Treating disruptions to motor control and motor learning are central to recovery from stroke, with basic and clinical researchers adopting multiple strategies to enhance recovery (Johansson, 2011). Effective therapeutic approaches can leverage and augment endogenous plasticity and other mechanisms involved in spontaneous recovery. Here we evaluate various strategies used in animal and clinical trials to increase functional recovery after stroke. We also review mechanisms underlying spontaneous recovery that are targeted by therapeutic strategies.
Rodent stroke models
Rodent models of ischemic stroke are well established and have high face validity with cerebral ischemic damage such as middle cerebral artery (MCA) occlusion. MCA occlusion or MCA branch occlusion can cause cortical damage to specific brain structures including the motor and sensory cortices (Liu and McCullough, 2011; Macrae, 2011; Tajiri et al., 2013). Tissue damage can occur within minutes and can vary in severity depending on the degree of blood flow reduction and the time and permanence of occlusion (Durukan and Tatlisumak, 2007). In animal ischemic models, cerebral blood flow can be continuously monitored using laser Doppler technology (Bishop et al., 1986; Dirnagl et al., 1989). Reduction in blood flow can be induced using suture where the branches of the MCA are tied off in mice and rats (Belayev et al., 1996; Belayev et al., 1999). Insertion of an intraluminal filament can also induce MCA occlusion in rodents (Dittmar et al., 2003; Trueman et al., 2011). Embolic stroke by injection of clotted blood into the MCA also induces ischemic injury in rodent models (Zhang et al., 1997; Ahn et al., 1999; Henninger et al., 2006). Embolic stroke models in rodents have been particularly effective in the evaluation of acute stroke prevention using tissue plasminogen activator (Sumii and Lo, 2002; Fan et al., 2013). Chemical approaches to vascular occlusion include the photothrombotic stroke model which uses intravascular photooxidation via a photosensitive dye such as rose-bengal (De Ryck et al., 1989; Liu et al., 2014). When irradiated, singlet oxygen species cause local endothelial damage and platelet activation, resulting in thrombotic ischemia (Carmichael, 2005). Hemorrhagic stroke models utilizing stereotaxic injections of bacterial collagenase also exist (Rosenberg et al., 1990; Klahr et al., 2014). A variety of potential treatments and rehabilitative strategies can be easily evaluated using these rodent models.
In addition to animal models that involve extensive ischemic damage to the cortex and subcortical tissue, small stroke models with more limited damage to cortical and subcortical brain tissue have drawn increasing attention due to their clinical significance. Postconditioning treatment has demonstrated neuroprotective benefits in these stroke models (Table 1). The incidence rate of small strokes per 1000 person-years has been estimated at 4.7 (Bos et al., 2007). We recently developed a whisker barrel cortex stroke model of rats and mice. This model involves ligations of distal branches of the MCA and transient occlusion of both common carotid arteries (CCAs), leading to selective and restricted damage to the ipsilateral sensorimotor cortex, including the whisker barrel cortex (Wei et al., 2001; Wei et al., 2004; Wei et al., 2006). This relatively small and selective ischemic injury allows morphological and functional assessments before, during and at different times after the ischemic insult. Interestingly, the whisker stimulation can induce activity-dependent repair in the rodent somatosensory cortex.
Table 1. Postconditioning treatment in stroke models.
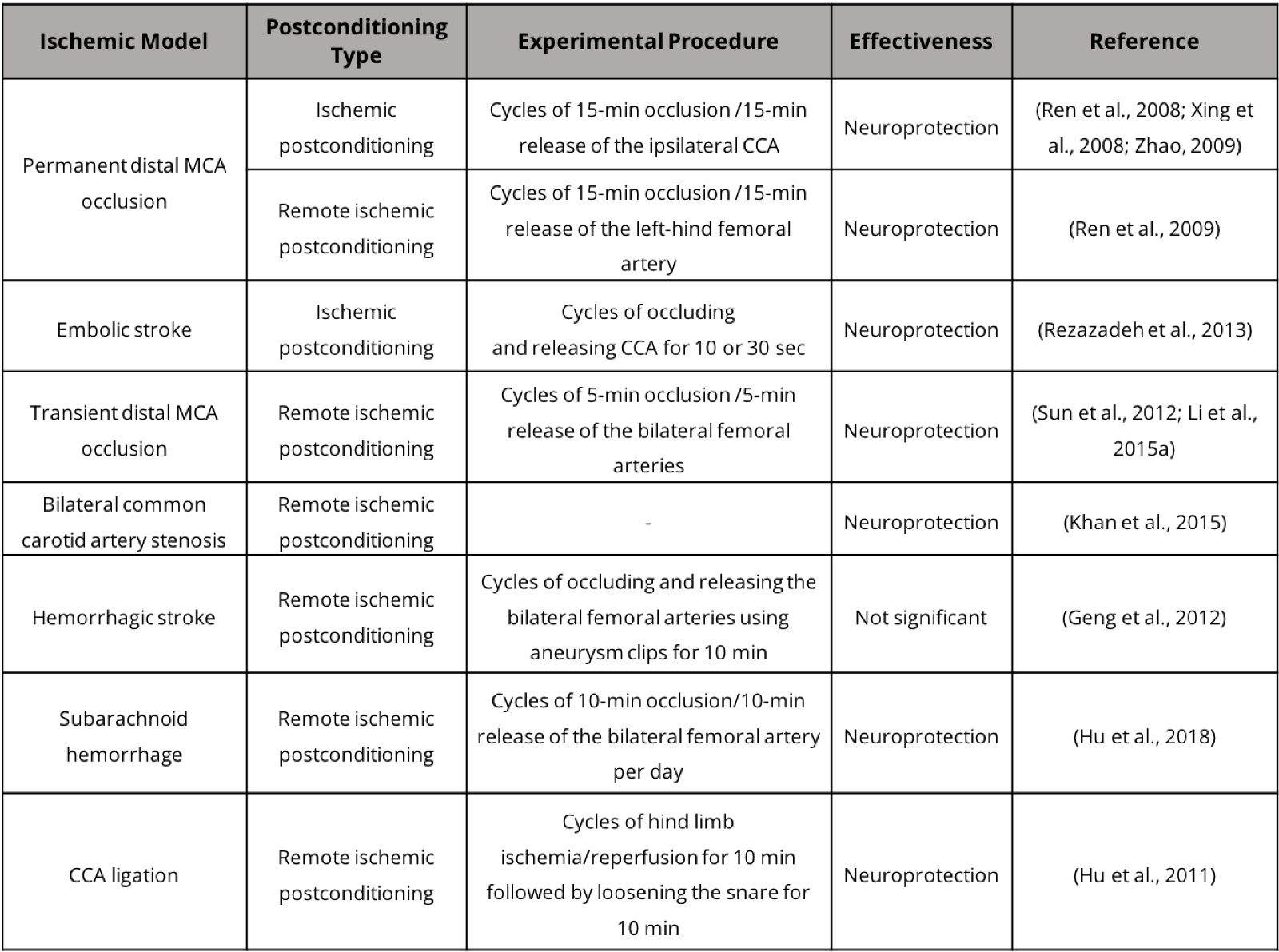
Functional recovery and clinical assessments of stroke recovery
While stroke patients experience some level of functional recovery following mild to severe stroke, such recovery is a mix of "true" recovery via restoration of neural circuitry and task-specific compensation (Kitago et al., 2012). In animal models, functional recovery is assessed by a wide array of behavioral tests that assess motor and sensory ability (Table 2). Assessment paradigms in animals for post-ischemic sensory and motor function include the neurological deficit score, which assesses a suite of behaviors including hind limb retraction, beam walking, forelimb flexion, and forelimb grasping. Other tests include forelimb reaching tasks including the Montoya staircase test, where animals must reach for food pellets on staircases (Klein and Dunnett, 2012). Tests have also been designed to measure asymmetry such as the cylinder test where the use of forelimbs for body support on the wall of a vertical cylinder can indicate deficits in motor ability and recovery (Fleming and Schallert, 2012; Clarkson et al., 2013). Typically, rodent models of stroke exhibit recovery within weeks of injury, while in humans post-stroke recovery may take years (Schaar et al., 2010). Neuroimaging techniques, including functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), can be effectively employed to investigate neurophysiological post-stroke recovery in human brains. Specifically, fMRI provides information regarding task/stimulus neuronal activation, functional connectivity, and neurotransmitter release, while DTI determines the integrity and connectivity of white matter (Dijkhuizen et al., 2012). Studying post-stroke recovery using animal models has both advantages and disadvantages due to the disparity in the recovery time window. Evaluating post-stroke repair in humans and in animal models presents challenges when determining the amount of recovery resulting from restoration of lost neuronal circuits versus compensatory behaviors that also improve performance in behavioral tests.
Table 2. Postconditioning and functional recovery in stroke models.
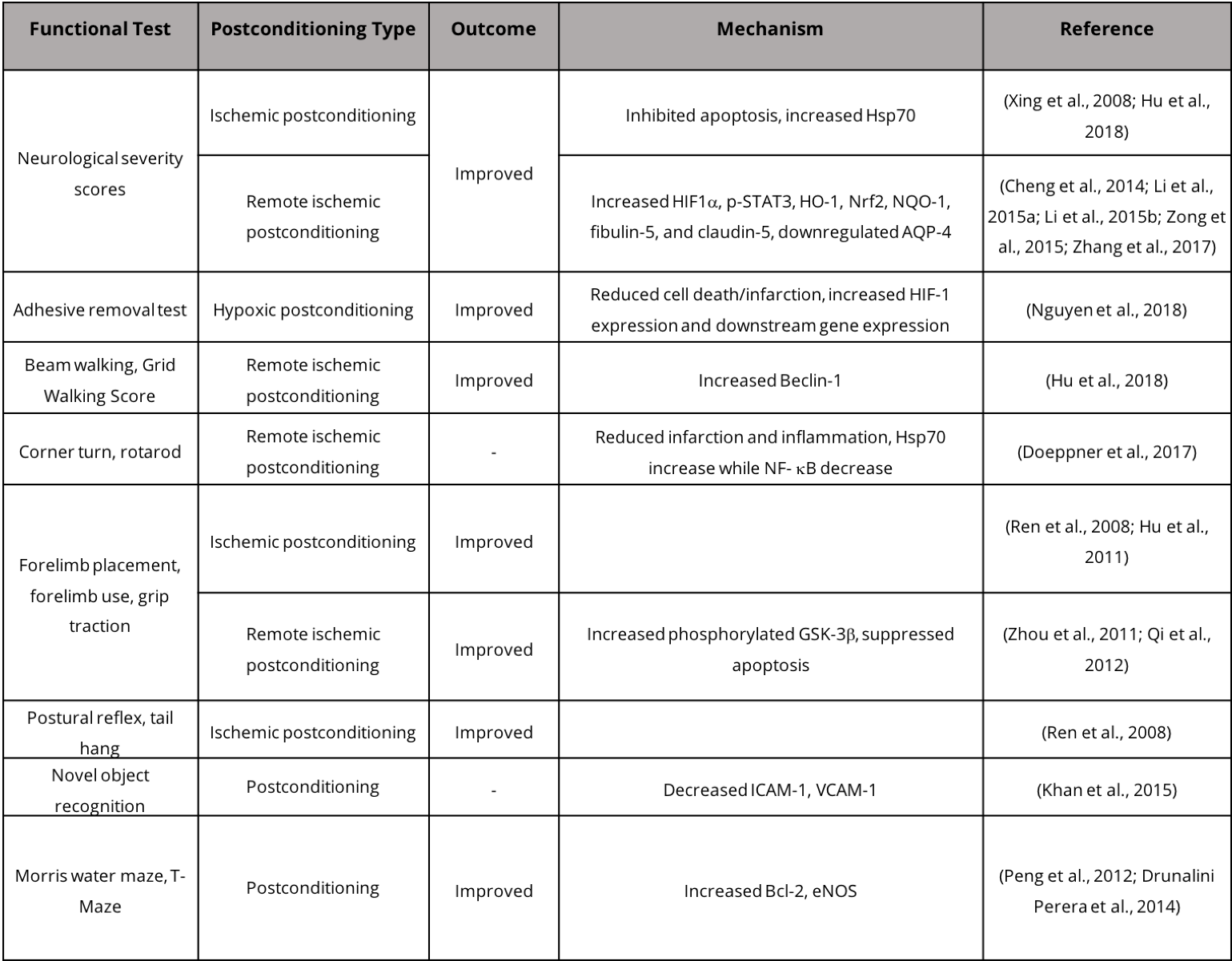
In humans, an important metric for evaluating recovery from stroke-induced upper extremity hemiparesis is a patient's performance on the Wolf Motor Function Test developed by Wolf et al. specifically for assessing the effectiveness of constraint-induced motor therapy (CIMT) (Wolf et al., 2001). The test assesses joints of the arm and hand in tasks of varying difficulty. Two assessments of strength and fifteen assessments of speed result in three scores: the Functional Ability Score, based on the quality of motor ability; the Time Score, based on speed; and the Grip Strength Score, based on strength. This test has been used widely to assess recovery using CIMT (Dromerick et al., 2000; Wolf et al., 2005; Dromerick et al., 2009). Another functional test of performance of the upper extremity after stroke used in CIMT trials is the Action Research Arm Test (ARAT). Developed in 1981, the ARAT uses a 4-point scale on 19 functional tasks including grasping, gripping, pinching, and gross movement (Lyle, 1981). The two aforementioned tests are often used in conjunction with the widely used Neurological Severity Score (Brott et al., 1989).
Neuroplasticity, circuit repair and spontaneous recovery
Cerebral ischemic stroke disrupts the somatotopic maps in the cortex where clusters of sensory and motor neurons form groups that are specifically coupled to their respective afferent nerves (Bernardo et al., 1990). Loss of cortical neurons disrupts the circuits required for sensory and motor control despite the preservation of neurons in unaffected brain regions and spinal cord (central pattern generators) and peripheral neurons. Recovery from such damage occurs with the restoration of these disrupted circuits or via functional takeover by nearby nerve tissue due to neuroplasticity. Unmasking and redundancy in the brain may be the two endogenous phenomena that form the basis for spontaneous neuroplasticity following stroke (Lee and van Donkelaar, 1995). The maintenance of synapses requires persistent neuronal activity, but there are also exit-latent sub-threshold synapses which do not normally induce action potentials (Isaac et al., 1995; Zeiler et al., 2013). Sudden loss of cortical neurons can unmask these "silent" synapses (Font et al., 2010; Otsuka et al., 2013). Without other synaptic competition, these once sub-threshold synaptic inputs are free to strengthen and establish new pathways resulting from motor learning (Font et al., 2010; Chipchase et al., 2011). A second characteristic of synapses in the CNS that contributes to stroke recovery is redundancy (Petersen et al., 2001; Chklovskii et al., 2004). Cortical projections are both specific and diffuse under healthy conditions (Chmielowska et al., 1989). Neurons in the sensory and motor cortices feature cortico-thalamic projections but also corticocortical projections (Hoffer et al., 2003). Competition and development define adult neural circuits, but diffuse projections are also developed (Arbib., 1981); Petreanu et al., 2012). As such, motor control lost from stroke can undergo some level of spontaneous recovery by utilizing backup and previously redundant pathways (Wen et al., 2014). For example, although motor control of any ipsilateral muscle group is commonly believed to be controlled by neurons in the contralateral cortex, ipsilateral afferent and efferent motor pathways exist. Recent research suggests that strengthening of these ipsilateral connections following stroke recovery is paired with intensive use, thus playing a key role in spontaneous recovery following stroke (Wen et al., 2014; Xerri et al., 2014).
Vascular plasticity in stroke recovery: angiogenesis and arteriogenesis
Vascular plasticity also plays an integral role in stroke recovery (Edvinsson and Povlsen, 2011; Hermann and Chopp, 2012). Restoration of lost blood supply by angiogenesis supports metabolic demand required in the peri-infarct region around the infarcted core of a stroke. Interestingly, general exercise and physical activity are known to induce physiological and metabolic processes after injury, which may generate and regulate the transient metabolic flow in specific brain regions (Shaughnessy et al., 2012; Chen et al., 2013). In rodent models of sensorimotor/whisker barrel cortex strokes, repetitive whisker stimulation using a bar or brush has demonstrated that therapeutic manipulation of sensory inputs promotes angiogenesis within the ischemic cortex (Whitaker et al., 2006; Li et al., 2008). Additionally, overuse of contralateral whiskers by trimming ipsilateral whiskers can increase use-dependent recovery in rodents (Whitaker et al., 2006). Whisker stimulation can significantly increase functional recovery from unilateral barrel cortex stroke in rats performing whisker texture discrimination tasks (Hoffman et al., 2003). Adult mice suffering loss of cortical whisker barrels have been shown to restore lost somatotopic maps over just weeks using voltage-sensitive dyes and peripheral stimulation (Brown et al., 2009). Specifically promotion of angiogenesis using vascular endothelial growth factor (VEGF) can reduce neurodegeneration and immune cell infiltration after stroke while increasing vessel volume. In a neonatal rat stroke model, a single 1.5 µg/kg intracerebroventricular injection of VEGF significantly ameliorated ischemic injury and promoted endothelial cell proliferation (Dzietko et al., 2013). Involvement of VEGF and other angiogenic factors in stroke, such as delayed HIF-1α/VEGF treatment, stem cell therapy, and physical exercise may become medical necessities to promote post-stroke vascular plasticity (Wagenaar et al., 2018).
Enriched environment for activity-dependent repair and stroke recovery
Rodent models can be used to study activity-dependent repair induced by social housing and enriched environments. Complex environmental settings have been established as an effective method for increasing capillary branching, synaptic density, and cortical thickness (Sirevaag et al., 1988; Saito et al., 1994; Diamond, 2001; Will et al., 2004). Enriched environments for rodents feature running wheels, complex housing, and toys. Rats given access to early rehabilitation during the acute phase after ischemia (5 to 14 days) experienced significant recovery compared to delayed (30 days after stroke) access. Importantly, at the physiological level, early access animals had greater branching of layer V cortical neurons (Murphy and Corbett, 2009). Rather than reducing ischemic volume, enriched environments promote beneficial post-stoke structural changes to neurons that include increased spine density, dendritic branching, and neurogenesis (Biernaskie and Corbett, 2001; Johansson and Belichenko, 2002; Komitova et al., 2002; Nygren and Wieloch, 2005). These effects are mediated by increased and sustained levels of neurological activity that upregulates expression of immediate early genes such as nerve growth factor-induced gene A (NGF-I) compared to control rats (Nygren and Wieloch, 2005). Targeting VEGF receptors, the angiopoietin-1 pathway, and endothelial nitric oxide synthase (eNOS) can also promote angiogenesis and arteriogenesis in the peri-infarct region to generate a more conducive microenvironment for therapy-induced neuroplasticity (Chen and Chopp, 2012).
In both animal models and human patients, motor learning after stroke is induced by intensive, repetitive, and meaningful exercises. Forced exercise is featured in a wide array of rehabilitation programs, but the parameters regarding training vary. Moderate- to high-intensity aerobic exercise releases in a dose-dependent manner brain-derived neurotrophic factor (BDNF), insulin-like growth factor-I (IGF-I), and nerve growth factor (NGF), and enhances diffuse synaptogenesis (Ploughman et al., 2014). These exercises can generate additional dendritic spines and synaptogenesis but also strengthen redundant and unmasked cortical connections. When exercises lack any of these three properties, physiological changes indicative of motor learning are significantly subdued (Daly and Ruff, 2006). Asynchronous motor therapy in rats administered concurrently with anti-Nogo-A antibody can produce marked recovery in paretic forelimbs. Treament with anti-Nogo-A along with intensive forelimb reaching (100 reaches per day), improved performance in both the Montoya staircase grasping test and the horizontal ladder crossing test within just two weeks (Wahl et al., 2014).
Constraint-induced motor therapy for stroke patients
For human patients, meaningful activities, including daily necessities such as reaching and grasping, when intensively and repetitively practiced every day, can generate permanent and significantly improved motor learning and skill function (Kleim and Jones, 2008; Hubbard et al., 2009). Early stroke rehabilitation developed by Bobath sought to maximize recovery using a neurophysiological approach which included therapy involving both the affected and unaffected limbs (Paci, 2003; Dobkin, 2004; Chan et al., 2006). This was due to the prevalent notion that motor skill was reflexive and hierarchical (Loeb et al., 1999). However, it is now evident that motor ability is distributed throughout the brain (Doyon and Benali, 2005; Shumway-Cook and Woollacott, 2007). Recovery of upper extremities has been particularly challenging as functional improvement has been demonstrated to be more difficult to achieve with and without therapy compared with lower extremity recovery (Rand and Eng, 2012). CIMT was initially developed by Edward Taub in response to the phenomenon known as "learned non-use" (Wen et al., 2014). Stroke patients suffering from hemi-paresis often preferentially use the unaffected limb while ignoring the paretic limb, even when the paretic limb still has some limited ability to move. To counteract this effect, clinicians recommended artificial restriction of the unaffected limb to force the use of the paretic limb. Early applications of CIMT involved putting the healthy limb in a sling, or patients were asked to wear a mitten that restricted use of the digits in order to promote the use of the paretic limb (Caimmi et al., 2008). Rigorous CIMT involves use of a splint for the patient’s waking hours (<90% of awake time) to force use of the paretic limb except when use of the healthy limb is absolutely necessary. CIMT has since developed into one of the most effective rehabilitative strategies for treating hemi-paretic limbs. Large multicenter randomized clinical trials investigating the efficacy of CIMT in human stroke patients with ipsilateral upper extremity paresis have concluded that this therapy effectively improves functional recovery up to two years after stroke (Wolf et al., 2006).
The reason for its success lies in how this approach leverages neuroplasticity following injury. CIMT induces detectable cortical reorganization after stroke in patients suffering from upper extremity hemiplegia (Levy et al., 2001). The extremity constraint-induced therapy evaluation (Wolf et al.) trial reported that compared with usual control care or no therapy, CIMT conferred greater improvements when evaluated using the Wolf motor function test and the Motor Activity Log (MAL) amount-of-use evaluation (Wolf et al., 2001; Van der Lee et al., 2004). Some fMRI studies provide further evidence as to the mechanism underlying functional recovery. Specifically, fMRI studies in patients demonstrated that CIMT enhances ipsilateral afferent and efferent pathways (Gauthier et al., 2014). CIMT's effectiveness has primarily been demonstrated in patients suffering in the chronic phase of stroke, but its success has merited investigation for acute stroke patients. Traditionally, treating acute or subacute stroke with rehabilitation presents more challenges than recruiting chronic stroke patients in a clinical trial. The 2009 Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS) trial with a total of 52 participants found that CIMT was effective compared to control but no different than traditional therapy after inpatient stroke rehabilitation. Meanwhile, it was reported that high intensity CIMT conferred less motor improvement 90 days after therapy (Dromerick et al., 2009). Strenuous activity soon after ischemia may cause damage or impede stroke recovery. As such, CIMT can be adapted to a lower intensity for acute administration compared with high intensity or regular CIMT. In a systemic review of five randomized clinical trials featuring 106 total patients, an overall positive trend was identified in the effects of both lower-intensity and high-intensity CIMT during the acute/subacute phase after stroke. Recovery was detected using the Action Research Arm Test, Motor Activity Log, and the grooved pegboard test with patients significantly improving in these tasks compared with no-therapy controls. A limitation of acute studies with CIMT is the existence of wide variation in inclusion criteria between studies. Some studies require both proximal and distal motor ability, which suggests the preservation of central to distal connections (Boake et al., 2007; Bonaiuti et al., 2007). Other studies included only proximal motor ability in their inclusion criteria where recovery of distal digit function is a more reliable indicator of recovery.
Evaluation of CIMT across clinical trials has yielded predominantly positive results for this therapeutic strategy. However, stroke recovery resulting from CIMT is often highly variable within studies. This may be a consequence of two factors, the first owing to the heterogeneity in severity and presentation of stroke-induced deficits, and the second being patient-specific factors including variable brain activation patterns. Chronic stroke patients recruited in clinical trials for CIMT experience variable degrees of recovery, which persist for months to years after therapy. fMRI data showed significantly increased activation in the motor cortex of CIMT-treated individuals that could be detected after 6 months (Rijntjes et al., 2011). Augmenting CIMT with an intensive, meaningful, and repetitive exercise regimen can bolster the effectiveness of the therapy. Patients participate in training programs that feature repetitive everyday tasks such as screwing, turning faucets, and flipping light switches. In a meta-analysis on the effect of exercise intensity and frequency, there is some support for the notion that a higher dose of the same exercise confers greater motor recovery following stroke (Cooke et al., 2010). A limitation of CIMT is that it is dependent on a minimum level of movement ability of the paretic limb. The recruitment of patients is highly dependent on stroke severity and thus eliminates those with complete paresis in the upper extremities. Human trials using CIMT cannot control for several important factors contributing to motor recovery such as patient motivation, initial level of motor ability, and family support (Winstein et al., 2003).
Exercise and transcranial magnetic stimulation for stroke recovery
Low-frequency repetitive transcranial magnetic stimulation (rTMS) has also been used in conjunction with intensive exercise training for upper extremity paresis (Conforto et al., 2012). In a multicenter trial with 204 patients, daily low-frequency rTMS applied over the motor cortex contralateral to the paretic limb followed by 120 min of training and 60 min of self-exercise resulted in a mean improvement in both the Fugl-Meyer assessment (Hoffman et al., 2003) and the Wolf motor function test. This effect was observed in 79 patients one month after discharge. Importantly, there were no differences in improvement as a function of age, nor did any patients experience adverse side effects (Kakuda et al., 2012).
Virtual reality is an emerging computer-based technique used for stroke rehabilitation by simulating multimodal sensory inputs to users with real-time feedback. While traditional neurophysiological techniques help improve motor recovery following stroke, there remains a gap for patients during the acute phase of stroke when participation in exercise programs is not possible. Furthermore, virtual reality addresses shortages of rehabilitation services for a group of stroke survivors without access to more expensive and intensive programs (Jutai and Teasell, 2003; Teasell et al., 2008). Virtual reality simulated exercise has the potential to apply the same concepts to stroke recovery and neuroplasticity as physical exercise programs and to apply similar approaches, which include simulations that are intensive, repetitive, and meaningful (Langhorne et al., 2009). To address accessibility issues, computer-based rehabilitation programs take advantage of common household entertainment systems such as the Nintendo Wii. Non-immersive video games are being adapted for clinical rehabilitation despite not having been designed with such a purpose in mind (Saposnik et al., 2010; Mouawad et al., 2011). A key advantage of virtual reality systems is their ability to facilitate recovery in the acute/subacute phase following stroke. Clinical trials investigating the efficacy during the acute phase (4-6 weeks) have demonstrated significant benefits of using virtual reality rehabilitation whether it is immersive (true virtual reality) or non-immersive (gaming) (Piron et al., 2003; Joo et al., 2010; Saposnik et al., 2010; Casserly and Baer, 2014). Virtual reality has also proven to significantly improve upper extremity recovery following stroke in the chronic phase. Specifically when compared to equal intensity conventional training, virtual reality conferred an equal benefit to recovery (Fritz et al., 2013; Prange et al., 2013).
Conditioning strategy as an effective stroke treatment
In recent years, stimulation of endogenous neuroprotective mechanisms has drawn increasing attention due to its well-demonstrated protective potency and a better understanding of the underlying cellular and molecular regulations. Brain cells inherently have the ability to adapt to pathological conditions by activating or recruiting stress-induced defense mechanisms. Specifically, the brain can tolerate adverse events such as brief cerebral ischemia, thereby enhancing its endurance when future, more severe insults occur (Riepe et al., 1997; Huber et al., 1999). The stress response of protective pathways to counter cell death depends on the strength and duration of the insults. Interestingly, it was shown that preconditioning-induced tolerance could be transferred between different cells/tissues. For example, neurons cultured with oxygen-glucose deprivation (OGD)-preconditioned astrocytes were significantly resistant to lethal ischemic injury compared to neurons incubated with sham-treated astrocytes, suggesting that preconditioned astrocytes passed tolerance to neurons (Narayanan et al., 2018). The protective effect of ischemic preconditioning of astrocytes may involve lactate production and the transcription factor nuclear erythroid 2-related factor 2 (Nrf2) (Narayanan et al., 2018). The adaptation process may involve the institution of a harmonic response at multiple cellular and molecular levels. On the other hand, beyond the limits of tolerance, cells may be doomed to go down in response to the activation of cell death pathways. Therefore, investigations on the regulation of the conditioning events and their underlying mechanisms are imperative for utilizing the endogenous defense system.
Ischemic/hypoxic conditioning is achieved via sublethal insults of minutes to several hours and repeated for days or a few weeks. Its aim is to reduce the severity or prevent the deleterious consequences of the pathological processes. Ischemic/hypoxic and pharmacological/chemical conditioning in cellular and preclinical models have demonstrated neuroprotective effects against ischemic, traumatic, or chronic neurodegenerative diseases. Other conditioning, such as hypothermic preconditioning or cooling (i.e., at 31.5 oC), may reduce cerebral ischemic damage and induce tolerance in animals and brain slices (Nishio et al., 2000). Another possibility is to aim at inducing neuroplasticity and repair in the central nervous system, which can be a part of the mechanisms induced by postconditioning.
Ischemic preconditioning
Since the first report of ischemic preconditioning in a rabbit model of heart ischemia (Murry et al., 1986), the protective effect of ischemic preconditioning has been validated in many experimental models (Bahjat et al., 2013; Thompson et al., 2013; Ayodele and Koch, 2017; Wei et al., 2017; Almohanna and Wray, 2018; Chen et al., 2018; Narayanan et al., 2018). Currently, the term ischemic conditioning covers three types of ischemic/hypoxic conditioning strategies spanning the three phases of before, during and after an ischemic attack (Zhao, 2009). Ischemic preconditioning refers to the application of brief non-lethal ischemia to activate protective mechanisms against future injurious insults. Ischemic preconditioning has proven so far to be one of the most effective neuroprotective strategies in basic research (Murry et al., 1986; Meller and Simon, 2013; Lehotsky et al., 2015; Yang et al., 2017). In addition, other types of preconditioning have been explored to provide protection against brain injuries. For example, an intensive exercise protocol for rats generated a conditioning benefit that induced VEGF expression and improved stroke outcomes (Rezaei et al., 2018). A recent study showed that low-dose ethanol preconditioning protected against OGD-induced neuronal injury, and involved activation of the large conductance Ca2+-activated K+ channels (Su et al., 2017).
Ischemic post- and per-conditioning
Ischemic postconditioning is defined as a strategy to induce brief interruptions in blood flow during reperfusion, thereby protecting organs from ischemia/reperfusion injury (Halkos et al., 2004). To date, ischemic postconditioning can be induced by bilateral arm/lower limb remote ischemia, bilateral common carotid artery occlusion, and many others in clinical and preclinical studies (Chen et al., 2018; Dugbartey and Redington, 2018). One postconditioning strategy, ischemic per-conditioning, results from application of brief ischemia during a prolonged ischemic insult. These conditioning strategies can induce tolerance and have demonstrated efficacies and therapeutic benefits.
Postconditioning can improve disrupted cerebral blood flow and prevent apoptotic events such as cytochrome c translation. Other mechanisms may involve the protein kinase Akt (Pignataro et al., 2008; Zhou et al., 2011) and phospoinositide 3 kinase linked pathway activation (Rehni and Singh, 2007). During conditioning, suppression of thousands of genes enhances tolerance to reperfusion injury by inducing endogenous responses including those involved in neuroprotective and repair mechanisms. Recent evidence suggests that these adaptive phenotypes are epigenetically mediated to reduce acute injury and the burden of chronic neurodegeneration. However, the mechanism of epigenetic responses during conditioning needs to be identified in neurological disease (Gidday, 2015). Due to its high feasibility and non-invasive nature, postconditioning provides a great opportunity to translate basic knowledge to clinical stroke treatments (Simon, 2014).
Remote conditioning
In addition to local pre- and postconditioning, remote ischemic preconditioning has been developed by subjecting a remote and more accessible organ, such as an arm or a leg, to a nonlethal ischemic insult to protect a vital organ such as the brain from more severe ischemic damage (Kitagawa et al., 1991; Meller and Simon, 2013; Lehotsky et al., 2015; Yang et al., 2017). In recent years, remote ischemic conditioning has been developed as a type of conditioning strategy. Remote conditioning is highly clinically relevant and is a preferred method of conditioning therapy (Dezfulian et al., 2013; Hoda et al., 2014; Pan et al., 2016; Chen et al., 2018). More information on the mechanisms of the three types of conditioning can be obtained in recent review articles (Wang et al., 2015; Pan et al., 2016; Chen et al., 2018).
Conditioning-induced neurovascular plasticity/remodeling and functional recovery
Ischemic/hypoxic conditioning can modulate neurovascular plasticity. In an endothelin-1-induced rat stroke model, postconditioning under 8% O2 1 hour per day for 5 days started during the subacute phase of stroke showed reduced infarct and further increased HIF-1 and the glucose transporter-1 (Nguyen et al., 2018). Infarct volume might be further reduced by a combination of remote ischemic postconditioning plus peripheral nerve electric stimulation (Xiao et al., 2015). Ischemic postconditioning also increased blood-brain barrier integrity, reduced brain leukocyte infiltration and upregulated Hsp70 (Doeppner et al., 2017). Postconditioning with daily remote limb ischemia following a rat model of acute cerebral stroke also showed reduced infarct and upregulation of endogenous tissue kallikrein levels in circulating blood and local ischemic brain regions (Liang et al., 2018). In a mechanism study, postconditioning showed increased levels of phosphorylated Akt (p-Akt) and Akt isoforms, and upregulated p-mTOR, p-S6K and p-4EBP1 in the mTOR pathway (Xie et al., 2013). In patients with symptomatic i ntracranial atherosclerotic stenosis, remote ischemia-induced postconditioning increased levels of serum VEGF and bFGF and collateral circulation (Wei et al., 2016). Some pathological conditions such as diabetic vascular changes may significantly reduce the protective effects induced by ischemic postconditioning (Altunkaynak and Ozcelikay, 2016).
Postconditioning can stimulate endogenous regenerative mechanisms. In a rat MCAO model, postconditioning with 10-minute reperfusion plus 10-minute reocclusion resulted in an increase in doublecortin+/BrdU+ and collagen-IV+/Ki67+ double-positive cells (Esposito et al., 2015). Remote ischemic postconditioning significantly increased the number of proliferating neural cells, as indicated by the colabeling of BrdU with Nestin, NeuN, or GFAP, as well as increased doublecortin-positive cells in the subgranular zone and subventricular regions (Huang et al., 2017). Endogenous neurogenesis may be improved by an enriched environment and circulating regenerative factors due to postconditioning treatment. This may be further combined with cell transplantation to promote brain repair (Doeppner et al., 2017). Whether brain recovery after stroke or during chronic neurodegeneration benefits from postconditioning is currently less investigated. The mechanisms underlying neuronal protection, regeneration and functional recovery for postconditioning treatments remain to be better delineated.
Conclusion and future directions
Stroke has been historically difficult to treat. Despite the brain's plasticity enabling some spontaneous recovery, clinicians and researchers now leverage and augment endogenous plasticity using a variety of techniques. These therapies have demonstrated effectiveness during both the acute and chronic phases of stroke recovery, leading to long-term permanent improvements in neurovascular structures and functional outcomes. Future work will involve better understanding of the protective and regenerative mechanisms, and this will ultimately help to improve stroke treatments such as conditioning therapy. Continued research in rodent models into the mechanisms of potential therapies and augmenting the brain’s capacity for repair are critical for the development of novel therapeutic strategies. The clinical translation of several effective stroke treatments including therapeutic hypothermia and conditioning methods is an intensive and promising research area. It is expected that some of these innovative therapies will be available for stroke patients in the near future, providing effective, safe and cost-efficient treatments for those suffering from stroke and other brain injuries.
Conflicts of interest
The authors claim no conflict of interest related to this work.
References
Michael Jiang1*
1Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA 30322
Zheng Zachory Wei1*
1Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA 30322
Ling Wei1,2
1Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA 30322
2Department of Neurology, Emory University School of Medicine, Atlanta, GA 30322
Shan Ping Yu1,3
1Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA 30322
3Center for Visual and Neurocognitive Rehabilitation, Atlanta Veterans Affairs Medical Center, Decatur, GA 30033
Corresponding author:
Shan Ping Yu
Email: spyu@emory.edu
*These authors contributed equally to this article.
Table 1. Postconditioning treatment in stroke models.

Table 2. Postconditioning and functional recovery in stroke models.

Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10642 | 16 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA