Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The role of peripherally derived monocytes in the aging injured brain
Time:2019-01-03
Number:7117
Susanna Rosi1,2,3,4,5
Author Affiliations


- 1Department of Physical Therapy and Rehabilitation Science, University of California, San Francisco, CA 94143, USA
- 2Brain and Spinal Injury Center, University of California, San Francisco, CA 94110, USA
- 3Department of Neurological Surgery, University of California, San Francisco, CA 94110, USA
- 4Weill Institute for Neuroscience, University of California, San Francisco, CA 94143, USA
- 5Kavli Institute of Fundamental Neuroscience, University of California, San Francisco, CA 94143, USA
Conditioning Medicine, 2018. 1(7):350-354.
Abstract
Traumatic brain injury (TBI) is a primary cause of neurological disability worldwide. TBI affects the elderly population at a higher rate than any other group, resulting in a diminished quality of life post-injury. The effects of age on the pathophysiology of TBI currently is inadequately comprehended. We demonstrated that peripherally-derived monocytes (C-C chemokine receptor type 2 [CCR2+]) permeate the brain and exacerbate cognitive impairments chronically post-TBI. Notably, the age of the animals was demonstrated to directly increase infiltration of peripherally-derived monocytes following TBI. Here we discuss the role of age on peripherally-derived monocytes’ (CD45hi; CCR2+) brain infiltration during the sub-acute stage of TBI and the effects of these monocytes on the progression of TBI-induced cognitive impairments. Following TBI, there was a surge of peripherally-derived monocytes into the aged brain in contrast to the young brain. In the aged brain, infiltration of peripherally-derived monocytes persisted sub-acutely after injury and was paralleled by an upregulation of CCR2 chemotactic ligands and a proliferation of CCR2+ monocytes. The myeloid cells present in the aged brain had diminished anti-inflammatory capacity versus the young brain. Notably, knockout of CCR2 was able to protect against TBI-associated spatial memory impairment in aged animals. Overall, these results establish the vital role that peripherally-derived monocyte infiltration plays during the sub-acute period post-TBI in the aged brain.
Abstract
Traumatic brain injury (TBI) is a primary cause of neurological disability worldwide. TBI affects the elderly population at a higher rate than any other group, resulting in a diminished quality of life post-injury. The effects of age on the pathophysiology of TBI currently is inadequately comprehended. We demonstrated that peripherally-derived monocytes (C-C chemokine receptor type 2 [CCR2+]) permeate the brain and exacerbate cognitive impairments chronically post-TBI. Notably, the age of the animals was demonstrated to directly increase infiltration of peripherally-derived monocytes following TBI. Here we discuss the role of age on peripherally-derived monocytes’ (CD45hi; CCR2+) brain infiltration during the sub-acute stage of TBI and the effects of these monocytes on the progression of TBI-induced cognitive impairments. Following TBI, there was a surge of peripherally-derived monocytes into the aged brain in contrast to the young brain. In the aged brain, infiltration of peripherally-derived monocytes persisted sub-acutely after injury and was paralleled by an upregulation of CCR2 chemotactic ligands and a proliferation of CCR2+ monocytes. The myeloid cells present in the aged brain had diminished anti-inflammatory capacity versus the young brain. Notably, knockout of CCR2 was able to protect against TBI-associated spatial memory impairment in aged animals. Overall, these results establish the vital role that peripherally-derived monocyte infiltration plays during the sub-acute period post-TBI in the aged brain.
Introduction
Traumatic brain injury (TBI) plays a key role in the development of neurological disabilities and is a major contributing factor in the onset of dementia and other neurodegenerative diseases (Johnson et al., 2010; Corrigan et al., 2010). An increase in the incidence of TBIs in the elderly population is extremely likely as a large portion of the world’s population begins to age (Stocchetti et al., 2012). TBI-related mortalities and hospitalizations are significantly increased for elderly patients, which is worrisome due to the diminished ability for endogenous cognitive repair and reduced quality of life post-TBI with age (Kameoka et al., 1984; Hukkelhoven et al., 2003; Schonberger et al., 2009). The difference in TBI incidence and outcome between older and younger populations also persists with mild TBIs (Susman et al., 2002). Efforts beyond acknowledging the role age plays in TBI outcomes are needed. Indeed, further investigations are required to elucidate how trauma affects elderly patients as the number of TBI incidents rise in the elderly population (Mushkudiani et al., 2007). Several studies have determined the reliability of rodent TBI models for studying the long-term effect of age on TBI (Itoh et al., 2008; Onyszchuk et al., 2008). Evidence has shown that aged mice subjected to TBIs have an increased level of neurodegeneration and diminished behavioral recovery versus young mice (Onyszchuk et al., 2008; Timaru-Kast et al., 2012). Age-related increases in injury cavitation and neuronal cell death following TBI have been linked to an elevated inflammatory cytokine response two days post-TBI (Sandhir et al., 2004; Kumar et al., 2012; Timaru-Kast et al., 2012). An increase in inflammatory markers and an amoeboid morphology present several weeks post injury demonstrates simultaneous intensification of macrophage/microglia (myeloid cells) activation (Sandhir et al., 2008; Kumar et al., 2013; Morganti et al., 2016).
Peripherally-derived monocytes invade the injured brain and differentiate into activated macrophages, which play a prominent role in inflammation and behavioral deficits post-TBI in adult animals (Semple et al., 2010; Hsieh et al., 2014; Morganti et al., 2015). Peripherally-derived monocytes are characterized by C-C chemokine receptor type 2 (CCR2) surface expression. Its ligand, C-C motif chemokine ligand 2 (CCL2), epitomize the primary signaling pathway of peripherally derived monocyte infiltration (Prinz and Priller, 2010). Following TBI, CCL2 increases in both rodent models and human patients (Semple et al., 2010; Shi and Pamer, 2011; Liu et al., 2013). Inhibition of CCR2 reduces monocyte infiltration by 80-90%, weakens inflammatory cytokine expression, protects neuronal density, and prevents chronic learning deficits in young animals following TBI (Hsieh et al., 2014; Gyoneva et al., 2015; Morganti et al., 2015). Our research demonstrated in aged brains, that monocyte infiltration within 24 hours of the injury is sevenfold greater than in young brains. Therefore we hypothesized that peripherally-derived monocytes in the brain play a vital role in age-associated differences following TBI (Morganti et al., 2016).
We recently showed that in aged brains there were increased levels of peripherally-derived monocytes following TBI for up to a week post-injury (Morganti et al., 2016). The increased rate of monocyte translocation in aged mice was maintained for 4 days post-TBI. Aging also elevated the sub-acute expression of CCR2 ligands responsible for monocyte permeation into the injured brain. Importantly, there was a substantial increase in peripherally-derived monocytes in the bloodstream of aged animals post-TBI. Critically, we identified that age limits the anti-inflammatory response of myeloid cells during the injury-driven inflammatory response. Hindering the infiltration of monocytes via CCR2 knockout in aged mice mitigates the progression of TBI-associated spatial memory impairments, indicating the importance of a healthy peripheral immune system in optimal TBI outcomes with aging.
Aging and Injured Brain: Role of Monocytes
Aging has been shown to create a surge in peripherally-derived monocytes into the injured brain and to modulate the sub-acute anti-inflammatory response leading to an increase in cognitive deficits following TBI. We elucidated an age-related surge in peripherally-derived monocytes into the injured brain as well as in the blood, which mirrored an increase in CCR2 chemotactic ligands. Interestingly, knockout of CCR2 diminished peripherally-derived monocyte infiltration and reduced spatial memory deficits post-TBI.
The development of myeloid cells within the injured brain has been previously linked to aging, as demonstrated by the increase in the myeloid markers, ionized calcium adaptor molecule 1 (Iba-1) and CD11b (Sandhir et al., 2008). Of note, the investigators of the aforementioned study failed to differentiate between resident microglia and peripherally-derived monocytes. However, the specific kinetics of monocyte translocation at sub-acute times post-TBI in the aged brain has subsequently been demonstrated for the first time. Indeed, it was shown that age-associated monocyte translocation remained through the subchronic stage.
Notably, an elevation of CCR2+ monocytes was also detected in the blood of aged animals post-TBI, which indicates that CCR2+ may serve as a potential biomarker for TBI patients. Currently, studies that examine biomarkers for TBI only analyze proteins that leave the brain and enter the blood, and they lack detailed comprehension of the role the proteins have on TBI outcomes (Kawata et al., 2016; Agoston et al., 2017). However, our lab and other investigators have shown peripherally-derived monocytes to be critical to the development of post-TBI deficits (Hsieh et al., 2014; Morganti et al., 2015). A recent study in human patients, spanning a variety of ages, revealed a significant elevation of monocytes in blood within the first 24 hours post-TBI (Liao et al. 2013; Hazeldine et al., 2015). The results of the previous study may have been moderately affected by the wide range of patient ages (24 to 62 years old), as the data were not stratified according to age. This study cannot definitively determine acute surges of monocyte population growth and leakage into the bloodstream, however it may show that older patients have increased monocytes in their blood at the sub-acute stage rather than at acute time point. The collected data cannot exclude the possibility that various other blood lymphocytes may influence the results. Additional studies with a much broader scope are required to determine if peripheral monocyte levels and TBI patient outcomes are related in order to validate the use of blood monocytes as a biomarker for patient outcomes.
Although the primary source of CCR2+ and CD45hi monocytes is bone marrow, alternative sites, for example the brain, contain endogenous macrophage populations and may offer a tolerant microenvironment for proliferation of peripherally-derived monocytes (Geissmann et al., 2010; Shi and Pamer, 2011). Importantly, peripherally-derived monocytes and endogenous macrophage populations have comparable function and markers, although they have markedly different origins (Alliot et al., 1999; Ginhoux et al., 2010). Following translocation, peripherally-derived monocytes reduce the expression of CCR2 and adopt a microglia-analogous form by upregulating the microglial marker CX3CR1 (Saederup et al., 2010; Morganti et al., 2015). As a result, infiltrated monocytes may proliferate into BrdU-marked monocytes in the brain post-TBI. Peripherally-derived monocytes have been demonstrated to proliferate in the peritoneum, an area with an endogenous macrophage population, during peritonitis (Davies et al., 2013). Additional studies need to evaluate the ability of the altered brain microenvironment to permit peripherally-derived monocytes to adopt the proliferative ability of microglia (Ajami et al., 2007; Yona et al., 2013). As several studies highlight the effects of age on microglia growth post-injury, age may also play a role in peripherally-derived monocytes (Sandhir et al., 2008; Kumar et al., 2013; Loane and Kumar, 2016).
We have previously demonstrated an elevation of the CCR2 ligands CCL7, CCL8, and CCL12 24 hours post-TBI in the aged brain (Morganti et al., 2016). We recently determined that the age-related disparities of CCL8 and CCL12 remain at 4 and 7 days post-TBI (Chou et al., 2018). This elevation plays a possible role in the intensified monocyte translocation in the aged brain post-TBI. Although young and aged animals showed similar levels of CCL2 and CCL7 4 days post-TBI, in the young brains these levels were reduced by day 7 post-TBI, whereas in the aged animals levels were still significantly elevated (Chou et al., 2018). In a prior study, we only monitored the F4/80hi monocyte population and determined that monocyte translocation ceased by 48 hours post-injury. However, when all F4/80+ subpopulations were included, we observed persistent monocyte translocation up to 4 days post-TBI in young animals. Additional studies are necessary to identify the precise moment monocyte infiltration occurs in both age groups in order to discern if monocyte infiltration is only magnified or if the window persists for a longer period. Further studies using immunohistochemistry could evaluate the regional presence of monocyte translocation into the aged brain. While the gating scheme used in our study selects most of the monocyte subpopulations, the data falls short of excluding all other cell types. Neutrophils, for example, also express CD45hi, CCR2, and CD11b, while possessing the ability to penetrate the injured brains of young animals (Jin et al., 2012; Hsieh et al., 2014). Although neutrophil penetration of the injured brain primarily occurs during the first 24 hours post-TBI, and subsides by day 3 to 7, further studies should determine if the neutrophil response is comparable in aged animals.
Prior studies have demonstrated that age boosts both anti- and pro-inflammatory responses in the first 24 hours post-injury (Timaru-Kast et al., 2012; Kumar et al., 2013; Morganti et al., 2016). Our data indicate that at 7 days post-TBI, the pro-inflammatory response is equivalent for aged and young animals, whereas the anti-inflammatory gene response is diminished in the myeloid cells of aged animals. Microglia/macrophage polarization is evident by the presence of both pro- and anti-inflammatory phenotypes (Gordon 2003; Kumar et al., 2016). Although it has been demonstrated that the myeloid population can exhibit both phenotypes concurrently post-TBI, the reduction in the anti-inflammatory markers, CD206 and Ym1, in aged animals indicates a less robust anti-inflammatory response following injury (Jablonski et al., 2015). Notably, the aged brain already prioritizes myeloid cells to pro-inflammatory gene expression, intensifying the effects of diminished anti-inflammatory function (Lee et al., 2013). Additionally, the reduction in IL-4Ra and TGF-β, a receptor and cytokine, respectively, that encourage the anti-inflammatory phenotype, indicates an overall deficiency in anti-inflammatory polarization in contrast to dysfunction in a particular cytokine pathway (Gordon 2003; Gong et al., 2012). The age-spurred physiologic drive away from the anti-inflammatory myeloid phenotype likely contributes additional inflammation post-TBI, resulting in worse outcomes (Kumar et al., 2013; Morganti et al., 2015). In contrast, peripherally-derived monocytes have demonstrated anti-inflammatory properties post-stroke or spinal cord injury resulting in improved functional deficits (Shechter et al., 2009; Gliem et al., 2016; Wattananit et al., 2016). Although monocyte permeation of the brain worsens functional recovery post-TBI, peripherally-derived monocytes have corresponded with increases in anti-inflammatory markers 7 days post-TBI in young mice (Semple et al., 2010; Hsieh et al., 2014; Gyoneva et al., 2015; Morganti et al., 2015). These data suggest that chronic deficits of anti-inflammatory properties in the aged animals may be due to diminished effectiveness of peripherally-derived monocytes. Rather than weakening the pro-inflammatory response, therapies that reestablish or supplement the anti-inflammatory pathways of peripherally-derived monocytes may provide more robust results for treating TBI in the elderly. Our results also indicate these treatments may have a prolonged treatment window post-injury (Chou et al., 2018).
The hinderance of CCR2+ monocyte permeation in young animals significantly lowers both anti- and pro-inflammatory responses and leads to overall cognitive improvements post-TBI (Hsieh et al., 2014; Morganti et al., 2015). Inhibiting monocyte infiltration utilizing a CCR2 agonist leads to a diminished acute inflammatory response in aged animals, post-TBI (Morganti et al., 2016). In our study, we found that radial arm water maze (RAWM) scores were significantly affected by age for both sham and TBI animals, agreeing with published studies in which age has been the sole factor in reduced cognition (Rosenweig and Barnes, 2003; Villeda et al., 2014). The avoidance of monocyte infiltration via CCR2 knockout improved TBI-related functional deficits for spatial memory in aged animals. However, RAWM scores reflected a persistent learning impairment on training days (Chou et al., 2018). This experimental design primarily accounted for the effects of peripherally-derived monocytes and disregarded microglia/macrophages. Microglia activation post-injury has led to chronic inflammation and exacerbated cognitive deficits, comparable to peripherally-derived monocytes (Jin et al., 2012; Loane and Kumar, 2016). The effects of age on the microglial response post-injury has yet to be differentiated from the peripherally-derived monocytes effect, as the endogenous brain microglia population may correct for the lack of monocyte permeation in our study. It is vital to acknowledge a possible secondary injury cascade, which could negatively impact TBI outcomes in conjunction with monocyte infiltration in aged animals.
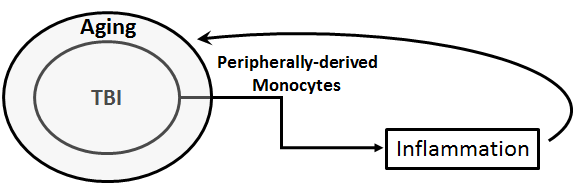
In a new window | Download PPT
Figure 1: Schematic diagram of monocytes as a biomarker for TBI. Peripherally-derived monocytes may serve as a biomarker for TBI, which is exacerbated by aging. These monocytes may reflect the TBI-aging effects on inflammation, and also the resulting inflammatory feedback response contributing to the pathology of TBI-aging condition.
Conclusion
To conclude, altogether these studies significantly widen our comprehension of how age modulates the peripherally-derived monocytes response, in particular the TBI inflammatory response (Figure 1), and open new possibilities for targeted therapies for elderly TBI patients. Specifically, we emphasized the potential of peripherally-derived monocytes as an age-specific biomarker and stressed the deficiencies of anti-inflammatory pathways post-TBI for older cohorts. We are optimistic these results can expedite translational research to increase positive outcomes for the population at highest risk for TBI.
References
Susanna Rosi1, 2, 3, 4, 5
1Department of Physical Therapy and Rehabilitation Science, University of California, San Francisco, CA 94143, USA.
2Brain and Spinal Injury Center, University of California, San Francisco, CA 94110, USA.
3Department of Neurological Surgery, University of California, San Francisco, CA 94110, USA.
4Weill Institute for Neuroscience, University of California, San Francisco, CA 94143, USA.
5Kavli Institute of Fundamental Neuroscience, University of California, San Francisco, CA 94143, USA.
Corresponding author:
Susanna Rosi
Email: susanna.rosi@ucsf.edu

In a new window | Download PPT
Figure 1: Schematic diagram of monocytes as a biomarker for TBI. Peripherally-derived monocytes may serve as a biomarker for TBI, which is exacerbated by aging. These monocytes may reflect the TBI-aging effects on inflammation, and also the resulting inflammatory feedback response contributing to the pathology of TBI-aging condition.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7117 | 9 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA