Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Role of inflammatory signaling pathways in the efficacy of immunization with neural-derived peptides in spinal cord injury
Time:2019-01-04
Number:8794
Antonio Ibarra1, Elisa García1
Author Affiliations


- 1Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, Mexico.
Conditioning Medicine, 2018. 1(7):346-349.
Abstract
Laboratory evidence suggests that immunization with neural-derived peptides (INDP) exerts neuroprotection against spinal cord injury (SCI). However, the degree of neuroprotection by INDP is affected by the severity of SCI, with lack of functional outcomes in severe SCI after INDP treatment. Because inflammation is closely associated with SCI, a close examination of the resulting inflammatory response may provide clues into the role of inflammatory signaling pathways in SCI pathology and the treatment with INDP. This review article reports on the recent observations that expression of inflammation-related genes (interleukin [IL]-6, IL-12, IL-1β, interferon gamma [IFNγ], tumor necrosis factor alpha [TNFα], IL-10, IL-4, and insulin growth factor-1 [IGF-1]) may differ between moderate and severe SCI-exposed animals treated with INDP peptides, namely A91 and Cop-1. Of interest, INDP treatment in moderate SCI triggers a significant reduction in the expression of pro-inflammatory genes IL-6, IL-1β, and TNFα, and an increase in the anti-inflammatory genes IL-10, IL-4, and IGF-1. In contrast, the reverse was observed with severe SCI in that INDP produced an increase in the expression of IL-12, IL-1β, IFNγ (pro-inflammatory genes), and IGF-1. An in-depth understanding of the inflammatory signaling pathways after SCI may guide optimization strategies for INDP to enhance protective autoimmunity.
Keywords: Immunization with neural-derived peptides, spinal cord injury, protective autoimmunity, inflammation, A91, Cop-1
Abstract
Laboratory evidence suggests that immunization with neural-derived peptides (INDP) exerts neuroprotection against spinal cord injury (SCI). However, the degree of neuroprotection by INDP is affected by the severity of SCI, with lack of functional outcomes in severe SCI after INDP treatment. Because inflammation is closely associated with SCI, a close examination of the resulting inflammatory response may provide clues into the role of inflammatory signaling pathways in SCI pathology and the treatment with INDP. This review article reports on the recent observations that expression of inflammation-related genes (interleukin [IL]-6, IL-12, IL-1β, interferon gamma [IFNγ], tumor necrosis factor alpha [TNFα], IL-10, IL-4, and insulin growth factor-1 [IGF-1]) may differ between moderate and severe SCI-exposed animals treated with INDP peptides, namely A91 and Cop-1. Of interest, INDP treatment in moderate SCI triggers a significant reduction in the expression of pro-inflammatory genes IL-6, IL-1β, and TNFα, and an increase in the anti-inflammatory genes IL-10, IL-4, and IGF-1. In contrast, the reverse was observed with severe SCI in that INDP produced an increase in the expression of IL-12, IL-1β, IFNγ (pro-inflammatory genes), and IGF-1. An in-depth understanding of the inflammatory signaling pathways after SCI may guide optimization strategies for INDP to enhance protective autoimmunity.
Keywords: Immunization with neural-derived peptides, spinal cord injury, protective autoimmunity, inflammation, A91, Cop-1
Introduction
Spinal cord injury (SCI) is followed by several auto-destructive events including intracellular calcium overload, neural fiber injury, metabolic impairment, destruction of microvessels, and breakdown of the blood-spinal cord barrier. In addition, the recruitment of immunological cells such as neutrophils, hematogenous macrophages, and T lymphocytes to the site of injury, along with the activation of resident microglia, triggers an inflammatory response. Several cells and pro-inflammatory cytokines are involved in the inflammatory response, which aggravates lipid peroxidation, free radical production, and demyelination, leading to extensive secondary tissue damage (Bethea et al., 2002; Hausmann et al., 2003; Ibarra et al., 2010). This intensified inflammatory reaction can trigger a pathological autoreactivity response mainly mediated by the activation of T lymphocytes. In this pathological condition, the activation of the pro-inflammatory Th1 phenotype increases demyelination and injury size. On the other side, the activation of a Th2 phenotype could trigger an anti-inflammatory response inducing a neuroprotective microenvironment (Ibarra et al., 2010; Schwartz et al., 2001a, b).
Regulation of immune response and protective autoimmunity for SCI treatment
Immunization with neural-derived peptides (INDP) elicits neuroprotective effects against SCI (Ibarra et al., 2018). The level of SCI may affect the therapeutic effects of INDP (Ibarra et al., 2018). Indeed, INDP is ineffective in severe SCI. Interestingly the expression of inflammation-related genes (interleukin [IL-6], IL-12, IL-1β, interferon gamma [IFNɣ], tumor necrosis factor alpha [TNFα], IL-10, IL-4, and insulin growth factor-1 [IGF-1]) has been analyzed by quantitative PCR in moderate and severe animal models of SCI subjected to INDP (Ibarra et al., 2018). In particular, the effects of INDP have been tested by using two different peptides: A91 and Cop-1 (Ibarra et al., 2018). After a moderate SCI, significant decreases in pro-inflammatory gene expression (IL-6, IL-1β, and TNFα) and increases in anti-inflammatory gene expression (IL-10, IL-4, and IGF-1) have been observed. On the contrary after severe SCI, INDP elicits an increase in the expression of IL-12, IL-1β, IFNɣ (pro-inflammatory genes), and IGF-1. Altogether these data indicate that severe injury is not conducive to the beneficial effects of protective autoimmunity (PA) (Ibarra et al., 2018).
The immune system has a central role in the pathophysiology secondary to SCI (Donnelly et al., 2008). Modulation, rather than inhibition, of the immune response has been shown to be advantageous and stimulates neurological recovery after injury (Garcìa et al., 2012; Rodríguez-Barrera et al., 2013).
PA is a novel approach characterized by the modulation of the immune response after traumatic injuries to the central nervous system (Berger et al., 1997; Hauben et al., 2000; Linington et al., 1993). PA is promoted by immunizing with non-encephalitogenic INDP, including A91 or Cop-1 (Schwartz et al., 2001a; Gaur et al., 1997). A91 is a myelin basic protein (MBP)-derived peptide (sequence of amino acids 87–99), derived by substitution of lysine with alanine at residue 91 (Gaur et al., 1997; Katsara et al., 2009; Samson et al., 1995). Cop-1 is another peptide able to regulate PA. Cop-1 is a random polypeptide synthesized from four amino acids (L-tyrosine, L-glutamic acid, L-alanine, and L-lysine) with an average molar fraction of 0.141, 0.427, 0.095, and 0.0338, respectively (Guan et al., 1997; Liu et al., 2007). However, the underlying mechanisms of the beneficial actions of PA are still unknown. Gene expression could be one of the main mechanisms that underlie the protective effects of PA. Inflammation-related genes like IL-6, IL-12, IL-1β, interferon γ (IFNɣ), TNFα, IL-10, IL-4, and IGF-1, could affect cell function over minutes to hours, or longer periods of time, in a pro- or anti-inflammatory way (Donnelly et al., 2008). However, the neuroprotective effects elicited by INDP have been to be ineffective against severe injuries (Martiñon et al., 2007).
Inflammatory response in moderate and severe SCI after INDP
It has been hypothesized that the absence of a neuroprotective effect exerted by INDP could be associated with the up-regulation of pro-inflammatory and down-regulation of anti-inflammatory genes (Figure 1). In this regard, Garcìa et al., (2018) analyzed the expression of eight inflammation-related genes in moderate and severe SCI animal models immunized with A91 or Cop-1 peptides (Garcìa et al., 2018). In different in vivo models of SCI, changes in gene expression have been reported as an early response (Carmel et al., 2001; Song et al., 2001; Tachibana et al., 2002). Changes in gene expression can be determined after a traumatic insult. Numerous studies have analyzed post-traumatic gene expression in order to identify the biological and functional outcome of SCI (Aimone et al., 2004; Di Giovanni et al., 2003). Garcìa et al., (2018) analyzed the expression of inflammatory-related genes such as IL-6, IL-12, IL-1β, IFNγ, TNFα, IL-10, IL-4, and IGF-1, in moderate and severe SCI (Garcìa et al., 2018). Interestingly, anti-inflammatory cytokines like IL-4 and IL-10 increased in moderate SCI after INDP, suggesting a crucial role for these two cytokines in the protection and restoration of neural tissue (Garcìa et al., 2018, Walsh et al., 2015; Zhou et al., 2009). Moreover, the cytokine profile induced by INDP in this moderate SCI model plays a key role in the modulation of the inflammatory response as it is involved in the activation, differentiation, and proliferation of Th2 lymphocytes (Hausmann et al., 2003). In the same way, this cytokine pattern stimulates M2 macrophage differentiation, promotes macrophage expression of the major histocompatibility complex class II (MHC-II), and reduces the pro-inflammatory cytokines IL-1, IL-6, and TNFα production (Becker and Daniel, 1990; Cao et al., 1989; Crawford et al., 1987; Gerrard et al., 1990; Hart et al., 1989). Indeed, in the study of Garcìa et al., (2018), the INDP-treated moderate SCI rats showed a significant reduction in inflammatory cytokines (Garcìa et al., 2018). The importance of this effect is underscored by the fact that regulation of TNFα, a pro-inflammatory cytokine that stimulates several factors that exacerbate inflammation, such as IL-8, IL-6, IL-1, nitric oxide, peroxide, and prostaglandin (Esposito and Cuzzocrea, 2009, 2011). Interestingly, evidence has shown that INDP can decrease inflammation and lipid peroxidation (Ibarra et al., 2003). On the contrary, INDP was ineffective after severe injury (Martiñón et al., 2012). Garcìa et al., (2018) examined the reason for this lack of effect and found a significant increase in pro-inflammatory cytokine expression in the severe SCI animal model after immunization with A91 or Cop-1 peptides (Garcìa et al., 2018). Notably, INDP increased the expression of IL-12, IL-1β, and IFNγ in this SCI model (Garcìa et al., 2018). However, the underlying mechanism of this cytokine pattern of expression induced by INDP is still unknown.
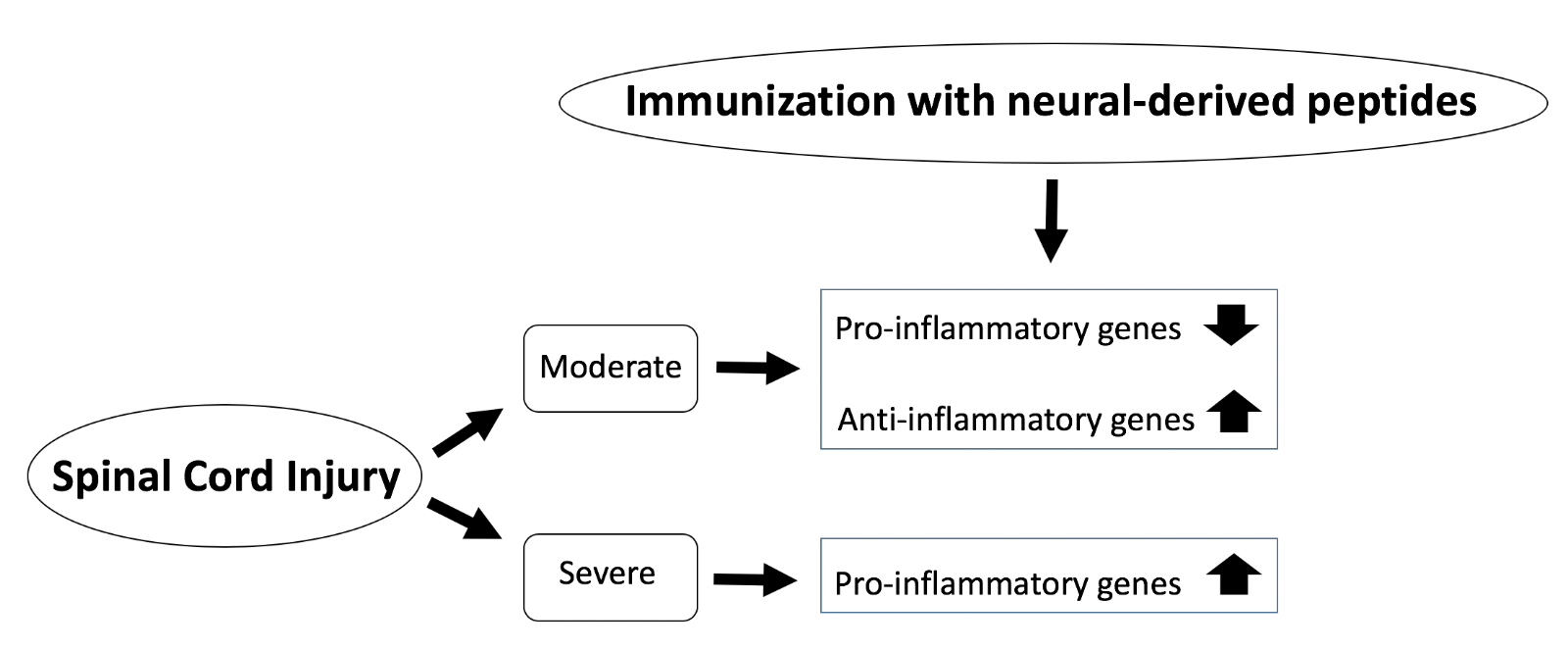
In a new window | Download PPT
Figure 1: Schematic diagram of SCI severity and effects of INDP on inflammation. Following moderate SCI, INDP treatment leads to a dampened pro-inflammatory response and an enhanced anti-inflammatory reaction. On the other hand, after severe SCI, INDP treatment drives the pro-inflammatory response.
High concentrations of tissue protein-like damage-associated molecular patterns (DAMPs) could activate that pattern of inflammatory gene expression by activating the NFkB signaling pathway (Kigerl et al., 2009; Mills et al., 2011). Indeed, severe SCI results in a higher release of DAMPs and neural constituents than moderate SCI. Therefore, a shift of the immune response towards DAMPs along with INDP could shift the immune response towards a Th1 encephalitogenic phenotype (Bielekova et al., 2010). The predominant Th1 phenotype could inhibit the proliferation of Th2 protective cells, and consequently its beneficial roles (Martiñón et al., 2012).
Previous studies have shown that severe injury or excessive administration of INDP can inhibit the beneficial effect of PA (Martiñon et al., 2007, 2012). However, a significant increase in IGF-1 in A91 and Cop-1 immunized animals with severe SCI, has been reported by Garcìa et al., (2018). IGF-1, as well as transforming growth factor-β, and platelet-derived growth factor, could be produced by peripheral and resident macrophages. IGF-1 is also released by astrocytes and endothelial cells. Interestingly IGF-1 down-regulates several pro-inflammatory cytokines, such as TNFα, IL-1β, and IL-6 (Higashi et al., 2010). The enhanced IGF-1 concentration could be a response to the high levels of pro-inflammatory cytokines (TNFα, IL-1β, and IL-6), especially in the severe SCI animal model.
Furthermore, the anti-inflammatory cytokine IL-10 can decrease IL-1α, IL-1β, IL-8, IL-12, IFNα, inducible nitric oxide synthase (iNOS), and TNFα production (Clemens et al., 1991; Howard and O’Garra, 1992; Moore et al., 2001). Previous reports suggested that the application of IL-10 could reduce the inflammatory response and increase neuron survival after SCI (Abraham et al., 2004; Brewer et al., 1999). Garcìa et al. (2018) showed that animals with INDP subjected to severe SCI did not have any significant production of IL-10 (Garcìa et al., 2018). On the other hand, rats with moderate SCI had a significant production of that cytokine (Garcìa et al., 2018). These results support the hypothesis that an intense inflammatory response in severe SCI influences the effects of INDP, while INDP promotes beneficial effects in moderate SCI in which the microenvironment is propitious for neuroprotection and neurogeneration.
Interestingly, A91 and Cop-1 can induce the production of neurotrophic factors such as brain derived neurotrophic factor, neurotrophin 3, and IGF-1 (Martiñón et al., 2012; Rodríguez- Barrera et al., 2017; Binbin et al., 2010). In turn, these neurotrophic factors could modulate cytokines production by either reducing IL-1β and TNFα or increasing IL-10 (Martiñon et al., 2016; Ji et al., 2015). Accordingly, the pattern of cytokine expression reported by Garcìa et al., (2018) could be due to neurotrophic factor production (Garcìa et al., 2018).
Conclusion and future directions
INDP is neuroprotective in SCI animal models by upregulating anti-inflammatory and down-regulating pro-inflammatory genes, correlating with a previous study that showed a reduction in iNOS expression and nitric oxide production after INDP treatment (Garcìa et al., 2012). Moreover, IL-10 and IL-4 can down-regulate iNOS expression (Garcìa et al., 2012). Finally, further studies on the use of INDP against SCI are needed to expand our knowledge into its neuroprotective effects and its potential to treat SCI.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
Antonio Ibarra1
1Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, Mexico.
Elisa García1
1Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Campus Norte; Huixquilucan, Edo. de México, Mexico.
Corresponding author:
Dr. José Juan Antonio Ibarra Arias
Email: jose.ibarra@anahuac.mx

In a new window | Download PPT
Figure 1: Schematic diagram of SCI severity and effects of INDP on inflammation. Following moderate SCI, INDP treatment leads to a dampened pro-inflammatory response and an enhanced anti-inflammatory reaction. On the other hand, after severe SCI, INDP treatment drives the pro-inflammatory response.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8794 | 15 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA