Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Potential role of c-Jun N-terminal kinase in ischemic preconditioning and neuroprotection
Time:2019-01-04
Number:7530
Joo Eun Jung1, Eng H. Lo2
Author Affiliations


- 1Department of Neurology, University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
- 2Neuroprotection Research Laboratory, Departments of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Conditioning Medicine, 2018. 1(7):327-336.
Abstract
Brain preconditioning, a sub-lethal insult that enables resistance to a subsequent lethal injury, has been widely studied as a neuroprotective therapy against several neurodegenerative diseases, various brain-associated injuries including cerebral ischemia, hemorrhage, neonatal hypoxic injury, and trauma. Clinical trials using ischemic preconditioning or remote ischemic preconditioning have investigated the beneficial effects of brain preconditioning in cerebrovascular-related injuries or diseases such as ischemic stroke and subarachnoid hemorrhage.
A wide spectrum of preconditioning stimuli, including transient global/focal cerebral ischemia, remote ischemia, hypoxia, hypothermia, and various pharmacological treatments, have been used to protect the brain in in vivo experimental animal studies, and in in vitro cell culture systems. The signaling molecules and pathways triggered by preconditioning stimuli, which are involved in cytoprotective mechanisms, have only been recently identified. c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinases (MAPKs) and is activated by various cellular stresses including ischemic injury. JNK signaling is well known as one of the key pathways for regulating neuronal death in many central nervous system injuries. However, JNK signaling has a dual role and is also involved in neuronal survival in brain preconditioning.
In this mini review, we discuss the current clinical trials for ischemic preconditioning and experimental brain ischemic preconditioning, and examine the role of JNK in effective preconditioning against cerebral ischemic stroke.
Keywords: Central nervous system (CNS) injury, Cerebral ischemic stroke, Brain ischemic preconditioning, Neuroprotection, c-Jun N-terminal kinase (JNK)
Abstract
Brain preconditioning, a sub-lethal insult that enables resistance to a subsequent lethal injury, has been widely studied as a neuroprotective therapy against several neurodegenerative diseases, various brain-associated injuries including cerebral ischemia, hemorrhage, neonatal hypoxic injury, and trauma. Clinical trials using ischemic preconditioning or remote ischemic preconditioning have investigated the beneficial effects of brain preconditioning in cerebrovascular-related injuries or diseases such as ischemic stroke and subarachnoid hemorrhage.
A wide spectrum of preconditioning stimuli, including transient global/focal cerebral ischemia, remote ischemia, hypoxia, hypothermia, and various pharmacological treatments, have been used to protect the brain in in vivo experimental animal studies, and in in vitro cell culture systems. The signaling molecules and pathways triggered by preconditioning stimuli, which are involved in cytoprotective mechanisms, have only been recently identified. c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinases (MAPKs) and is activated by various cellular stresses including ischemic injury. JNK signaling is well known as one of the key pathways for regulating neuronal death in many central nervous system injuries. However, JNK signaling has a dual role and is also involved in neuronal survival in brain preconditioning.
In this mini review, we discuss the current clinical trials for ischemic preconditioning and experimental brain ischemic preconditioning, and examine the role of JNK in effective preconditioning against cerebral ischemic stroke.
Keywords: Central nervous system (CNS) injury, Cerebral ischemic stroke, Brain ischemic preconditioning, Neuroprotection, c-Jun N-terminal kinase (JNK)
Introduction
Several clinical trials have examined the neuroprotective effects of preconditioning against cerebral ischemic vascular- or subarachnoid hemorrhage (SAH)-related brain conditions. Indeed, ischemic preconditioning and remote limb ischemic preconditioning have been employed to attenuate damage induced by ischemic stroke, SAH, multiple sclerosis, subcortical vascular dementia, and brain injury carotid endarterectomy. Many preclinical studies using in vivo animal models and in vitro cell culture systems have demonstrated the neuroprotective effects of ischemic preconditioning in brain ischemia- or hemorrhage-related injuries, as well as many other central nervous system (CNS) injuries. However, there are still several gaps in our current knowledge that must be addressed to translate the preclinical results into clinical applications for humans.
Preclinical studies aiming to uncover the central molecule or signaling pathway that plays a pivotal role in ischemic preconditioning-mediated neuroprotective effects have successfully identified multiple molecules and signaling pathways of interest. One such pathway is the c-Jun N-terminal kinase (JNK) signaling pathway, which is involved in both neuronal death and survival in various CNS injuries, and thus, has been widely studied as a therapeutic target for brain ischemia and many other brain diseases.
In this mini review, we examine the role of JNK in the neuroprotective effects of preconditioning against cerebral ischemic stroke. A deeper understanding of the molecular mechanisms surrounding brain preconditioning will enable us to develop better therapeutic strategies that more effectively protect the brain against acute or chronic CNS injury.
1. History of the translational approach of ischemic preconditioning to the bedside; clinical trials of preconditioning in cerebrovascular diseases.
Since preclinical studies and human meta-analysis have demonstrated potential neuroprotective and cytoprotective effects of limb ischemic preconditioning (Lehotsky et al., 2009; Ayodele and Koch, 2017), many clinical trials have examined the safety and feasibility of preconditioning against various injuries (Lehotsky et al., 2009; Ayodele and Koch, 2017) (find more information at clinical.org by cross-referencing “Limb preconditioning” as a keyword). So far, several clinical trials examining the efficacy of remote ischemic preconditioning (RIPC) against cerebral ischemic vascular disease or SAH have been completed (Table 1). In addition, there are still several ongoing (as of October 6, 2018) clinical trials using ischemic preconditioning or limb RIPC to determine if preconditioning improves stroke rehabilitation or SAH outcomes, and to investigate the beneficial effects or feasibility of using preconditioning to treat ischemic stroke, SAH, multiple sclerosis, subcortical vascular dementia, and brain injury carotid endarterectomy (Table 2). Among the cerebrovascular diseases, SAH may be a reasonable model to test whether preconditioning is an effective clinical remedy for reducing SAH-induced brain damage (Koch, 2010; Koch et al., 2011). SAH is caused by an initial primary hemorrhage that is followed by other SAH hallmarks, such as cerebral vasospasm and delayed cerebral ischemia, which are mainly amplified by increased inflammation and activation of various cellular signaling pathways that leads to vasoconstriction (Macdonald et al., 2007). After a SAH, it normally takes 4-10 days until delayed cerebral ischemia is apparent, so limb ischemic pre-, per-, or post-conditioning could be employed during this period (Pluta et al., 2009; Vergouwen et al., 2010).
Table 1. Completed clinical trials (stroke- and subarachnoid hemorrhage-related).
|
Identifier |
Title |
Aims descripted in the study |
Actual Enrollment |
|
NCT01118000 |
Study on the Cardioprotection and Humoral Mechanism of Limb Ischemia Preconditioning |
To assess whether limb ischemic preconditioning protects remote tissue or organs through a humoral mechanism |
60 |
|
NCT03072914 |
Effects of Remote Ischemic Preconditioning With Postconditioning on Neurologic Outcome |
To evaluate whether RIPC with RIPostC reduce the major neurocomplication in patients undergoing STA-MCA anastomosis |
108 |
|
NCT01110239 |
Preconditioning for Aneurismal Subarachnoid Hemorrhage |
To determine if RIPC can be safely and effectively instituted in patients with SAH |
34 |
|
NCT02602977 |
the Influence of Remote Ischemic Preconditioning on Inflammation During Human Endotoxemia (RISPENDO) |
To investigate the effects of (repeated) ischemic preconditioning on inflammation during human endotoxemia |
30 |
|
NCT01158508 |
Remote Ischemic Preconditioning in Subarachnoid Hemorrhage (RIPC-SAH) |
To study effect of RIPC on cerebral vasospasm following SAH |
20 |
|
NCT01658306 |
Clinical Trial on Remote Ischemic Preconditioning and Cerebral Small Vessel Disease (RIPC-SVD) |
To test if RIPC might have a beneficial effect on outcomes of cerebral small vessel disease |
30 |
|
NCT02177981 |
Impact of Remote Ischemic Preconditioning Preceding Coronary Artery Bypass Grafting on Inducing nEeuroprotection (RIPCAGE) |
To determine whether RIPC can reduce the adverse impact of cardiopulmonary bypass on neurological outcome |
70 |
|
NCT01321749 |
The Neuroprotective Effect of Remote Ischemic Preconditioning on Ischemic Cerebral Vascular Disease |
To observe the effect of RIPC on ischemic cerebral vascular disease |
196 |
Several clinical trials using RIPC to investigate any beneficial effects in cerebral ischemic vascular disease or SAH have been completed (found at ClinicalTrials.gov).
Table 2. Current clinical trials.
|
Identifier |
Title |
Condition or disease |
Aims descripted in the study |
Estimated Enrollment (As of Oct. 6th, 2018) |
|
NCT02411266 |
Preconditioning With Limb Ischemia for Subarachnoid Hemorrhage (PreLIMBS) |
Ischemic Preconditioning |
A study of limb preconditioning in subjects with SAH who are at high risk of cerebral ischemia in the first 2 weeks after hemorrhage |
60 |
|
NCT03023150 |
Ischemic Preconditioning as an Intervention to Improve Stroke Rehabilitation – Froedtert |
Ischemic Preconditioning |
A study to use ischemic preconditioning as an intervention to improve stroke rehabilitation |
50 (active, not recruiting) |
|
NCT02381522 |
Remote Ischemic Pre-conditioning in Subarachnoid Hemorrhage (RIPC-SAH) |
Brain Aneurysms |
To investigate the effect of limb ischemia preconditioning on SAH outcome (cerebral vasospasm) |
100 |
|
NCT02997748 |
Remote Ischemic Preconditioning After Cardiac Surgery (RIPCRenal) |
Cardiac Surgery, Aortocoronary Bypass |
To reduce the incidence of AKI by implementing RIPC and to evaluate the dose-response relationship using the biomarkers urinary [TIMP-2] *[IGFBP7] in high risk patients undergoing cardiac surgery |
180 |
|
NCT02169739 |
Remote Preconditioning Over Time To Empower Cerebral Tissue (REM-PROTECT) |
Ischemic Stroke |
To assess feasibility of ischemic preconditioning by preconditioning device |
60 |
|
NCT03153553 |
Ischemic Preconditioning, Exercise Tolerance and Multiple Sclerosis |
Multiple Sclerosis |
To see whether it is feasible to use ischemic preconditioning to improve exercise performance in people with multiple sclerosis |
40 |
|
NCT03022149 |
Remote Ischemic Preconditioning for Subcortical Vascular Dementia (RIPSVD) |
Subcortical Vascular Dementia |
To determine whether the RIPC are effective in the treatment of mild to moderate vascular dementia |
52 (active, not recruiting) |
|
NCT03624452 |
Remote Ischaemic Preconditioning Combined With Exercise Training on Vascular Function. |
Cardiovascular Disease Risk |
To investigate whether combining 8 weeks of exercise and RIPC is more beneficial to systemic vascular function/ (Cerebro) vascular function than exercise alone |
60 |
|
NCT02553655 |
Remote Ischemic Limb Preconditioning In Healthy Volunteers |
Cerebrovascular Disease |
To determine if remote ischemic leg preconditioning in healthy volunteers improves cerebral vasomotor reactivity |
30 |
|
NCT03598855 |
Ischemic Preconditioning and Type 2 Diabetes |
Type 2 Diabetes |
To determine the impact of 7 days of daily ischemic preconditioning on vascular function and insulin sensitivity in type 2 diabetes mellitus |
21 (recruitment completed) |
|
NCT03027011 |
Remote Ischemic Preconditioning on Brain Injury in Carotid Endarterectomy |
Remote Ischemic Preconditioning |
To test an intervention (RIPC) in patients undergoing carotid endarterectomy |
40 |
|
NCT03474952 |
Effects of Remote Ischemic Preconditioning During Free Flap Reconstruction |
Remote ischemic preconditioning (RIPC) |
To evaluate the effect of RIPC on tissue oxygen saturation and skin temperature of the flap |
50 |
Several on-going clinical trials (which are “recruiting or completed recruitment”) to assess the beneficial effects or feasibility of ischemic preconditioning or RIPC in cerebral ischemia, SAH, multiple sclerosis, subcortical vascular dementia, and brain injury (found at ClinicalTrials.gov).
Koch and colleagues initiated a clinical trial to determine the feasibility and safety of a limb ischemic preconditioning regimen (three instances of the following cycle: 5 min of inflation to 200 mm Hg/5 min of reperfusion using a blood pressure cuff) against SAH (Koch et al., 2011). In a phase 2b study (total of 33 participating patients), the research team reported that limb preconditioning is safe and well tolerated, and that even an increased duration of ischemia (to 10 min) is safe (Koch et al., 2011). The phase 2 study (PreLIMBS) is being conducted with a relatively large patient population (60 patients) to further evaluate the safety and feasibility (as the primary outcome measurement), as well as the clinical outcome (as the secondary outcome measurement) of limb ischemic preconditioning against SAH (ClinicalTrials.gov Identifier: NCT02411266). Other clinical trials have been conducted to explore the feasibility and safety of limb preconditioning in other conditions, such as acute kidney injury in patients undergoing coronary artery bypass graft surgery, symptomatic intracranial stenosis (Wang et al., 2017), heart and lung injury after abdominal aortic aneurysm repair (Li et al., 2013), carotid artery stenting (Zhao et al., 2017), and other vascular surgeries (find more information at clinical.org).
However, there are still gaps in our current knowledge that need to be filled in order to successfully translate preclinical findings from in vivo animal and in vitro cell models to patients. For instance, pertinent biomarkers must be found and assessed to determine when the effects of preconditioning will be most beneficial and how long these effects last in the human body. Additionally, it is imperative to optimize the ischemic preconditioning protocols by choosing the appropriate stimulus and duration of the preconditioning stress. Furthermore, age, sex, and comorbidities, which elderly patients may have, should be considered to generate a scientifically proven, well-designed protocol that can be applied in clinical trials. Indeed, comorbidities such as hyperglycemia and hyperlipidemia may interfere with the beneficial effects of preconditioning (Kersten et al., 1998; Kehl et al., 2002; Ungi et al., 2005; Giricz et al., 2006; Yang et al., 2013; Schenning et al., 2015; Ayodele and Koch, 2017). Moreover, older patients (over 65 years of age) do not receive myocardial protection induced by pre-infarction angina, (a form of clinical preconditioning), suggesting that advanced age may nullify the beneficial effects of preconditioning (Abete et al., 1997).
2. Experimental brain ischemic preconditioning in vivo or in vitro
Cerebral ischemic stroke caused by blocking blood flow and oxygen to the brain is one of the major CNS injuries, leading to severe damage and death of brain tissue. While many studies have attempted to develop a neuroprotective drug against stroke in animal models, only tissue plasminogen activator (tPA) has been applied clinically.
An alternative approach to stroke therapy, ischemic preconditioning that induces tolerance to subsequent severe focal or global ischemia, has been largely studied for many years (Liu et al., 1992; Matsushima and Hakim, 1995; Stagliano et al., 1999; Cardenas et al., 2002; Zhou et al., 2004; Stetler et al., 2014; Chen et al., 2018b). The primary brain ischemia model using rodents is transient or permanent middle cerebral artery occlusion (MCAO) induced by insertion of an intraluminal suture (Stetler et al., 2014; Fluri et al., 2015). After a certain duration of occlusion, the suture is withdrawn, allowing reperfusion to occur (transient focal ischemia) (Stetler et al., 2014; Fluri et al., 2015). Alternatively, permanent ligation of the MCA without reperfusion (permanent focal ischemia) generates a severe cerebral ischemic injury (Stetler et al., 2014; Fluri et al., 2015). The MCAO model is commonly used in rodent stroke research and generates a reproducible infarction (extent of injury is dependent on occlusion time) in the cortex and striatum, forming an ischemic core and a penumbra surrounding the core (Stetler et al., 2014; Fluri et al., 2015). In addition, the MCAO model is similar to an ischemic stroke in humans because the infarction generated by thrombotic occlusion is localized mainly in the MCA region of the human brain (Fluri et al., 2015; Sommer, 2017). To produce ischemic tolerance against subsequent lethal ischemia, the MCAO method (either transiently or permanently induced) has been commonly used as a preconditioning stimulus (Stagliano et al., 1999; Cardenas et al., 2002; Chen et al., 2018b). One or three 5 min incidents of a brief MCAO before a severe 1h MCAO significantly reduces infarct volume (Stagliano et al., 1999). A short ischemic preconditioning event caused by 10 min of transient MCAO reduces the infarct volume in rat brains subjected to subsequent permanent MCAO (Cardenas et al., 2002). In rats treated with ischemic preconditioning involving 10 min of MCAO prior to 90 min of subsequent severe MCAO, neurological functional deficits, cerebral infarct size, and neuronal death decrease (Chen et al., 2018b).
Cardiac arrest induced by obstructing blood circulation can also cause brain ischemia (Safar, 1986; Petito et al., 1987; Mangus et al., 2014; Stetler et al., 2014). Global ischemia has generally been used in animal stroke models to simulate cerebral ischemic injury caused by cardiac arrest (Petito et al., 1987; Stetler et al., 2014), and this model specifically induces delayed neuronal degeneration in the hippocampal CA1 region. In this model, carotid arteries are occluded transiently, and vertebral arteries are then permanently ligated by electrocoagulation.
Global ischemia has also been used to induce ischemic preconditioning, producing ischemic tolerance to subsequent severe ischemic damage from either focal or global ischemic insults (Liu et al., 1992; Matsushima and Hakim, 1995; Zhou et al., 2004; Stetler et al., 2014). The brief bilateral common carotid artery occlusion (BCCAO) method with vertebral artery ligation or BCCAO with induction of systemic hypotension has also been used to induce global ischemic preconditioning (Liu et al., 1992; Matsushima and Hakim, 1995; Zhou et al., 2004; Stetler et al., 2014). In adult rats, 2 min of global ischemia by BCCAO as an ischemic preconditioning stimulus generates robust neuroprotection in the hippocampal CA1 region following 30 min of reperfusion, 10 min of lethal global ischemia, and 3 days of reperfusion (Perez-Pinzon et al., 1997). Additionally, BCCAO as a preconditioning stimulus 30 min before subsequent MCAO significantly reduces infarct volumes in mice brains, suggesting that the optimal time between the preconditioning stimulus and subsequent lethal focal ischemia is 30 min (Speetzen et al., 2013).
As discussed above, ischemic preconditioning in animal focal or global stroke models have shown neuroprotective effects. However, in clinical situations, cerebral ischemic preconditioning is difficult to apply due to potential safety and ethical issues in patients (Leape, 2005; Dave et al., 2006; Ren et al., 2008; Hu et al., 2012). Therefore, the use of remote ischemic preconditioning (RIPC) has been considered as an alternative method to ischemic preconditioning (Dave et al., 2006; Ren et al., 2008; Hu et al., 2012). RIPC is minimally invasive and potentially provides a wider therapeutic time window (Dave et al., 2006; Ren et al., 2008; Hu et al., 2012). Non-invasive RIPC of a limb induces neuroprotection against MCAO via changes in the composition of peripheral immune cells (Liu et al., 2016). Four repeated cycles of brief blood flow constriction in the hind-limbs produced an immunomodulatory effect in the spleen during RIPC-mediated neuroprotection against MCAO (Chen et al., 2018a).
Ischemic preconditioning in in vitro cell culture models has also been widely studied. The oxygen/glucose deprivation (OGD) model is the most common method for mimicking ischemic preconditioning in vitro. Short term OGD preconditioning (0.5 h duration) in cultured rodent cerebral endothelial cells prior to a lethal 2.5 h OGD exposure increased cell viability and blood-brain barrier (BBB) integrity via stabilization of tight junction proteins (An and Xue, 2009). Cultured hippocampal neurons exposed to brief OGD as the ischemic preconditioning stimulus significantly reduced neuronal death after a subsequent lethal OGD injury (Keasey et al., 2016). Additionally, ischemic OGD preconditioning protected cultured rodent astrocytes from a subsequent lethal OGD exposure (Narayanan et al., 2018). Exposure to hypoxic conditions (experimental animals or cells in 8% O2 chamber) is another method for inducing ischemic preconditioning against ischemic brain injury that has been largely studied in in vitro and in vivo systems (Gidday et al., 1994; Miller et al., 2001; Bernaudin et al., 2002; Lin et al., 2003; Tang et al., 2006; Zhang et al., 2006a; Zhang et al., 2007b; Zhan et al., 2010; Fan et al., 2011; Yang et al., 2017; Zhan et al., 2017; Zhan et al., 2018). Pre-exposure to hypoxia during the perinatal period protects the neonatal rat brain from hypoxic-ischemic injury (Gidday et al., 1994). Additionally, hypoxic preconditioning is neuroprotective against global cerebral ischemia in adult rats (Zhan et al., 2017; Zhan et al., 2018), and protects rats from cerebral ischemic injury in MCAO models (Wacker et al., 2009; Yang et al., 2017).
3. Ischemic preconditioning for neuroprotection and JNK signaling
Many studies have demonstrated the neuroprotective effects of ischemic preconditioning and other preconditioning treatments against severe subsequent cerebral ischemia, as well as the molecular mechanisms involved in this neuroprotection. C-Jun N-terminal kinase (JNK) is a family of mitogen-activated protein kinases (MAPKs) associated with the cell death accompanying various brain injuries (Ip and Davis, 1998; Irving and Bamford, 2002; Brecht et al., 2005; Kaiser et al., 2005; Kuan and Burke, 2005; Bogoyevitch and Kobe, 2006; Johnson and Nakamura, 2007; Atochin et al., 2016; Kim et al., 2017; Shvedova et al., 2018). This signaling pathway is activated by various cellular stresses such as oxidative stress, inflammation, heat, and osmotic shock (Ip and Davis, 1998; Irving and Bamford, 2002; Brecht et al., 2005; Kaiser et al., 2005; Kuan and Burke, 2005; Bogoyevitch and Kobe, 2006; Johnson and Nakamura, 2007; Atochin et al., 2016; Kim et al., 2017; Shvedova et al., 2018). The JNK signaling pathway plays a central role in cerebral ischemic injury, as well as in myocardial ischemic injury (Kuan et al., 2003; Kaiser et al., 2005; Johnson and Nakamura, 2007; Pei et al., 2008; Shvedova et al., 2018). JNK phosphorylation is induced and its signaling pathways are triggered after cerebral ischemia in rodent models (Hayashi et al., 2000; Irving and Bamford, 2002; Borsello et al., 2003; Ferrer et al., 2003; Okuno et al., 2004; Tian et al., 2005; Kamada et al., 2007; Atochin et al., 2016), and JNK activation exacerbates brain damage following stroke (Davis, 2000; Okuno et al., 2004; Okami et al., 2013). In JNK1 knockout mice subjected to permanent MCAO, brain infarct size significantly increase relative to wild-type mice subjected to MCAO (Brecht et al., 2005). JNK activation caused by an ischemic injury contributes to neuronal cell death through phosphorylation of Bcl-2-associated death promoter (Bad), induction of apoptosis regulator Bim and Fas, activation of caspases -3, -8, and -9, and release of mitochondrial cytochrome c (Kuan et al., 2003; Okuno et al., 2004; Carboni et al., 2005; Kamada et al., 2007; Li et al., 2010). However, JNK also plays a dual role in cell death and survival during cerebral ischemia (Waetzig and Herdegen, 2005).
Here, we discuss the dual role of JNK in ischemic preconditioning and various other forms of cerebral preconditioning to subsequent cerebral ischemia. Currently, around 140 studies have reported the relationship between the JNK signaling pathway and preconditioning in various cells, tissues, and organs under many different physiological and pathophysiological conditions (found by searching PubMed with keyword “JNK and preconditioning”). Among these studies, 41 reports involve the connection between JNK and brain preconditioning in various brain diseases and injuries (Table 3) (found by searching PubMed with keyword “JNK and preconditioning and brain”). Pretreatment with 3 min of ischemic preconditioning via four-vessel occlusion prevents the phosphorylation of JNK1 induced by 6 min of lethal ischemia in rat brains (Gu et al., 2000). While 6 min of lethal ischemia significantly reduces neuronal density in the CA1 hippocampal region, 3 min of ischemic preconditioning rescues these CA1 pyramidal cells (Gu et al., 2000). Moreover, in Mongolian gerbils, after ischemic injury by 6 min of bilateral carotid occlusion, JNK expression is highly increased and histological damage occurs in the hippocampal CA1 region. However, animals receiving 2 min of ischemic preconditioning do not experience an increase in JNK expression and lack histological damage in hippocampal and cortical regions (Colangelo et al., 2004). In the rat global ischemia model, 3 min of ischemic preconditioning generated by four-vessel occlusion down-regulates activation of JNK1/2 via N-methyl-D-aspartate (NMDA) receptor-mediated Akt1 activation, and induces neuroprotection in the hippocampal CA1 region (Miao et al., 2005). Similarly, 3 min of ischemic preconditioning with four-vessel occlusion in rat brains subjected to transient global ischemia protects pyramidal neurons in the hippocampal CA1 region and down-regulates activation of c-Jun (Wang et al., 2016b). Additionally, in rat brains, 3 min of ischemic preconditioning by four-vessel occlusion significantly reduces JNK3 activation and apoptotic neuronal death in the CA1 hippocampal region after 8 min of subsequent lethal ischemia (Zhang et al., 2009). This protective effect is abolished by a NMDA receptor antagonist, suggesting that the neuroprotection caused by ischemic preconditioning needs NMDA receptor-mediated JNK3 inhibition (Zhang et al., 2009). In in vitro models of NMDA preconditioning in primary rat cultured neurons, NMDA preconditioning-induced neuroprotection against glutamate toxicity is mediated through JNK inactivation (Navon et al., 2012). Furthermore, in in vitro models of OGD-induced cell death in cultured microglia, isoflurane preconditioning inhibits activation of TLR4 and JNK, attenuates OGD-induced cell death, and significantly reduces the level of pro-inflammatory cytokines from cultured microglia (Xiang et al., 2014).
Table 3. Studies on the role of JNK in brain preconditioning.
Among 140 studies (found by searching PubMed with keyword “JNK and preconditioning”), 41 studies involve the connection between JNK and brain preconditioning in various brain diseases or injuries (found by searching PubMed with keyword “JNK and preconditioning and brain”). The table was categorized by brain injury model and preconditioning stimulus.
However, there are several contradicting studies regarding the role of JNK in ischemic preconditioning-mediated neuroprotection. In Mongolian gerbil brains, 2.5 min of ischemic preconditioning via bilateral common carotid artery occlusion, followed by 5 min of lethal ischemia, induces c-Jun expression in the hippocampal CA1 region and prevents delayed neuronal cell death (Sommer et al., 1995). Fifteen minutes of ischemic preconditioning via OGD in cultured astrocytes significantly reduces cell death generated by 8 hrs of ischemic injury. Of note, this ischemic preconditioning also promotes JNK activation, and the neuroprotective effects are eliminated upon JNK inhibition (Pang et al., 2015). Moreover, ischemic preconditioning protects primary astrocytes against ischemic injury by upregulating the protective protein 14-3-3γ. However, inhibiting JNK with SP600125, a specific inhibitor of JNK, diminishes the ischemic preconditioning-associated up-regulation of 14-3-3γ (Pang et al., 2015). Thrombin-induced ischemic tolerance protects neurons against subsequent OGD-induced ischemia in vitro, and protects the brain from MCAO-related damage in vivo, through JNK activation (Granziera et al., 2007). For example, pre-treatment with a low dose of thrombin (0.01U/ml) for 1 h prior to 24 hrs of OGD prevents neuronal cell death in rat hippocampal organotypic slice cultures, and JNK inhibition prevents this thrombin preconditioning-induced neuroprotective effect (Granziera et al., 2007). In addition, intracerebroventricular injection of thrombin 1 day before MCAO significantly reduces the lesion size in mice MCAO brains, and improves functional recovery (Granziera et al., 2007). Again, JNK inhibition blocks this thrombin preconditioning-induced neuroprotective effect and prevents the reduction in infarct volume observed in the MCAO mice brains pretreated with thrombin, thus, indicating that JNK is involved in thrombin preconditioning-induced neuroprotection against transient cerebral ischemia (Granziera et al., 2007). In Mongolian gerbil brains, preconditioning via intraperitoneal administration of a low dose (3 mg/kg) of 3-nitropionic acid induces JNK activation in the hippocampal CA1 region 2 days after injection and prevents delayed neuronal cell death against subsequent ischemia in the hippocampus (Sugino et al., 2000). Preconditioning with 30 min of hypothermia (33 °C) also generates neuroprotective effects in the cerebral cortex of rats following traumatic brain injury through early activation of JNK (Lotocki et al., 2006). In addition, hypothermic preconditioning decreases tumor necrosis factor (TNF) ligand-receptor-1 expression via early JNK activation, triggering neuroprotective signal cascades and suppressing both caspase-3 activation and cleavage of X-linked inhibitor of apoptosis protein (XIAP) during later time points (Lotocki et al., 2006). However, it is also possible that JNK signaling has no role in cerebral ischemic preconditioning-induced ischemic tolerance in the brain. Cerebral ischemic preconditioning in the rat brain up-regulates glial glutamate transporter 1 (GLT-1), which is important for brain ischemic tolerance, and significantly increases the phosphorylation of p-38 MAPK, but does not induce any changes in p-ERK1/2 or p-JNK in the hippocampal CA1 region (Zhang et al., 2017). Moreover, inhibition of p-p38 MAPK suppresses ischemic tolerance in the brain, suggesting that p-p38 MAPK is involved in GLT-1-mediated ischemic tolerance following cerebral ischemic preconditioning (Zhang et al., 2017). When MAPKs and PI3K/Akt were examined in cultured rat cerebral cortical neurons preconditioned with 1 h of OGD prior to 3 hrs of lethal OGD, no relative changes in the phosphorylation or expression of JNK, ERK1/2, and p38 were observed (Bhuiyan et al., 2011). Only Akt showed significant changes in its phosphorylation after ischemic preconditioning with OGD, and Akt inhibition abolished these ischemic preconditioning-induced neuroprotective effects (Bhuiyan et al., 2011).
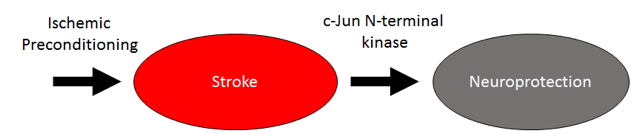
In a new window | Download PPT
Figure 1: Signaling pathway of ischemic preconditioning. There is scientific evidence to suggest that JNK mediates the neuroprotective effects of ischemic preconditioning in stroke.
Conclusion
Many clinical and pre-clinical studies have been conducted to develop therapeutic treatments for cerebral ischemia and various other CNS injuries. Ischemic preconditioning, including RIPC, is one of main approaches to protect brain or neuronal cells against cerebrovascular ischemic-related injury. However, in order to translate the findings to the clinic, many factors need to be elucidated and identified, such as age/gender-dependency, comorbidities, patient medication history, and standard biomarkers. Essentially, clinical trials or pre-clinical studies should be more “personalized and categorized” in relation to these factors. In addition, experimental studies aimed to identify the key molecule that plays a central role in ischemic preconditioning-mediated neuroprotection against cerebral ischemia should also consider factors such as the extent of the injury, time point, brain tissue area, and cell type. JNK has a dual role in regulating neuronal cell death and survival following a brain injury. Therefore, a therapeutic approach that targets JNK activity in ischemic preconditioning should be carefully developed. A detailed understanding about JNK-mediated molecular mechanisms involved in ischemic preconditioning-induced neuroprotection will lead to the development of improved therapeutic strategies for cerebral ischemia and other CNS injuries.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
Joo Eun Jung1
1Department of Neurology, University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
Eng H. Lo2
2Neuroprotection Research Laboratory, Departments of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Corresponding author:
Joo Eun Jung
Email: Joo.Eun.Jung@uth.tmc.edu
and
Eng H. Lo
Email: Lo@helix.mgh.harvard.edu
In a new window | Download PPT
Figure 1: Signaling pathway of ischemic preconditioning. There is scientific evidence to suggest that JNK mediates the neuroprotective effects of ischemic preconditioning in stroke.
Table 1. Completed clinical trials (stroke- and subarachnoid hemorrhage-related).
|
Identifier |
Title |
Aims descripted in the study |
Actual Enrollment |
|
NCT01118000 |
Study on the Cardioprotection and Humoral Mechanism of Limb Ischemia Preconditioning |
To assess whether limb ischemic preconditioning protects remote tissue or organs through a humoral mechanism |
60 |
|
NCT03072914 |
Effects of Remote Ischemic Preconditioning With Postconditioning on Neurologic Outcome |
To evaluate whether RIPC with RIPostC reduce the major neurocomplication in patients undergoing STA-MCA anastomosis |
108 |
|
NCT01110239 |
Preconditioning for Aneurismal Subarachnoid Hemorrhage |
To determine if RIPC can be safely and effectively instituted in patients with SAH |
34 |
|
NCT02602977 |
the Influence of Remote Ischemic Preconditioning on Inflammation During Human Endotoxemia (RISPENDO) |
To investigate the effects of (repeated) ischemic preconditioning on inflammation during human endotoxemia |
30 |
|
NCT01158508 |
Remote Ischemic Preconditioning in Subarachnoid Hemorrhage (RIPC-SAH) |
To study effect of RIPC on cerebral vasospasm following SAH |
20 |
|
NCT01658306 |
Clinical Trial on Remote Ischemic Preconditioning and Cerebral Small Vessel Disease (RIPC-SVD) |
To test if RIPC might have a beneficial effect on outcomes of cerebral small vessel disease |
30 |
|
NCT02177981 |
Impact of Remote Ischemic Preconditioning Preceding Coronary Artery Bypass Grafting on Inducing nEeuroprotection (RIPCAGE) |
To determine whether RIPC can reduce the adverse impact of cardiopulmonary bypass on neurological outcome |
70 |
|
NCT01321749 |
The Neuroprotective Effect of Remote Ischemic Preconditioning on Ischemic Cerebral Vascular Disease |
To observe the effect of RIPC on ischemic cerebral vascular disease |
196 |
Several clinical trials using RIPC to investigate any beneficial effects in cerebral ischemic vascular disease or SAH have been completed (found at ClinicalTrials.gov).
Table 2. Current clinical trials.
|
Identifier |
Title |
Condition or disease |
Aims descripted in the study |
Estimated Enrollment (As of Oct. 6th, 2018) |
|
NCT02411266 |
Preconditioning With Limb Ischemia for Subarachnoid Hemorrhage (PreLIMBS) |
Ischemic Preconditioning |
A study of limb preconditioning in subjects with SAH who are at high risk of cerebral ischemia in the first 2 weeks after hemorrhage |
60 |
|
NCT03023150 |
Ischemic Preconditioning as an Intervention to Improve Stroke Rehabilitation – Froedtert |
Ischemic Preconditioning |
A study to use ischemic preconditioning as an intervention to improve stroke rehabilitation |
50 (active, not recruiting) |
|
NCT02381522 |
Remote Ischemic Pre-conditioning in Subarachnoid Hemorrhage (RIPC-SAH) |
Brain Aneurysms |
To investigate the effect of limb ischemia preconditioning on SAH outcome (cerebral vasospasm) |
100 |
|
NCT02997748 |
Remote Ischemic Preconditioning After Cardiac Surgery (RIPCRenal) |
Cardiac Surgery, Aortocoronary Bypass |
To reduce the incidence of AKI by implementing RIPC and to evaluate the dose-response relationship using the biomarkers urinary [TIMP-2] *[IGFBP7] in high risk patients undergoing cardiac surgery |
180 |
|
NCT02169739 |
Remote Preconditioning Over Time To Empower Cerebral Tissue (REM-PROTECT) |
Ischemic Stroke |
To assess feasibility of ischemic preconditioning by preconditioning device |
60 |
|
NCT03153553 |
Ischemic Preconditioning, Exercise Tolerance and Multiple Sclerosis |
Multiple Sclerosis |
To see whether it is feasible to use ischemic preconditioning to improve exercise performance in people with multiple sclerosis |
40 |
|
NCT03022149 |
Remote Ischemic Preconditioning for Subcortical Vascular Dementia (RIPSVD) |
Subcortical Vascular Dementia |
To determine whether the RIPC are effective in the treatment of mild to moderate vascular dementia |
52 (active, not recruiting) |
|
NCT03624452 |
Remote Ischaemic Preconditioning Combined With Exercise Training on Vascular Function. |
Cardiovascular Disease Risk |
To investigate whether combining 8 weeks of exercise and RIPC is more beneficial to systemic vascular function/ (Cerebro) vascular function than exercise alone |
60 |
|
NCT02553655 |
Remote Ischemic Limb Preconditioning In Healthy Volunteers |
Cerebrovascular Disease |
To determine if remote ischemic leg preconditioning in healthy volunteers improves cerebral vasomotor reactivity |
30 |
|
NCT03598855 |
Ischemic Preconditioning and Type 2 Diabetes |
Type 2 Diabetes |
To determine the impact of 7 days of daily ischemic preconditioning on vascular function and insulin sensitivity in type 2 diabetes mellitus |
21 (recruitment completed) |
|
NCT03027011 |
Remote Ischemic Preconditioning on Brain Injury in Carotid Endarterectomy |
Remote Ischemic Preconditioning |
To test an intervention (RIPC) in patients undergoing carotid endarterectomy |
40 |
|
NCT03474952 |
Effects of Remote Ischemic Preconditioning During Free Flap Reconstruction |
Remote ischemic preconditioning (RIPC) |
To evaluate the effect of RIPC on tissue oxygen saturation and skin temperature of the flap |
50 |
Several on-going clinical trials (which are “recruiting or completed recruitment”) to assess the beneficial effects or feasibility of ischemic preconditioning or RIPC in cerebral ischemia, SAH, multiple sclerosis, subcortical vascular dementia, and brain injury (found at ClinicalTrials.gov).
Table 3. Studies on the role of JNK in brain preconditioning.
|
Brain Injury Model |
Cerebral Ischemia Focal & Global ischemia |
(Gu et al., 2000; Gu et al., 2001; Nozaki et al., 2001; Colangelo et al., 2004; Miao et al., 2005; Zhang et al., 2006b; Yang et al., 2008; Du et al., 2009; Zhang et al., 2009; Liang et al., 2011; Maslov and Lishmanov Iu, 2012; Simao et al., 2012; Mirante et al., 2013; Zhang et al., 2013; Wang et al., 2016a; Wang et al., 2016b; Zhang et al., 2017; Zhuang et al., 2017; Shvedova et al., 2018) |
|
Hemorrhage |
(Chang et al., 2015) |
|
|
Hypoxia |
(Jones and Bergeron, 2004; Gustavsson et al., 2007; Zhang et al., 2007a; Nilsson et al., 2015) |
|
|
Trauma |
(Lotocki et al., 2006) |
|
|
Oxygen Glucose Deprivation |
(Meloni et al., 2006; Bhuiyan et al., 2011; Xiang et al., 2014; Pang et al., 2015) |
|
|
Pharmacological reagent-induced brain or neuronal injury |
(Sugino et al., 2000; Leak et al., 2006; Granziera et al., 2007; Price et al., 2010; Navon et al., 2012; Tripathi et al., 2014; Yang et al., 2015; Fu et al., 2017) |
|
|
Preconditioning Stimulus |
Focal or Global ischemia or Remote ischemia |
(Gu et al., 2000; Gu et al., 2001; Nozaki et al., 2001; Colangelo et al., 2004; Miao et al., 2005; Zhang et al., 2006b; Yang et al., 2008; Du et al., 2009; Zhang et al., 2009; Maslov and Lishmanov Iu, 2012; Wang et al., 2016b; Zhang et al., 2017) |
|
Hypoxia |
(Jones and Bergeron, 2004; Gustavsson et al., 2007; Zhang et al., 2007a; Nilsson et al., 2015) |
|
|
Oxygen Glucose Deprivation |
(Bhuiyan et al., 2011; Pang et al., 2015) |
|
|
Hypothermia |
(Lotocki et al., 2006) |
|
|
Pharmacological Reagent (Melatonin, Sevoflurane, Pitavastatatin, Isoflurane, Riboflavin, Remifentanil, Protease Nexin-1, Resveratrol, Lipopolysaccharide, NMDA, Thrombin, 6-hydroxydopamine, Erythropoietin, 3-nitropropionic acid). |
(Sugino et al., 2000; Leak et al., 2006; Meloni et al., 2006; Granziera et al., 2007; Price et al., 2010; Liang et al., 2011; Navon et al., 2012; Simao et al., 2012; Mirante et al., 2013; Zhang et al., 2013; Tripathi et al., 2014; Xiang et al., 2014; Chang et al., 2015; Wang et al., 2016a; Fu et al., 2017) |
Among 140 studies (found by searching PubMed with keyword “JNK and preconditioning”), 41 studies involve the connection between JNK and brain preconditioning in various brain diseases or injuries (found by searching PubMed with keyword “JNK and preconditioning and brain”). The table was categorized by brain injury model and preconditioning stimulus.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7530 | 15 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA