Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Potential role of omega-3 polyunsaturated fatty acids in ischemic stroke: review on clinical and preclinical studies
Time:2019-09-04
Number:13424
Hong Shi1,2, Di Chen2, Yanqin Gao2
Author Affiliations


- 1Department of Anesthesiology of Shanghai Pulmonary Hospital, Tongji University, Shanghai 200433, China.
- 2State Key Laboratory of Medical Neurobiology, MOE Frontiers Center for Brain Science and Institute of Brain Sciences, Fudan University, Shanghai 200032, China.
Conditioning Medicine, 2019, 2(4). 152-163.
Abstract
Dietary supplementation with omega-3 polyunsaturated fatty acids (n-3 PUFAs) has been associated with reduced incidence of stroke and accumulated experimental data show potent neuroprotective effects of n-3 PUFAs. However, epidemiological and clinical observational studies are inconsistent. In this review, we summarize the current clinical trials and potential mechanisms of n-3 PUFAs to clarify the optimal method(s) for utilization of dietary supplementation to lower the risk and improve neurofunctional recovery after stroke.
Keywords: Omega-3 polyunsaturated fatty acids, Stroke, Docosahexaenoic acid, Eicosapentaenoic acid, Neuroprotection, Neuroinflammation
Abstract
Dietary supplementation with omega-3 polyunsaturated fatty acids (n-3 PUFAs) has been associated with reduced incidence of stroke and accumulated experimental data show potent neuroprotective effects of n-3 PUFAs. However, epidemiological and clinical observational studies are inconsistent. In this review, we summarize the current clinical trials and potential mechanisms of n-3 PUFAs to clarify the optimal method(s) for utilization of dietary supplementation to lower the risk and improve neurofunctional recovery after stroke.
Keywords: Omega-3 polyunsaturated fatty acids, Stroke, Docosahexaenoic acid, Eicosapentaenoic acid, Neuroprotection, Neuroinflammation
Background
Stroke remains one of the leading causes of death and long-term disability worldwide (Mozaffarian et al., 2015). Although the use of thrombolytic tissue plasminogen activator (tPA) has been approved for the treatment of acute ischemic stroke, only 3-5% of stroke patients benefit from it due to its narrow therapeutic time window (Jauch et al., 2013; Hirano, 2015). Therefore, there is an urgent need to develop new methods to reduce the incidence of stroke and improve neurofunctional recovery.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), commonly called fish oils, are essential fatty acids for humans and other animals, which maintain cellular membrane structural and functional integrity. Epidemiological analyses suggest that deficiencies in n-3 PUFAs in the diet are associated with acute stroke (Yaemsiri et al., 2013), whereas n-3 PUFA supplementation decreases the risk of ischemic stroke and the prevalence of subclinical infarcts (He et al., 2002; Virtanen et al., 2008). Moreover, some clinical studies have shown that a diet enriched in marine fish is associated with a lower risk of ischemic stroke (He et al., 2002; Sauvaget et al., 2003; Mozaffarian et al., 2005; Bazan et al., 2005). However, the clinical data across groups has not been consistent. Indeed, in 2019 Manson et al. (2019a; 2019b) demonstrated that n-3 PUFAs did not change the risks for stroke. Given this background, we summarize the current clinical research on n-3 PUFAs in stroke and potential mechanisms that might underlie their improved use in the treatment and prevention of stroke.
Introduction of n-3 PUFAS
n-3 PUFAs are essential nutritional compounds that cannot be synthesized de novo in animals, thus they must be obtained from dietary sources. There are three distinct classes of n-3 PUFAs in humans (Fig. 1): α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA is found in many plant oils, such as flaxseeds, canola, and pumpkin seeds. ALA can be converted to EPA, and further to DHA by a desaturase enzyme (Gomez-Candela et al., 2015). The portion of ALA that converts to DHA and EPA is affected by the competitive inhibition of linoleic acid and negative feedback onto DHA and EPA (Goyens et al., 2006). Fish oil is the main source of DHA and EPA. Dietary supplementation with fish oil is believed to be a reliable method for increasing n-3 PUFA levels in humans.
Blood n-3 PUFAs levels are highly correlated with the dietary intake of n-3 PUFAs (Sands et al., 2005) and unesterified DHA readily diffuses from plasma to the brain (Ouellet et al., 2009; Rapoport et al., 2011). Thus, n-3 PUFA supplements to the diet elevate levels in the brain (Berger et al., 2001; Wood et al., 2010; Zhang et al., 2015; Cai et al., 2017). On the contrary, n-3 PUFAs depletion in the diet (Akbar et al., 2006; Desai et al., 2014; Desai et al., 2016) or chronic ethanol exposure lowers levels of DHA in the central nervous system (Wen and Kim, 2004; Akbar et al., 2006).
DHA is the most abundant fatty acid found in brain membranes and is necessary to maintain the normal physical conformation of ion channels, receptors, and transporters in cell membranes and for their functional integrity (Bazan, 2007). DHA levels in the brain vary with different extrinsic and intrinsic factors.
DHA levels in the elderly are much lower than in younger adults (Moranis et al., 2012; Ledesma et al., 2012; Labrousse et al., 2012). So many randomized control trial studies are conducted in elderly populations that try to find an effective means to lower the incidence of stroke. Despite age, the levels of DHA and EPA may be affected by gender distinction. Independently of the effects of dietary n-3 PUFAs, DHA is higher in females than in males (Bakewell et al., 2006; Harris et al., 2007; Childs et al., 2008; Crowe et al., 2008; Block et al., 2008; Kitson et al., 2010; Sala-Vila et al., 2011; Lohner et al., 2013; Lin et al., 2016). In addition, Walker et al. (2014) observed that an increase in EPA concentrations in plasma was significantly greater in females than in males following 12-month supplementation with EPA and DHA. Thus, it’s reasonable to suppose that females are more efficient than males at converting the precursor n-3 PUFA into the long-chain n-3 PUFAs (Pawlosky et al., 2001; Burdge et al., 2002; Burdge and Wootton, 2002; Walker et al., 2014).
Clinical trials of n-3 PUFAS
Since the American Heart Association (AHA) published a statement on n-3 PUFAs in 2002, a large number of studies have been performed to assess the influence of n-3 PUFAs on cardiovascular events, including stroke. However, inconsistent results were observed in different groups. Thus in this review, we discuss the effects of n-3 PUFAs on stroke (Fig. 1).
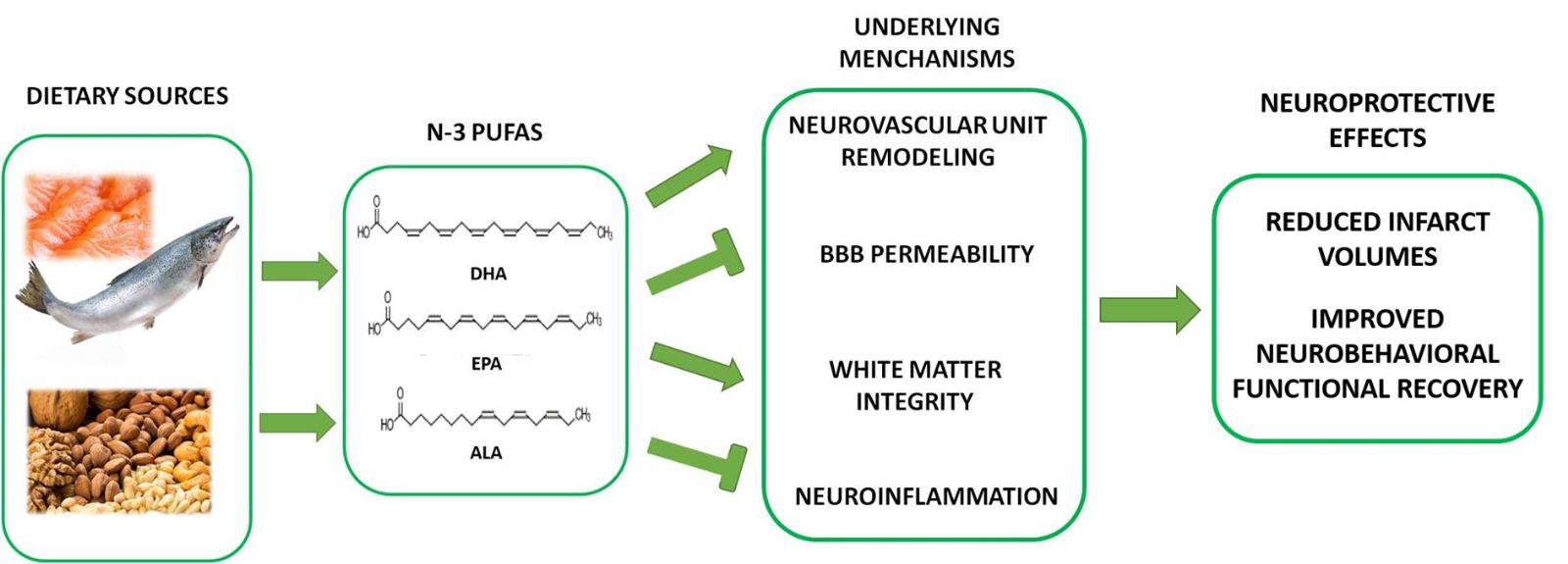
In a new window | Download PPT
Figure 1: Schematic outline of the mechanisms underlying n-3 PUFAs-mediated neuroprotective effects against ischemic stroke. Plant oil contains ALA and fish oil is the primary dietary source of DHA and EPA. n-3 PUFAs exert prominent neuroprotective effects on ischemic stroke, including reduced total infarct volumes and improved long-term behavioral recovery. Related mechanisms include increased neurovascular unit remodeling, decreased BBB and white matter damage, and decreased neuroinflammation.
1. To reduce the incidence of stroke
Because of the positive effect of n-3 PUFAs on cardiovascular diseases, consumption of seafood, namely fish, is assumed to be useful for reducing stroke risks. In 2011, Larsson and Orsini (2011) conducted a systematic review, including fifteen prospective studies from the United States, Europe, Japan, and China that included 9,360 stroke events across 383,838 participants. This meta-analysis revealed that for each three-servings/week increase in fish consumption, the risk of stroke decreased by 6%. Xun et al. (2012) found that consumption of one serving of seafood per week was associated with a 14% decreased risk of ischemic stroke compared to no or infrequent consumption of seafood. In 2013, Ikeya et al. (2013) compared common clinical parameters among 65 patients with ischemic stroke and 65 control subjects in Japan and found the plasma EPA concentrations and EPA/arachidonic acid (AA) ratios were potential predictive risk factors for ischemic stroke. In 2016, Kippler et al. (2016) assessed in a prospective population-based Cohort of Swedish Men, including 39,948 middle-aged and elderly men, and observed a protective association with dietary EPA-DHA intake. Similar associations were seen in a report by Ward et al. (2019) who analyzed 197,761 Million Veteran Program (MVP) participants (66 ± 12 years old, median fish intake: 1 serving/week), and found that n-3 PUFAs supplement was associated with 12% lower risk of stroke among males. As well, Manson et al. (2019a) reported that participants with low fish consumption (<1.5 serving/week) may be sensitive to n-3 PUFA supplements for cardiovascular protection.
Regrettably, epidemiological investigations have found unclear results with regard to n-3 PUFAs reduction in stroke risk. Recently, Manson et al. (2019a; 2019b) conducted a randomized, placebo-controlled trial of n-3 PUFAs (DHA 380mg/day, EPA 460mg/day) and vitamin D3 (2000IU/day) among 25,871 participants (men ≥ 50 years old, women ≥ 55 years old) in the United States, and found n-3 PUFA supplementation generally did not result in a lower incidence of total stroke. Similar results were attained by Bowman et al. (2018) who studied diabetes mellitus. Therefore, n-3 PUFAs supplements may afford no reliable benefit to reduce total stroke risk, however, in some subgroups n-3 PUFAs appear to be helpful, such as in those individuals without fish eating habits (< 1-1.5 serving/ week).
People of different races or regions are inclined to have different dietary habits and various reactions to n-3 PUFAs, which may account for the inconsistent incidence of stroke. In 2011, Sekikawa et al. (2011) used a population-based cross-sectional study designed to assess cognitive functioning with dietary changes in 608 Japanese and American white men aged 40 to 49. These authors found that more than 40% of Japanese males ate fish four times/week or more as opposed to only 3% of white American males. The Japanese had markedly elevated serum levels of both EPA and DHA, and lower intima-media thickness than white Americans (Sekikawa et al., 2011). Furthermore, Kappus et al. (2017) reported a study in African American and white American participants in 2017, which demonstrated that racial differences confer diverse effects on oxidative stress (OS) and vascular function. Thus, study subjects’ backgrounds, including age, sex, race, and living environment should be considered before performing a study.
2. To improve cognitive function in the elderly
Although inconsistent data were reported about the influence of n-3 PUFAs on stroke prevention, n-3 PUFAs still have the potential to improve neurofunction in the elderly, because of the importance of DHA in the brain and its decline with age.
In 2008, Johnson et al. (2008) reported the cognitive benefits of DHA (800 mg/day) and lutein (12 mg/day) in unimpaired older women. Similarly, Yurko-Mauro et al. (2010) found that 900 mg/day of DHA improved learning and memory function in a cohort of 485 healthy elderly individuals. Within the next several years, some groups reported that an elevated intake of fish oil with 880 mg/day or more of DHA and EPA in healthy adults resulted in significant improvements in cognitive functioning (Danthiir et al., 2011; Nilsson et al., 2012; Witte et al., 2014; Kulzow et al., 2016). These studies used 800-900 mg/day of DHA for 4-6 months in aged adults (age: >55 years). DHA supplementation appears to play a greater role in healthy aged brains when compared to EPA (Table 1).
|
Article |
DHA (mg/day) |
EPA (mg/day) |
CO-TREATMENT |
INTERVENTION LENGTH |
AGE (yr) |
NATIONALITY |
COGNITIVE FUNCTION |
|
880 |
1320 |
Vitamin E 15 mg |
26 weeks |
50-80 |
Germany |
Memory function↑ |
|
|
880 |
1320 |
Vitamin E 15 mg |
26 weeks |
50-75 |
Germany |
Executive function↑ |
|
|
300 |
100 |
ARA 120 mg |
4 weeks |
55-64 |
Japan |
Mood↑ |
|
|
300 |
100 |
ARA 120 mg |
4 weeks |
55-64 |
Japan |
Cognitive function↑ |
|
|
92/251 |
193/491 |
none |
12 weeks |
61-72, male |
Japan |
Cognitive function↑ |
|
|
1050 |
1500 |
none |
5 weeks |
51-72 |
Sweden |
Cognitive function↑ |
|
|
900 |
0 |
none |
24 weeks |
>54 |
USA |
Learning ↑memory↑ |
|
|
800 |
0 |
Lutein 12 mg |
4 months |
60-80, female |
USA |
Learning ↑memory↑ |
|
|
1720 |
600 |
none |
18 months |
65-90 |
Australia |
Cognitive function↑ |
|
|
240/480 |
240/480 |
multivitamin |
16 weeks |
50-70 |
Australia |
No change |
|
|
252 |
60 |
Vitamin E 10 mg |
90 days |
45-77 |
UK |
No change |
|
|
500 |
200 |
none |
24 months |
70-79 |
UK |
No change |
|
|
847/176 |
1093/226 |
none |
26 weeks |
>64 |
Holland |
No change |
|
|
380 |
460 |
VitaminD3 2000IU |
3.8-6.1 years |
men≥50, women≥55 |
USA |
No change |
Van de Rest et al. (2008) observed no overall effect of 26 weeks of EPA (1093/226 mg/day) and DHA (47/176 mg/day) supplementation on cognitive performance in 302 cognitively healthy Netherlandish individuals aged 65 years or older. Similarly, negative cognitive function with a low dose of EPA and DHA supplementation for 3/24 months was found in older individuals in the UK (Dangour et al., 2010; Stough et al., 2012). Similar results were found in an Australia cohort. Pase et al. (2015) observed no benefits of fish oils, with or without a daily multivitamin, on cognitive function in healthy elderly adults. Collectively, these results indicate that a low dose of DHA and EPA dietary supplementation has no reliable beneficial effect on cognitive performance in elderly adults. Different DHA:EPA ratios, dosage, and intervention durations may affect the final results of these studies.
Altogether, evidence from the prospective studies included here indicates that fish consumption or n-3 PUFAs intake may not be the sole factor influencing risk for ischemic stroke. DHA:EPA and EPA:AA ratios, dietary habits, living environment, vessel intima thickness, and race may also account for inconsistent results with n-3 PUFAs dietary supplementation.
3. To improve prognosis of stroke patients
Up until now, whether n-3 PUFAs supplements benefit stroke patients remains unclear. Does DHA or EPA improve functional recovery in stroke patients? Does supplementation with n-3 PUFAs lead to hemorrhage or other adverse side effects? Here, we review the literature relevant to these questions.
In 1985, Green et al. (1985) demonstrated that the ingestion of fish oil concentrate (1800 mg EPA/day) for 6 weeks had little influence on risk for excess cholesterol concentrations and platelet function in 11 patients with stroke or transient ischemic attack (TIA). This indicates that stroke patients may consume fish oils safely without risk of bleeding. Yoneda et al. (2008) also found that EPA (1800 mg/day) inhibited symptomatic cerebral vasospasms and cerebral infarctions and improved clinical prognoses in 101 patients with subarachnoid hemorrhage subjected to craniotomy and clip application. However, Poppitt et al. (2009) conducted a trial in 95 patients, some with ischemic stroke, in New Zealand, and observed no effect of 12 weeks of moderate-dose fish oil (669 mg/day DHA + 246 mg/day EPA) supplementation on mood.
To date, limited randomized controlled trials have been conducted in stroke patients. But normal doses, such as 1 g fish oils, do not appear to have adverse effects in stroke patients. Additionally, multiple factors may affect the final results of these studies, including differing dosages and proportions of EPA and DHA, as well as intervention length. We searched multiple websites and clinical trial registries, and found there are many ongoing and just completed studies of n-3 PUFAs and stroke that address the urgent need for larger standardized, randomized studies of their effects and underlying therapeutic mechanisms.
Function of n-3 PUFAs in clinical studies
The Mediterranean diet is often recommended by nutritionists for their positive cardio- and neuro-vascular health benefits. Considering the abundance of n-3 PUFAs in the Mediterranean diet, such as fish and nuts, this diet is supposed to lead to higher levels of n-3 PUFAs in plasma and brain, and furthermore confer protective effects on the brain. Fung et al. (2009) conducted a study of the effect of the Mediterranean diet on stroke in 74,886 American women over 20 years (1984 to 2004), and found a greater adherence to the Mediterranean diet was associated with a lower risk of stroke. Moreover Yau and Hankey (2011) reported the Mediterranean diet might help reduce the incidence of ischemic stroke. Reduced blood triglyceride concentrations, modulation of endothelial function, and inhibition of inflammation may be among the protective functions of n-3 PUFAs (Fig. 2).

In a new window | Download PPT
Figure 2: Timeline of n-3 PUFA conditioning before (preconditioning) or after (postconditioning) ischemic stroke and potential outcomes.
Hypertriglyceridemia might be an independent risk factor for stroke (Lisak et al., 2013). Growing evidence supports the role for plasma triglycerides (TGs) in dietary n-3 PUFA supplementation (Harris, 1997; Lewis et al., 2004; Harris et al., 2008; Eslick et al., 2009; Lopez-Alvarenga et al., 2010; Musa-Veloso et al., 2010; Shearer et al., 2012; Pounis et al., 2014; Hansen-Krone et al., 2014). DHA can elevate levels of high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol, and LDL particle size (Suzukawa et al., 1995; Harris, 1997; Balk et al., 2006; Eslick et al., 2009; Neff et al., 2011). Furthermore, DHA induces greater reduction than EPA does in TG concentrations and total cholesterol/HDL levels (Wei and Jacobson, 2011; Allaire et al., 2016; Innes and Calder, 2018).
Improvements in endothelial function are associated with the neuroprotective effects of n-3 PUFAs. With cerebral ischemic insult, endothelial dysfunction occurs, which is characterized by elevated expression of pro-inflammatory cytokines and adhesion molecules. Supplementation with n-3 PUFAs improves endothelial function (Wang et al., 2012; Xin et al., 2012). Additionally, in vitro studies have found that n-3 PUFAs stimulates nitric oxide (NO) production and induces endothelial nitric oxide synthase (eNOS) expression and activation (Zanetti et al., 2015; Colussi et al., 2017).
n-3 PUFAs have been postulated to alleviate the progression of stroke and stabilize atherosclerotic plaques by decreasing the infiltration and activity of inflammatory cells surrounding plaques (He, 2009). Dietary n-3 PUFA supplementation can reduce production of pro-inflammatory cytokines from macrophages, as well as circulating levels of endotoxin-induced inflammatory molecules, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (Shahbakhti et al., 2004; Tartibian et al., 2010; Rangel-Huerta et al., 2012; Haghiac et al., 2015). In addition, inhibition of NFκB-mediated cytokine production may be the primary mechanism underlying the efficacy of n-3 PUFAs (Thota et al., 2018).
There are increasingly more clinical studies examining the effect of n-3 PUFAs against cardiovascular disease, which have lead to more basic research into the effects of n-3 PUFA in stroke. However, many mechanisms remain to be explored. Therefore, in the following section we summarize the neuroprotective mechanisms of n-3 PUFAs in animal and cell models.
Neuroprotective mechanisms in experimental models
Stroke survivors usually experience long-term functional neurobehavioral disability. Growing evidence has demonstrated that n-3 PUFAs display pleiotropic effects on cognitive and sensorimotor deficits in in vivo and in vitro models (Chauveau et al., 2011; Eady et al., 2012; Williams et al., 2013; Eady et al., 2013; Hu et al., 2013; Wang et al., 2014; Eady et al., 2014; Hong et al., 2015; Zhang et al., 2015; Pu et al., 2016; Jiang et al., 2016; Cai et al., 2017; Belayev et al., 2018). However, the exact protective mechanisms underlying these effects remain unclear. Multiple roles have been revealed, including enhanced neurovascular unit remodeling, decreased blood-brain-barrier (BBB) permeability, and improved white matter integrity involving the protective and repair processes underlying n-3 PUFAs role in stroke insult (Fig. 2).
1. Enhancing neurovascular unit remodeling
Promoting neurogenesis and angiogenesis are among the most important repair mechanisms underlying recovery from ischemic brain injury (Zhang et al., 2014; Kuptsova et al., 2015; Dela et al., 2015; Zhao et al., 2015). Several labs have demonstrated that dietary supplementation with n-3 PUFAs promotes neurovascular remodeling and improves sensorimotor and cognitive functional recovery after stroke (Kan et al., 2007; Dagai et al., 2009; Crupi et al., 2013; Dyall, 2015; Zhang et al., 2015; Pu et al., 2016; Cai et al., 2017; Belayev et al., 2018). However, a recent study demonstrated that n-3 PUFA post-stroke treatment enhances long-term spatial cognitive function in young mice but not in aged mice (Jiang et al., 2019).
An important component of biological cell membranes, n-3 PUFAs play a vital role in neurodevelopment and neurological function (Lauritzen et al., 2016). Additionally, the DHA content of the brain and nerve tissues is higher than in other tissues. Lo et al. (2018; 2019) found that DHA is an effective neurogenesis inducer in adult neural stem progenitor cells (NSPCs) under ischemic conditions. DHA might shift the tendency for NSPCs to differentiate into neurons instead of astrocytes and foster neurite growth in hippocampal neurons (Calderon and Kim, 2004). These studies suggest that n-3 PUFAs contribute to neurogenesis by improving the survival of neuroblasts, promoting the migration of neuroblasts toward ischemic sites, and promoting the maturation of newly-generated NSPCs following ischemic insult. This subsequently results in elevated mature neuron numbers in the ischemic penumbra (Zhang et al., 2015; Cai et al., 2017), which can contribute to significant functionional benefits. Jiang et al. (2019), however, reported that post-stroke n-3 PUFA treatments promote neuron survival but not neurogenesis 35 days after distal medial artery occlusion (dMCAO).
Moreover, n-3 PUFAs have specific neuroprotective effects on the hippocampal loop. n-3 PUFA supplementation increased hippocampal neurogenesis, dendritic complexity of newborn neurons in the dentate gyrus areas, hippocampal volume, and neuronal density, while it decreased apoptosis in the hippocampus (Cutuli, 2014). n-3 PUFA supplementation also enhanced hippocampal functionality in aged mice (Cutuli, 2014). Furthermore, n-3 PUFAs are endogenous ligands of the retinoid receptor and peroxisome proliferator-activated receptors (PPARs), which are transcription factors involved in many cellular processes including learning and memory. Dyall et al. (2010) found that n-3 PUFAs restored age-induced reductions in retinoid receptors and PPARs, as well as the rate of hippocampal neurogenesis in the dentate gyrus, which may be a potential mechanism underlying the neuroprotective effects of n-3 PUFAs.
Angiogenesis may also be induced by ischemic insults. Our lab has found that supplementation with n-3 PUFAs enhances ischemia-induced angiogenesis. Indeed, the proliferation of endothelial cells and the density and length of cerebral blood vessels were all positively correlated with improved long-term sensorimotor activities (Cai et al., 2017). In 2019, Jiang et al. (2019) found that post-stroke n-3 PUFA treatment promotes angiogenesis in the peri-infarct CTX and STR after dMCAO, and that the efficacy declines with aging.
Other mechanisms have also been hypothesized. For instance, the accumulation of n-3 PUFAs in the brain also upregulated the expression of angiopoietin 1 (Ang1) and angiopoietin 2 (Ang2), which contribute to enhanced angiogenesis and neurogenesis after ischemic stroke (Hu et al., 2013; Wang et al., 2014; Zhang et al., 2015). n-3 PUFAs stimulated astrocytes to produce Ang2, which in turn promotes endothelial cell proliferation and barrier formation by stimulating vascular endothelial growth factor-rous sarcoma (VEGF-Src) signaling (Hu et al., 2013; Wang et al., 2014). Ang1 is an agonist at the endothelial-specific receptor tyrosine kinase, while Tie 2 promotes angiogenesis through the receptor. Ang1 promotes endothelial cell migration, sprouting, branching, and organization into tubules (Moss, 2013). Considering these mechanisms, angiopoietin-Tie 2 interactions are a potential target for future therapies for human vascular disease (Zhang et al., 2002).
2. Promoting white matter integrity and oligodendrogenesis
With the development of neuroimaging techniques, n-3 PUFAs have been shown to exert significant benefits on white matter in people. White matter occupies about half of the lesion volume (Ho et al., 2005; Kissela et al., 2009; Khan et al., 2015) and its injury results in long-term sensorimotor and cognitive deficits. We previously reported that n-3 PUFAs attenuated ischemia-induced white matter injury, improved white matter integrity, and significantly promoted neurological improvements following ischemic brain injury (Zhang et al., 2015; Jiang et al., 2016; Cai et al., 2017). An additional study revealed that elevated n-3 PUFA intake was associated with fewer white matter abnormalities and reduced risk for developing subclinical infarcts (Virtanen et al., 2008).
White matter integrity might be partially reestablished by oligodendrogenesis and replenishment of lost oligodendrocytes (Mandai et al., 1997; Chu et al., 2012). In this process, oligodendrocyte precursor cells (OPCs) proliferate and migrate to the demyelinated region where they differentiate into mature oligodendrocytes and restore the white matter lost to ischemic stroke. OPCs in the adult brain have the ability to replace lost oligodendrocytes, engage in white matter repair, and thus affect neurological recovery (Franklin and Ffrench-Constant, 2008; Duncan et al., 2009; Liu et al., 2014; Han et al., 2015). n-3 PUFAs support myelin-producing cells, oligodendrocyte survival, and oligodendrogenesis (Hu et al., 2013; Zhang et al., 2015; Jiang et al., 2016). Jiang et al. (2019) demonstrated that post-stoke n-3 PUFA treatment enhances oligodendrogenesis and promotes the survival of oligodendrocytes in both young and aged mice 35 days after dMCAO.
3. Attenuating BBB permeability
Clinically, BBB disruptions occur in more than one third of all stroke patients. Ischemic brain injury disrupts the BBB and then triggers a cascade of events, leading to edema formation and secondary brain injury (Belayev et al., 1996; Mogoanta et al., 2010; Yang and Rosenberg, 2011). BBB permeability is also associated with poor outcomes and lower survival rates following stroke (Rosenberg, 2012).
Several groups have described the beneficial effects of n-3 PUFAs including DHA treatment for ischemia-induced BBB permeability and brain edema (Belayev et al., 2011; Eady et al., 2012; Hong et al., 2014; Hong et al., 2015; Belayev et al., 2018). Mechanisms related to n-3 PUFA actions on the BBB include preservation of endothelial tight junction proteins, inhibition of matrix metalloproteinase (MMP) production and activity (Hong et al., 2015; Zhang et al., 2016), and suppression of glial scar formation in the peri-infarct area, which may be associated with a favorable microenvironment. Our group has shown that n-3 PUFA stimulate the expression of Ang1 after focal cerebral ischemia (Zhang et al., 2015). Ang1 antagonizes VEGF-induced BBB leakage by activating Tie2 to promote inter endothelial junction integrity after focal cerebral ischemia (Zhang et al., 2002; Moss, 2013).
Cellular and molecular mechanisms related to n-3 PUFAs
In the context of ischemia, neurons are injured and destructive cascades are often initiated, including excess excitatory amino acid release, the generation of reactive oxygen species (ROS), increased expression of proapoptotic factors, mitochondrial dysfunction, and inflammation (Bazan et al., 2005). n-3 PUFAs act on multiple cell types in the brain and attenuate pro-death (Shi et al., 2015) or pro- inflammatory (Lalancette-Hebert et al., 2011) mechanisms, as well as elicit cytoprotective responses (Zhang et al., 2014).
1. Inhibiting ROS activation
Ischemic insults contribute to the rapid accumulation of free fatty acids, such as arachidonic acid and DHA, which are released from the cell membrane and result in the depletion of n-3 PUFAs in the brain (Bazan et al., 2005). The brain is vulnerable to apoptosis and defective gene expression (Kirkland et al., 2002), as well as OS, which triggers multiple signaling pathways, leading to cell damage and death. Ischemia-reperfusion (I/R) injury also induces OS and produces large amounts of ROS, which cause additional neural tissue injury and the breakdown of cellular integrity.
DHA also modulates neuroprotection by increasing glutathione (GSH) expression in brain. GSH-peroxidase (Px), a member of the enzymatic antioxidant system, has been widely used as an antioxidant (Janaky et al., 1999; Sugawara and Chan, 2003). A remarkable elevation in GSH-Px generation was found in fat-1 neurons compared to wild type neurons after oxygen-glucose deprivation/reperfusion (OGD/R) injury. Therefore, the enhanced generation of GSH-Px and the inhibition of ROS activation are likely involved in the protective effects of fat-1 neurons with high endogenous n-3 PUFAs in OGD/R injury. DHA and GSH have consistently exhibited obvious antioxidant effects by scavenging ROS, which eventually prevents neuronal apoptosis. Therefore, it is conceivable that endogenous n-3 PUFAs possess the same antioxidant properties and thus exert neuroprotective effects against ischemic injury.
Transcription factors that mediate neuro hormesis include NF-κB, nuclear factor erythroid 2-related factor 2 (Nrf2), and hypoxia-inducible factor 1 (HIF1). Many of these transcription factors act on different antioxidant-responsive elements (AREs). Previous work by our group revealed that the Nrf2/heme oxygenase-1 (HO-1) signaling pathway was a neuroprotective mechanism underlying n-3 PUFAs action against brain ischemia. DHA and its neuroactive mediators also exhibit anti-oxidative properties by activating Nrf2 signaling pathways and up-regulating HO-1 expression in in vivo and in vitro models of brain ischemia and oxidative injury, respectively. In both, 4-hydroxy-hexenal, a specific end production of n-3 PUFAs, was linked to the activation of Nrf2, which combined with the antioxidant response element (ARE) of phase 2 genes resulting in the up-regulation of HO-1 and other enzymes (Zhang et al., 2014; Liu et al., 2014).
Expression of HO-1, which is an antioxidant enzyme, increases in response to OS, acting to protect against ischemia. Studies have shown that phosphoinositide 3-kinase (PI3K)/Akt, extracellular signal regulated kinase 1/2 (ERK1/2) and p38 also increase HO-1 expression induced by DHA via Nrf2 nuclear translocation (Yang et al., 2013; Bu et al., 2016). OS is one factor that leads to neurodegeneration by limiting the production of anti-oxidants such as biliverdin, which is produced via cleavage of heme by HO-1. This mechanism is a potential target for further therapeutic investigation. Furthermore, HO-1 also produces carbon monoxide (CO) by degrading heme, which also protects against inflammation, oxidation, and apoptosis (Zhang et al., 2014; Cheng and Rong, 2017). Neuroprotection against cerebral ischemic stroke, which is mediated by DHA, includes improving neuronal defense capacity and inhibition of cellular inflammatory mechanisms by increasing the expression of Nrf2 and HO-1.
2. Modulation of microglial function and neuroinflammation
Inflammatory responses triggered by microglia play a crucial role in the pathogenesis of stroke (Lee et al., 2014; Xia et al., 2015; Ritzel et al., 2015). Microglial phenotypes influence the protective role of n-3 PUFAs following cerebral ischemia. After cerebral ischemia, initial M2 microglial polarization is gradually overwhelmed by destructive M1 polarization, which may lead to progressive tissue injury (Hu et al., 2012). M2 microglia activation is significantly correlated with post-stroke sensorimotor recovery (Suenaga et al., 2015; Jiang et al., 2016) while M1 microglia activation is poorly correlated with post-stroke sensorimotor deficits (Jiang et al., 2016).
In mice with n-3 PUFAs post-treatment, we also found enhanced M2 polarization of microglia/macrophages 14 days following cerebral ischemia (Jiang et al., 2016). A previous study reported that DHA and EPA both promote microglial M2 polarization by down-regulating M1 signature genes (e.g. TNF-α, IL-1α, CCL5) and up-regulating M2 signature genes (e.g. CD206, TGF-β) in microglia cultures (Chen et al., 2014). Bazan (2018) demonstrated that docosanoids and elovanoids from n-PUFAs are pro-homeostatic modulators of inflammatory responses, cell damage, and neuroprotection. Cai et al. (2018) reported that DHA treatment significantly inhibited immune cell infiltration and promoted macrophage polarization toward an anti-inflammatory M2 phenotype in the ischemic brain in in vivo and in vitro model.
Studies have shown that n-3 PUFAs attenuate neuroinflammation through multiple pathways and directly and indirectly suppress the activity of nuclear transcription factors such as NF-κB and reduce levels of proinflammatory mediators, including COX-2, TNF-α, and IL-1β (Kang and Weylandt, 2008; Calder, 2013; Chen et al., 2014). n-3 PUFAs also exhibit potent immunomodulatory effects by inhibiting the expression of adhesion molecules. DHA acts as an anti-inflammatory agent by activating the Nrf2/HO-1 pathway to reduce the expression of intercellular adhesion molecule 1 (ICAM-1), which is induced by TNF-α and mediates leukocyte recruitment (Yang et al., 2013). DHA also reduces the expression of the pro-inflammatory transcription factor NF-κB (Pan et al., 2009) and that of pro-inflammatory genes encoding COX, TNF-α, HIF-1α (Zendedel et al., 2015), and interleukin 6 (Pan et al., 2009) in in vitro experiments on stroke models.
DHA is the precursor for neuroprotectin D1 (NPD1). Multiple studies have suggested that this anti-inflammatory property is due to DHA conversion into its metabolites, and into NPD1 in the brain, specifically (Marcheselli et al., 2003; Bazan et al., 2005; Serhan et al., 2006). Under stroke conditions, DHA is released from membrane phospholipids into the cytoplasm by the action of the enzyme phospholipase A2. DHA is subsequently transformed into NPD1 by the 15-lipoxygenase enzyme. (Bazan et al., 2005; Bazan, 2006). In brain ischemia, NPD1 acts as an anti-inflammatory agent by inhibiting leukocyte infiltration and proinflammatory gene expression (Serhan et al., 2006; Belayev et al., 2009).
3. Anti-apoptosis
Numerous studies have focused on the anti-apoptotic properties of DHA and its mediators, demonstrating their neuroprotective activity in the brain. For instance, Akbar et al. found that PI3K/Akt signaling positively mediated DHA’s anti-apoptotic effects in vitro (Akbar et al., 2005). Elevated Bcl-2 levels can negatively impact neuroprotection against stroke-induced injury (Cao et al., 2002; Zhao et al., 2003; Yin et al., 2006). We also demonstrated that n-3 PUFAs provide neuroprotection by activating Bcl-2 anti-apoptotic cascades. Additionally, we found that n-3 PUFAs enhance Akt-dependent pro-survival signaling by promoting the biosynthesis of phosphatidylserine in neuronal cell membranes (Zhang et al., 2015). Pan et al. (2009) found that DHA conditioning for 6 weeks prior to the induction of ischemia elevated the expression of anti-apoptotic proteins such as Bcl-2 and attenuated damage mechanisms via ERK- and Bcl-2-mediated pro-survival cascades (Pan et al., 2009).
In addition to improving cell survival by mediating survival-related cascades, DHA is also able to reduce brain apoptosis by blocking apoptosis, upregulating antiapoptotic protein expression, downregulating apoptotic protein expression, and maintaining mitochondrial integrity and function (Mayurasakorn et al., 2011). Florent et al. (2006) also observed upregulation of ERK activity when DHA was provided to stressed neuronal cells. In vitro studies of human neurons and glia cultured with NPD1 similarly revealed that NPD1 decreased amyloid beta-42-induced neurotoxicity by acting on Bcl-2 expression (Lukiw et al., 2005).
Additionally, several studies have demonstrated that modulating Bcl-2 family proteins results in the maintenance of Ca2+ homeostasis in the endoplasmic reticulum (ER) (Pinton and Rizzuto, 2006; Rong et al., 2009). It can be inferred that these confined alterations to intracellular Ca2+ contents have limited contributions to intracellular Ca2+ homeostasis. Furthermore, n-3 PUFAs significantly inhibit increased intracellular Ca2+ (Shi et al., 2016). Another astrocytes culture study reported that n-3 PUFAs inhibited ER Ca2+ release in an in vitro ischemia model (Begum et al., 2012).
Conclusion
Dietary supplementation of n-3 PUFAs, especially DHA and EPA, has shown unreliable association with stroke in epidemiologic and clinical studies. More factors must be considered to further translational studies, including race, sex, and basic food habits.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
Hong Shi1,2
1Department of Anesthesiology of Shanghai Pulmonary Hospital, Tongji University, Shanghai 200433, China. 2State Key Laboratory of Medical Neurobiology, MOE Frontiers Center for Brain Science and Institute of Brain Sciences, Fudan University, Shanghai 200032, China.
Di Chen2
2State Key Laboratory of Medical Neurobiology, MOE Frontiers Center for Brain Science and Institute of Brain Sciences, Fudan University, Shanghai 200032, China.
Yanqin Gao2
2State Key Laboratory of Medical Neurobiology, MOE Frontiers Center for Brain Science and Institute of Brain Sciences, Fudan University, Shanghai 200032, China.
Corresponding author:
Yanqin Gao
Email: yqgao@shmu.edu.cn

In a new window | Download PPT
Figure 1: Schematic outline of the mechanisms underlying n-3 PUFAs-mediated neuroprotective effects against ischemic stroke. Plant oil contains ALA and fish oil is the primary dietary source of DHA and EPA. n-3 PUFAs exert prominent neuroprotective effects on ischemic stroke, including reduced total infarct volumes and improved long-term behavioral recovery. Related mechanisms include increased neurovascular unit remodeling, decreased BBB and white matter damage, and decreased neuroinflammation.

In a new window | Download PPT
Figure 2: Timeline of n-3 PUFA conditioning before (preconditioning) or after (postconditioning) ischemic stroke and potential outcomes.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 13424 | 41 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA